Abstract
Aging is characterized by a progressive impairment of (a) cardiac structure including fibrosis and cardiomyocyte density, and (b) cardiac function including stroke volume, ejection fraction, and cardiac output. The cardiac remodeling involves loss of cardiac myocytes, reactive hypertrophy of the remaining cells, and increased extracellular matrix (ECM) and fibrosis in the aging heart, especially left ventricles. Fibrosis (i.e., accumulation of collagen) with aging is very critical in impairing cardiac function associated with increased myocardial stiffness. The balance of ECM remodeling via ECM synthesis and degradation is essential for normal cardiac structure and function. Thus an understanding of upstream ECM regulatory factors such as matrix metalloproteinases (MMPs), tissue inhibitors of metalloproteinases (TIMPs), tumor necrosis factor-α (TNF-α), transforming growth factor-β (TGF-β), and myofibroblasts is necessary for gaining new insights into managing cardiac remodeling and dysfunction with aging. In contrast, exercise training effectively improves cardiac function in both young and older individuals. Exercise training also improves maximal cardiovascular function by increasing stroke volume and cardiac output. However, limited data indicate that exercise training might attenuate collagen content and remodeling in the aging heart. We recently found that 12 weeks of exercise training protected against geometric changes of collagen ECM in the aging heart and ameliorated age-associated dysregulation of ECM in the heart, as indicated by up-regulation of active MMPs as well as down-regulation of TIMPs and TGF-β. This review will provide a summary and discussion of aging and exercise effects on fibrosis and upstream regulators of ECM in the heart.
Keywords: Aging, Exercise, Extracellular matrix, Collagen, Heart
INTRODUCTION
Myocardial tissue is composed of cardiac myocytes, nonmyocytes (e.g., fibroblasts, endothelial cells, vascular smooth muscle cells, etc), and extracellular matrix (ECM) proteins (Baudino et al., 2006; Bowers and Baudino, 2012; Camelliti et al., 2005; Curtis and Russell, 2011; Souders et al., 2009). Myocardial ECM is essential for proper cardiac structural integrity and pump function (Curtis and Russell, 2011). The ECM a) provides a scaffold for myocytes, fibroblasts, and endothelial cells, and b) transmits mechanical forces and signals to myocardial fibers (Baudino et al., 2006). The ECM also provides mechanical stability, physical strength, stiffness, ductility, and energy absorption to tissues. The ECM is essential for efficient cardiac function via myocyte alignment, regulating blood flow during contraction, and compliance. Moreover, the ECM is an important mediator of growth-related factor and in modulating the cardiac phenotype during development and hypertrophy. Therefore, the disruption of ECM homeostasis is a key factor for the progression of cardiac dysfunction (Baudino et al., 2006).
Myocardial ECM is composed of collagens (e.g., fibril-forming collagens and non-fibril forming collagens), glycoproteins (e.g., fibronectins, elastin, laminins, etc), proteoglycans, extracellular proteases, and ECM receptors (Corda et al., 2000; Goldsmith and Borg, 2002). ECM in the heart is linked to cellular cytoskeleton by transmembrane molecules, mainly integrins, which provides a physical connection between cytoskeleton and ECM proteins (Corda et al., 2000; Sarasa-Renedo and Chiquet, 2005). The interactions among ECM, cytoskeleton, and cell through integrins might be very important during cardiac remodeling (Goldsmith and Borg, 2002; Jane-Lise et al., 2000; Rosso et al., 2004). Although glycoproteins and proteoglycans are essential in proper cardiac geometry and various functions of the ECM, the most abundant structural components of the ECM are collagens (Bowers and Baudino, 2012), which are produced primarily by fibroblasts either on the membrane-bound ribosomes of the rough endoplasmic reticulum (ER) or placed within the ECM, respectively (Kjaer, 2004). The ability to synthesize the ECM components depends on cell types in the heart. For example, fibroblasts and smooth muscle cells synthesize collagen types I and III and fibronectin, whereas cardiac myocytes and endothelial cells produce collagen type IV (Corda et al., 2000). In addition, laminin is produced by cardiac myoctyes, smooth muscle cells, and endothelial cells (Corda et al., 2000). Alterations in the profile of ECM proteins can play a profound influence on the form and function of heart.
