Abstract
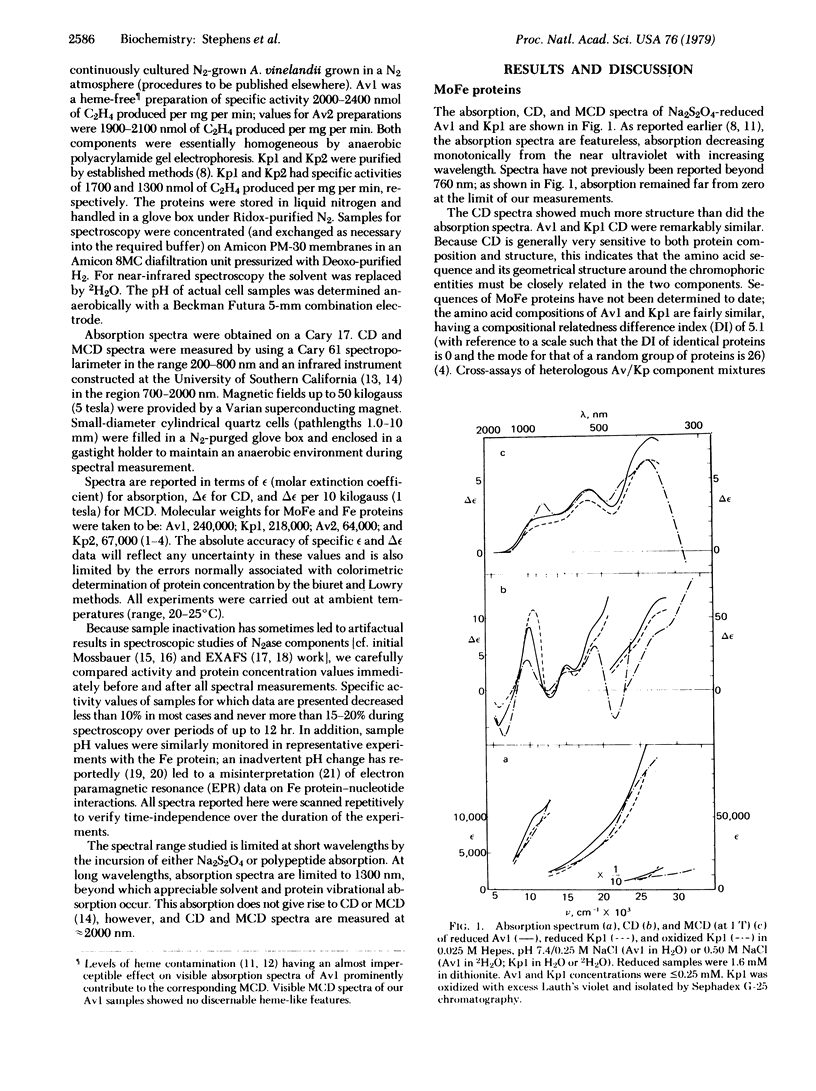
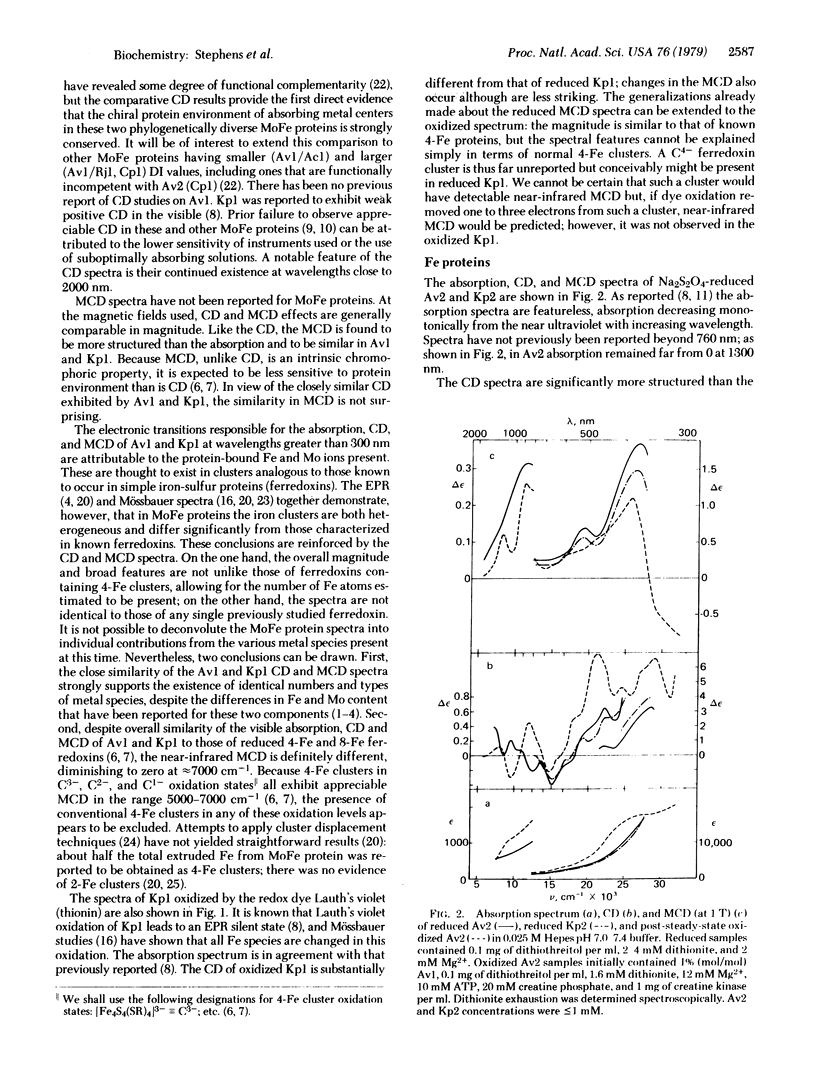
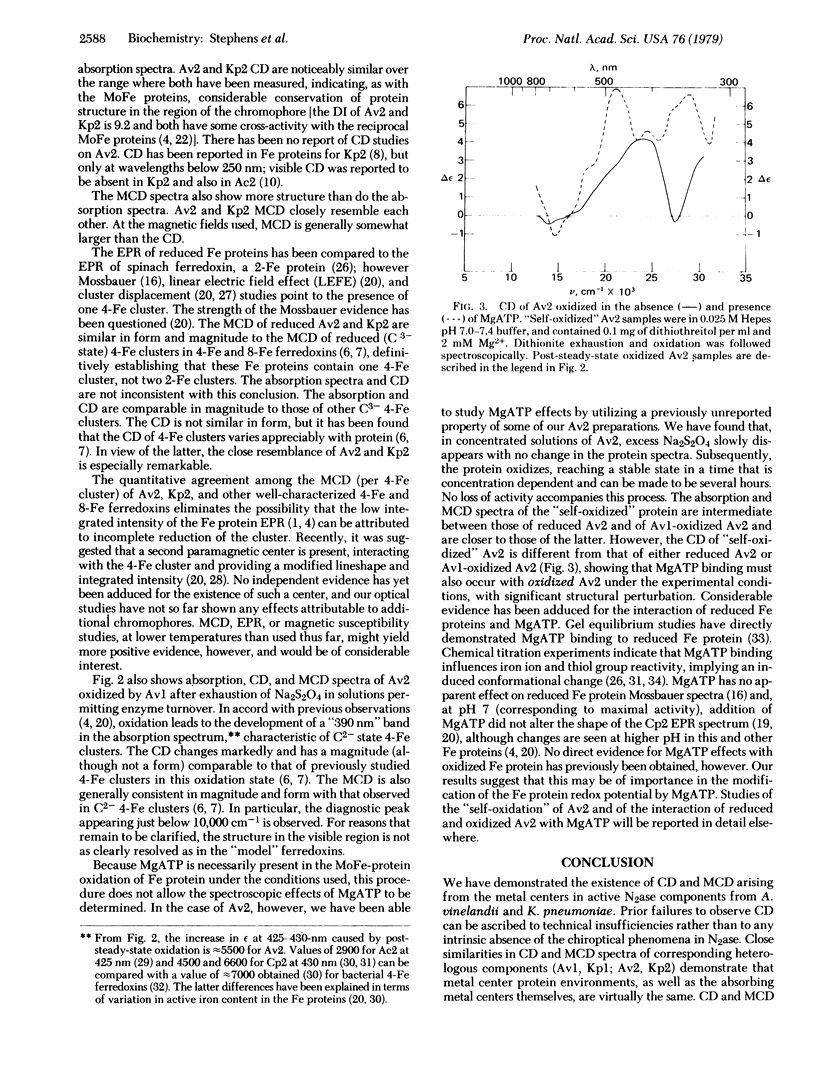
Circular dichroism (CD) and magnetic circular dichroism (MCD) spectra of nitrogenase components (MoFe protein and Fe protein) from Azotobacter vinelandii (Av) and Klebsiella pneumoniae (Kp) have been obtained in the near infrared—visible—near ultraviolet spectral region. Previously, visible CD was reported to be absent or barely detectable in nitrogenase proteins; MCD spectra have not been reported. The chiroptical spectra can be measured in solution at room temperature, an advantage relative to spectroscopic methods requiring cryogenic sample temperatures. Absorption spectra were also obtained. The CD and MCD are markedly more structured, and thus interpretively more useful, than the corresponding absorption spectra. The dithionite-reduced MoFe proteins (Av1, Kp1) have nearly identical CD and MCD, demonstrating identical numbers and types of metal centers in similar protein environments. The CD and MCD cannot be explained solely in terms of contributions from known 4-Fe or 2-Fe clusters; the near-infrared MCD is inconsistent with the presence of known 4-Fe clusters. CD and MCD spectra of Lauth's violet-oxidized Kp1 are also reported. The reduced Fe proteins (Av2, Kp2) have similar CD and MCD, again indicating significant conservation of chromophore environment. The spectra clearly demonstrate the presence of a reduced bacterial ferredoxin-like (C3-) 4-Fe cluster. No obvious evidence of additional chromophores is observed. CD, MCD, and absorption spectra of Av1-oxidized Av2 are reported. The absorption spectrum shows the expected shoulder near 390 nm. The CD and MCD are characteristic of a C2- 4-Fe cluster; in particular, the diagnostic near-infrared MCD peak is observed at ≈8300 cm-1. The CD of Av2 oxidized in the presence and absence of MgATP are radically different, providing the first direct evidence for MgATP interaction with Fe protein in this oxidation state.
Keywords: nitrogen fixation, chiroptical spectroscopy, molybdoenzymes, iron-sulfur proteins