The aging heart is characterized by decreased myocyte number, increased myocyte size, and increased extracellular matrix compared with younger heart (Kwak et al., 2006). Cell death by apoptosis or necrosis is very critical determinant of ECM remodeling because it induces a loss of contractile tissue, reactive compensatory hypertrophy of remaining cardiomyocytes, and accumulation of collagen (i.e., fibrosis) and other ECM proteins (Jugdutt, 2003). These phenotypic changes of the myocardium with aging occur in the mainly left ventricle. For example, apoptosis, programmed cell death, is localized into the left ventricle, suggesting that it is initiated by mechanical factors (Kajstura et al., 1996). Overall, myocardial remodeling is determined by the consequence of changes in cardiac myocytes and disruption of ECM homeostasis. The ECM remodeling caused by aging results in myocardial remodeling, contributing to rearrangement of normally existing structures (Swynghedauw, 1999). The ECM remodeling also occurs in dilated cardiomyopathy (Pauschinger et al., 2002) and myocardial infarction (Lindsey et al., 2003). The ECM is a fibrillar network that embeds cardiomyocytes and the whole cardiac structure. The ECM remodeling is a critical part of mortality in the elderly. Furthermore, aging seriously affects the myocardial structure and function, as the fundamental biological process of aging is associated with an increased arterial hypertension, atherosclerosis, and decreased physical activity.
Fibrosis is a complicated tissue response that causes the excessive deposition of ECM, especially collagens (Krieg and LeRoy, 1998; Souders et al., 2009). Fibrosis, one of the major biological determinants of cardiac remodeling, is an increased collagen content and concentration, resulting in increased myocardial stiffness and cardiac dysfunction. Fibrosis is multifactorial, and it is resulted from aging, myocardial ischemia, inflammatory processes, hormones, vasoactive peptides, or diabetes (Swynghedauw, 1999). There are converging reports suggesting that myocardial fibrosis occurs in senescent hearts both in rats and humans (Kwak et al., 2011). Furthermore, there is emerging evidence that aging is associated with increased cardiac fibroblasts (Camelliti et al., 2005; Krenning et al., 2010). The ECM remodeling with aging including modifications of ECM protein synthesis and degradation would suggest that the aging heart might be unable to adapt to an increased load well (Burgess et al., 2001; Debessa et al., 2001; Mendes et al., 2012; Nguyen et al., 2001).
COLLAGENS IN THE HEART
Collagens are a regulated family of ECM proteins that provide structure and optimize function of the heart (Baudino et al., 2006; Souders et al., 2009). Presently, more than 20 collagen types have been identified in various vertebrate tissues. Collagen is the most abundant protein in ECM and forms the essential mechanical building blocks, providing tensile strength and resisting stretch (Jugdutt, 2003). The collagen is composed of three α-chains called triple helix or tropocollagen molecule. The common structure of collagens is repeating amino acid sequence (Gly-X-Y) that comprises the collagen chain. Most of collagens are present in the forms of polypeptide chains called collagen molecule or α-chain, consisting of glycine, proline, and hydroxyproline with hydroxylysine (Jugdutt, 2003). The network of collagens structurally exists at three levels named a) endomysium surrounding individual muscle fibers, b) perimysium surrounding groups of myocytes, and c) epimysium surrounding the entire muscle (DeSouza, 2002). Connective tissue consists mainly of collagen, and to a much lesser extent, fibronectin, laminin, and elastic fibers (Carvalho Filho et al., 1996).
The collagens can be divided into two major classes, the fibrillar-forming and non-fibrillar-forming collagens (Jugdutt, 2003). Among collagens, five of collagens (I, II, III, V, and XI) form fibrils (Jugdutt, 2003). The fibril-forming collagens provide the structural framework of tissues. In particular, collagen types I and III in myocardial collagens are predominantly interstitial collagens in the heart that surround cardiac myocytes and the coronary microcirculation, providing structural integrity for the cardiomyocytes (Goldsmith and Borg, 2002; Jugdutt, 2003; Kassiri and Khokha, 2005). Type I collagen type makes up approximately 85% and type III collagen 11% of total collagen in the heart (DeSouza, 2002; Jugdutt, 2003). Although collagen types I and III coexist in the ECM, especially both in the perimysium and endomysium (DeSouza, 2002), there are some differences due to the composition of the α-chains that comprise the collagen triple-helix. Collagen type I is a hybrid, consisting of two identical α1 (I) chains and one α2 (I) chain that form the superhelix (Debessa et al., 2001). Collagen type I is thick, yellow or red, strong fibers and is thought to play an essential role in providing structural stability to tissues, whereas collagen type III contains three identical α1 (III) chains (Debessa et al., 2001). Collagen type III is thin, greenish fibers, and fine reticular network in most soft connective tissue unlike the larger fibers that are derived from collagen type I molecules (Debessa et al., 2001). Collagen type I provides high tensile strength and stiffness to tissues, whereas collagen type III provides high compliance to tissues (Jugdutt, 2003). So, the ratio of collagen type III to I has been implicated in functional properties of the heart, with a higher ratio of collagen type III to I indicating more compliant tissue and a lower ratio of collagen type III to I indicating a stiffer, less compliant tissue (Jugdutt, 2003).
REGULATION OF CARDIAC COLLAGEN ECM
Collagen ECM plays an important role in cardiovascular function, and remodeling in the ECM contributes to myocardial dys-function (Goldsmith and Borg, 2002; Porter and Turner, 2009). Myocardial failure and remodeling are usually characterized by collagen accumulation, collagen fibril disruption, myocyte loss via apoptosis or necrosis, and impaired rearrangement of structure (Swynghedauw, 1999). In particular, accumulation of collagen ECM with aging in the heart could create a mechanical environment and stress distribution that contributes diminished systolic performance, decreased compliance, and diastolic dysfunction (DeSouza, 2002). Therefore, the balance of ECM remodeling via collagen ECM synthesis and degradation is essential for normal cardiac structure and function (Jugdutt, 2003; Souders et al., 2009). Collagen ECM remodeling is modulated by regulatory proteins, hormonal factors, cytokines, and growth factors (Baudino et al., 2006; Camelliti et al., 2005). Thus an understanding of upstream ECM regulatory factors such as MMPs, TIMPs, TNF-α, TGF-β, and myofibroblasts provides therapeutic strategies to protect against cardiac remodeling and dysfunction with aging (Fig. 1).
Fig. 1.
Collagen ECM turnover signaling in the heart. Altered mechanical stress (σ) and oxidative stress may stimulate TNF-α, TGF-β, and MMP. TNF-α may stimulate MMP and inhibit TIMP. However, TGF-β may inhibit MMP and stimulate TIMP and myofibroblast. Finally, MMP degrades collagens, but TIMP and myofibroblast inhibit collagen degradation and promote collagen synthesis, which determine collagen ECM remodeling.
Cardiac MMPs and TIMPs
ECM depends on a balance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs), which determines cardiac remodeling (Ahmed et al., 2006; Jugdutt, 2003; Benjamin and Khalil, 2012). MMPs are an endogenous family of enzymes that degrade ECM proteins, which are responsible for ECM remodeling in a number of physiological and pathological process (Ahmed et al., 2006; Benjamin and Khalil, 2012; Tsuruda et al., 2004). To date, the MMP family consists of more than 20 unique proteins in vertebrates. Most of MMPs are inactive enzymes that are activated in ECM. It has been shown that MMPs highly related with myocardial remodeling are collagenases (e.g., MMP-1 and MMP-13), gelatinases (e.g., MMP-2 and MMP-9), stromelysin (e.g., MMP-3), and the membrane-type MMP (e.g., MMP-14) (Kassiri and Khokha, 2005). These kinds of MMPs degrade predominantly collagen types I and III in the ECM of the heart (Schupp et al., 2006).