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burns R. C., Hardy R. W. Purification of nitrogenase and crystallization of its Mo-Fe protein. Methods Enzymol. 1972;24:480–496. doi: 10.1016/0076-6879(72)24094-0. [DOI] [PubMed] [Google Scholar]
- Chen J. S., Multani J. S., Mortenson L. E. Structural investigation of nitrogenase components from Clostridium pasteurianum and comparison with similar components of other organisms. Biochim Biophys Acta. 1973 May 17;310(1):51–59. doi: 10.1016/0005-2795(73)90007-x. [DOI] [PubMed] [Google Scholar]
- Cramer S. P., Eccles T. K., Kutzler F. W., Hodgson K. O., Mortenson L. E. Letter: Molybdenum X-ray absorption edge spectra. The chemical state of molybdenum in nitrogenase. J Am Chem Soc. 1976 Mar 3;98(5):1287–1288. doi: 10.1021/ja00421a053. [DOI] [PubMed] [Google Scholar]
- Davis L. C., Orhme-Johnson W. H. Nitrogenase IX. Effect of the MgATP generator on the catalytic and EPR properties of the enzyme in vitro. Biochim Biophys Acta. 1976 Nov 8;452(1):42–58. doi: 10.1016/0005-2744(76)90056-5. [DOI] [PubMed] [Google Scholar]
- Eady R. R., Smith B. E., Cook K. A., Postgate J. R. Nitrogenase of Klebsiella pneumoniae. Purification and properties of the component proteins. Biochem J. 1972 Jul;128(3):655–675. doi: 10.1042/bj1280655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D. W., Burris R. H. Complementary functioning of the component proteins of nitrogenase from several bacteria. J Bacteriol. 1978 Jun;134(3):936–943. doi: 10.1128/jb.134.3.936-943.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillum W. O., Mortenson L. E., Chen J. S., Holm R. H. Quantitative extrusions of the Fe4S4 cores of the active sites of ferredoxins and the hydrogenase of Clostridium pasteurianum. J Am Chem Soc. 1977 Jan 19;99(2):584–595. doi: 10.1021/ja00444a044. [DOI] [PubMed] [Google Scholar]
- Hageman R. V., Burris R. H. Nitrogenase and nitrogenase reductase associate and dissociate with each catalytic cycle. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2699–2702. doi: 10.1073/pnas.75.6.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly M., Lang G. Evidence from Mossbauer spectroscopy for the role of iron in nitrogen fixation. Biochim Biophys Acta. 1970 Nov 3;223(1):86–104. doi: 10.1016/0005-2728(70)90134-9. [DOI] [PubMed] [Google Scholar]