MMPs are Ca2+-and Zn2+-dependent proteases that are usually synthesized as an inactive form or pro-MMP, which is activated by the cleavage of an amino-terminal propeptide domain either by autoproteolysis, another MMP, or serine protease (Jugdutt, 2003). For example, MMP-14 activates MMP-2, which requires TIMP-2 binding to its active place (Lafleur et al., 2003). MMPs in the heart are expressed primarily by fibroblasts (Chapman et al., 2003) and cardiomyocytes (Coker et al., 1999). Most pro-MMPs are stored extracellular bound to different ECM components. Upon stimulation, activated MMPs degrade the ECM proteins including collagens, fibronectin, laminin, gelatin, and proteoglycan. Therefore, MMPs are significant regulators of ECM turnover in the heart, thus contributing to physiological function as well as pathology. In contrast, activity of MMPs is in part regulated by endogenous inhibitors (Benjamin and Khalil, 2012; Jugdutt, 2003). TIMPs are specific MMP inhibitors in the ECM (Lovelock et al., 2005). The role of TIMPs is to prevent excessive ECM degradation by MMPs. There are 4 TIMPs identified in vertebrates, TIMP-1, -2, -3, and -4, acting as the natural inhibitors of active forms of all MMPs through binding to MMPs in a 1:1 ratio (Cleutjens and Creemers E, 2002). Among them, TIMP-1, -2, and -4 are soluble forms, whereas TIMP-3 binds to the ECM via heparan sulfate proteoglycans within the ECM (Yu et al., 2000). The balance between MMPs and TIMPs plays a critical role in the process of cardiac ECM remodeling which contributes to cardiac function. Based on previous findings, it appears that cardiac ECM remodeling is generally associated with enhanced MMP and reduced TIMP activities (Jugdutt, 2003). However, there are differences in the studies. For example, the levels of TIMP-1 were either repressed (Tyagi et al., 1996) or increased (Thomas et al., 1998) in dilated cardiomyopathy patients.
It has been shown that inhibition of MMP activity is beneficial during cardiac remodeling and wall stress following injury due to myocardial infarction (Benjamin and Khalil, 2012; Peterson, 2006). For example, Rohde et al. (1999) indicated that a broad range MMP inhibitor attenuated left ventricular dilatation 4 days after infarction in a mouse myocardial infarction. In addition, inhibition of MMP-9 activity attenuated left ventricular enlargement and collagen content after myocardial infarction (Ducharme et al., 2000). Therefore, MMP inhibition might be a new therapeutic treatment to control cardiac dysfunction and failure. A few publications indicated that MMP levels increased, and TIMP levels decreased in the rat heart with advancing age. For example, Lindsey et al. (2005) found that the levels of MMP-3, MMP-8, MMP-9, MMP-12, and MMP-14 increased, and the levels of TIMP-3 and TIMP-4 decreased in the insoluble fraction of old mice, compared with young adult mice, suggesting that aging is associated with increased ECM degradative capacity. However, much different findings were reported by Robert et al. (1997). Their results indicated a 40–45% decrease in both MMP-2 and pro-MMP-1 activity and mRNA in 24-month-old rat heart, suggesting that the reduction of ECM degradation pathway by MMP allows accumulation of collagen and promotion of age-associated fibrosis. Thus, the current literature is unclear about MMP or TIMP expression with aging in the heart.
Cardiac TNF-α
Upstream regulators of MMPs and TIMPs include inflammatory cytokines in the heart (Siwik and Colucci, 2004; Tsuruda et al., 2004). It seems likely that the cytokines may lead to an imbalance in myocardial MMP/TIMP ratio resulting in altered myocardial ECM architecture and development of left ventricle remodeling and dysfunction (Jugdutt, 2003; Siwik and Colucci, 2004). Among cytokines, tumor necrosis factor-α (TNF-α), a pro-inflammatory cytokine, can increase the matrix collagen degradation by upregulating MMP activity and downregulating TIMPs (Jugdutt, 2003; Murray et al., 2010; Siwik and Colucci, 2004). TNF-α has a variety of different biological capacities in response to one or more different forms of environmental stress in heart failure, including LV dysfunction, cardiomyopathy, LV remodeling, abnormalities of mitochondrial energetics, increased production of reactive oxygen, and cardiac myocyte apoptosis (Mann, 2002). In particular, LV remodeling by TNF-α is involved in alterations in the biology of the cardiac myocyte, progressive myocyte loss, and alterations in ECM including synthesis and degradation of collagen matrix (Mann, 2002; Murray et al., 2010).
Significantly increased levels of TNF-α have been demonstrated in patients with dilated or ischemic cardiomyopathy (Oral et al., 1999) and in animal models of myocardial infarction (Irwin et al., 1999). Furthermore, Li et al. (2000) suggested that cardiac over-expression of TNF-α in transgenic mice caused increases in MMP-2 and MMP-9 activity as well as marked diastolic dysfunction. In isolated cardiac fibroblasts, TNF-α decreases collagen synthesis, increases MMP expression, and decreases TIMP expression (Siwik et al., 2000). In contrast, Sivasubramanian et al. (2001) reported that there were significant decreases in total MMP activity and elevated TIMP-1 levels in the cardiac overexpression of TNF-α in transgenic mice, suggesting a possible mechanism for the increase in myocardial fibrosis. Mann (2002) also showed that TNF-α promoted cardiac fibroblast proliferation and fibrosis. Although the mechanisms by which TNF-α affect MMP and TIMP may depend on in vitro and in vivo models, TNF-α may indeed induce an imbalance in MMP/TIMP ratio, remodeling and fibrosis in the heart.
Cardiac TGF-β
Transforming growth factor-β (TGF-β) is a multifunctional cytokine that plays an important role in cell migration, proliferation, differentiation, apoptosis, and ECM protein production (Annes et al., 2003; Baudino et al., 2006; Hinck, 2012; Sales et al., 2006). TGF-β, an anti-inflammatory cytokine, is a potent stimulator of collagen synthesis (Siwik and Colucci, 2004). It consists of three isoforms, TGF-β1, TGF-β2, and TGF-β3 that are structurally and functionally closely related to one another (Annes et al., 2003). The TGF-β released from platelets and leukocytes stimulates the synthesis of ECM components including collagens, fibronectin, proteoglycans, and integrins in tissue repair after injury. It mediates collagen synthesis through increasing transcription and decreasing collagen degradation via reduced MMPs or enhanced TIMPs, thus favoring an accumulation of ECM and especially of collagen (Siwik and Colucci, 2004). For example, Seeland et al. (2002) suggested that the overexpression of TGF-β1 in transgenic mice resulted in increased protein expression of collagen types I and III, reduced interstitial collagenase protein activity and mRNA expression, and increased TIMP-1, -2, and -4 protein levels in the heart. Additionally, in cardiac fibroblasts, procollagen formation was stimulated by mechanical loading and TGF-β.
Acute exercise or mechanical loading may stimulate TGF-β synthesis in the heart (Calderone et al., 2001), smooth muscle (Gutierrez and Perr, 1999), skeletal muscle (Gavin and Wagner, 2001), and circulating blood (Heinemeier et al., 2003) as a physiological response. For example, Calderone et al. (2001) reported that TGF-β1 mRNA increased in the left ventricle of a voluntary exercise rat model of physiological cardiac hypertrophy. However, excessive and chronic expression of TGF-β is associated with many fibrotic diseases including cardiac fibrosis after infarction, lung fibrosis, and scarring (Annes et al., 2003). TGF-β may play a role in stimulating abnormal accumulation signaling of ECM proteins in the cardiovascular diseases. Rosenkranz et al. (2002) showed that TGF-β overexpression in the transgenic mice heart resulted in cardiac hypertrophy and fibrosis. Similarly, Brooks and Conrad (2000) found that TGF-β1 deficient old mice heart exhibited a decrease in myocardial fibrosis and reduced myocardial stiffness, indicating the role of TGF-β to contribute to ECM component synthesis in the heart.