- Ljones T., Burris R. H. Nitrogenase: the reaction between the Fe protein and bathophenanthrolinedisulfonate as a probe for interactions with MgATP. Biochemistry. 1978 May 16;17(10):1866–1872. doi: 10.1021/bi00603a010. [DOI] [PubMed] [Google Scholar]
- Ljones T. Nitrogenase from Clostridium pasteurianum. Changes in optical absorption spectra during electron transfer and effects of ATP, inhibitors and alternative substrates. Biochim Biophys Acta. 1973 Sep 15;321(1):103–113. doi: 10.1016/0005-2744(73)90064-8. [DOI] [PubMed] [Google Scholar]
- Lowe D. J. Simulation of the electron-paramagnetic-resonance spectrum of the iron-protein of nitrogenase. A prediction of the existence of a second paramagnetic centre. Biochem J. 1978 Dec 1;175(3):955–957. doi: 10.1042/bj1750955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Iron-sulfur proteins: structure and function. Annu Rev Biochem. 1973;42(0):159–204. doi: 10.1146/annurev.bi.42.070173.001111. [DOI] [PubMed] [Google Scholar]
- Que L., Jr, Holm R. H., Mortenson L. E. Letter: Extrusion of Fe2S2 and Fe4S4 cores from the active sites of ferredoxin proteins. J Am Chem Soc. 1975 Jan 22;97(2):463–464. doi: 10.1021/ja00835a064. [DOI] [PubMed] [Google Scholar]
- Rawlings J., Shah V. K., Chisnell J. R., Brill W. J., Zimmermann R., Münck E., Orme-Johnson W. H. Novel metal cluster in the iron-molybdenum cofactor of nitrogenase. Spectroscopic evidence. J Biol Chem. 1978 Feb 25;253(4):1001–1004. [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Isolation of an iron-molybdenum cofactor from nitrogenase. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3249–3253. doi: 10.1073/pnas.74.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah V. K., Brill W. J. Nitrogenase. IV. Simple method of purification to homogeneity of nitrogenase components from Azotobacter vinelandii. Biochim Biophys Acta. 1973 May 30;305(2):445–454. doi: 10.1016/0005-2728(73)90190-4. [DOI] [PubMed] [Google Scholar]