Cardiac myofibroblasts
Myofibroblast is a differentiated cell type from fibroblast characterized by increased ECM protein synthesis called fibrosis formation, providing an essential role for ECM remodeling during normal and pathological wound healing (Cleutjens and Creemers, 2002; Powell et al., 1999; Tomasek et al., 2002). Myofibroblasts as a smooth-muscle like fibroblasts might be produced from progenitor stem cells in the heart or from the circulation, and secret cytokines (e.g., TNF-α), growth factors (e.g., TGF-β), chemokines, and inflammatory mediators (Porter and Turner, 2009; Powell et al., 1999). In addition, differentiation to the myofibroblast may be induced by transforming growth factor-β1 (TGF-β1) (Tomasek et al., 2002). Myofibroblast expression may be not detectable in the normal healthy adult hearts, while myofibroblasts are often associated with injured heart such as myocardial infarction for wound healing (Baudino et al., 2006; Poobalarahi et al., 2006). In particular, Poobalarahi et al. (2006) reported that increased type I collagen synthesis by myofibroblasts was accompanied by a significant increase in collagen deposition into insoluble ECM in the heart. Accordingly, myofibroblasts appear to play a critical role in production of cardiac ECM in response to injury (Porter and Turner, 2009). In addition, differentiated myofibroblasts are unique in that they express α-smooth muscle actin (α-SMA) unlike adult fibroblasts (Chaponnier and Gabbiani, 2004; Powell et al., 1999).
Expression of α-SMA positive myofibroblasts appears to be regulated by TGF-β1 (Chaponnier and Gabbiani, 2004). A similar finding was also reported that TGF-β1 promoted the conversion of myofibroblasts in vitro (Gabbiani, 2003). Additionally, Kuwahara et al. (2002) found that TGF-β1 function-blocking antibodies administered to pressure-overload rats prevented the myofibroblasts conversion in cardiac interstitium and subsequent increases in mRNA of type I collagen as well as diastolic heart failure. Interestingly, Porter et al. (2004) showed that TNF-α via a TNF-R1 receptor also increased myofibroblast proliferation in human heart.
AGING, EXERCISE, AND COLLAGEN ECM IN THE HEART
Aging and cardiac collagens
Myocardial remodeling during aging is related with changes in the amount and organization of ECM components (Swynghedauw, 1999; Kwak et al., 2011). In particular, myocardial collagens in ECM undergo remodeling with aging. A healthy arrangement of collagens provides a framework for myocyte sheath sliding, transmittance of force from myocyte to the ventricular chamber, prevents excessive stretch and damage, and preserves heart function (DeSouza, 2002). However, excessive accumulation of collagen matrix is up-regulated in a number of cardiovascular diseases. Moreover, aging also increases the rate of ventricular collagen turnover and deposition by fibroblasts called fibrosis (Baudino et al., 2006; Mendes et al., 2012; Thomas et al., 2000, 2001). Fibrosis with aging is characterized by increased collagen content (Hwang et al., 2007), decreased collagen solubility, and increased collagen cross-linking (Thomas et al., 2000, 2001). This increase in collagen deposition during aging may be thought to result from a combination of cellular events including increased collagen synthesis and decreased degradation (Kwak et al., 2011). The collagen might become more resistant to collagenase degradation with aging (Jugdutt, 2003). Excessive accumulation of collagen in the heart could lead to tissue stiffness, increase the incidence of arrhythmias, disrupt electronic communication between myocytes, and result in diastolic and systolic dysfunction and heart failure (Baudino et al., 2006; Mendes et al., 2012).
Previous studies have demonstrated age-related changes in cardiac collagen concentration (Hwang et al., 2007; Lindsey et al., 2005; Mendes et al., 2012; Nguyen et al., 2001; Thomas et al., 2000, 2001). Debessa et al. (2001) indicated that the number and thickness of Type I collagen increased from adulthood to old age in human heart. Studies in animal hearts also provided consistent evidence of an increase in myocardial collagen concentration with aging (Nguyen et al., 2001; Thomas et al., 2000, 2001). These findings were confirmed by Nguyen et al. (2001), who examined the collagen concentration in the left ventricles of Fischer 344 rats at 6, 18, and 24 months of age. Their results revealed that the collagen concentration, as determined by hydroxyproline assay, progressively increased during aging with greatest increments from 6 to 18 months, then leveling off at 24 months. Similar findings were previously described by Mays et al. (1988), who found a gradual increase in collagen concentration, based on hydroxyproline levels between 2 weeks and 24 months of age. In addition, Lindsey et al. (2005) reported that total collagen levels increased with advancing age. Taken together, the increased collagen content/ concentration might be an integral part of ECM remodeling that takes place in the left ventricle consequent to the natural aging process leading to an increase in myocardial passive stiffness and impaired contractile function.