- Smith B. E., Lang G. Mössbauer spectroscopy of the nitrogenase proteins from Klebsiella pneumoniae. Structural assignments and mechanistic conclusions. Biochem J. 1974 Feb;137(2):169–180. doi: 10.1042/bj1370169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens P. J., Thomson A. J., Dunn J. B., Keiderling T. A., Rawlings J., Rao K. K., Hall D. O. Circular dichroism and magnetic circular dichroism of iron-sulfur proteins. Biochemistry. 1978 Oct 31;17(22):4770–4778. doi: 10.1021/bi00615a026. [DOI] [PubMed] [Google Scholar]
- Stephens P. J., Thomson A. J., Keiderling T. A., Rawlings J., Rao K. K., Hall D. O. Cluster characterization in iron-sulfur proteins by magnetic circular dichroism. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5273–5275. doi: 10.1073/pnas.75.11.5273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneley R. N., Yates M. G., Lowe D. J. Nitrogenase of Azotobacter chroococcum. Kinetics of the reduction of oxidized iron-protein by sodium dithionite. Biochem J. 1976 Apr 1;155(1):137–144. doi: 10.1042/bj1550137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tso M. Y., Burris R. H. The binding of ATP and ADP by nitrogenase components from Clostridium pasteurianum. Biochim Biophys Acta. 1973 Jun 6;309(2):263–270. doi: 10.1016/0005-2744(73)90024-7. [DOI] [PubMed] [Google Scholar]
- Winter H. C., Burris R. H. Nitrogenase. Annu Rev Biochem. 1976;45:409–426. doi: 10.1146/annurev.bi.45.070176.002205. [DOI] [PubMed] [Google Scholar]
- Yates M. G., Planqué K. Nitrogenase from Azotobacter chroococcum. Purification and properties of the component proteins. Eur J Biochem. 1975 Dec 15;60(2):467–476. doi: 10.1111/j.1432-1033.1975.tb21025.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann R., Münck E., Brill W. J., Shah V. K., Henzl M. T., Rawlings J., Orme-Johnson W. H. Nitrogenase X: Mössbauer and EPR studies on reversibly oxidized MoFe protein from Azotobacter vinelandii OP. Nature of the iron centers. Biochim Biophys Acta. 1978 Dec 20;537(2):185–207. doi: 10.1016/0005-2795(78)90504-4. [DOI] [PubMed] [Google Scholar]
- Zumft W. G., Mortenson L. E., Palmer G. Electron-paramagnetic-resonance studies on nitrogenase. Investigation of the oxidation-reduction behaviour of azoferredoxin and molybdoferredoxin with potentiometric and rapid-freeze techniques. Eur J Biochem. 1974 Aug 1;46(3):525–535. doi: 10.1111/j.1432-1033.1974.tb03646.x. [DOI] [PubMed] [Google Scholar]