A few studies also have showed age-related increases in collagen cross-linking in cardiac muscle (Thomas et al., 2000, 2001). Increased collagen cross-linking could be implicated as a potential mechanism for an impaired extensibility and increased stiffness in aged heart. For example, Thomas et al. (2000, 2001) reported that there were significant overall age–related increases in collagen cross-linking in the both left ventricle and septum. In addition to the heart, skeletal muscle also showed the same phenomena that collagen content and collagen cross-linking significantly increased from young to senescence in skeletal muscle (Gosselin et al., 1998).
Exercise and cardiac collagens
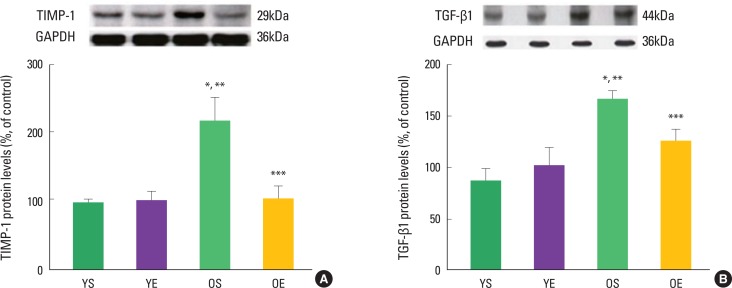
Alterations in collagen profile have been shown to occur following exercise training in heart (Thomas et al., 2000, 2001) and skeletal muscle (Gosselin et al., 1998). For example, Thomas et al. (2001) observed that ten weeks of treadmill exercise training reduced age-induced up-regulation of collagen concentration (percent collagen) in the left ventricle septum of rats. The collagen cross-linking (HP) of left ventricle free wall was significantly lower in old trained rats, compared with their sedentary counterparts (Thomas et al., 2000, 2001). In addition, our research group recently demonstrated that 12 weeks of exercise training in rats significantly ameliorated age-associated increases in extramyocyte space and collagen-positive staining (Kwak et al., 2006, 2011). We also found that exercise training protected against age-related down-regulation of active MMPs (e.g., MMP-1, MMP-2, MMP-3, and MMP-14). Consistent with the inhibitory effects of TIMP-1 on MMP activation, aging dramatically increased TIMP-1 protein levels, whiles exercise training alleviated age-induced increase in TIMP-1 protein levels (Fig. 2). Exercise training also mitigated age-associated increase of TGF-β1 in the heart (Fig. 2). TGF-β1 is a potent stimulator of TIMP-1 and a potential contributor to fibrosis in the aging heart.
Fig. 2.
Effects of aging and exercise training on TIMP-1 and TGF-β1 in the heart. (A) TIMP-1 protein levels and (B) TGF-β1 protein levels of left ventricles in young sedentary (YS), young exercise (YE), old sedentary (OS), and old exercise (OE) groups (Kwak et al., 2011).
Conflicting results were also reported by others. Burgess et al. (1996) suggested that total collagen concentration (hydroxylproline) of rat left ventricle did not change by 10 weeks of treadmill exercise training. In addition, collagen type III -to-I ratio was not altered by exercise training in the rat heart (Burgess et al., 1996). In addition, Woodiwiss et al. (1998) found that 16 weeks of habitual voluntary wheel running had no effects on myocardial collagen concentration and cross-linking in the rat left ventricle, although cardiac stiffness was reduced. Similarly, Jin et al. (2000) showed that mRNA levels of collagen types I and III did not change with 13 week treadmill exercise training in the rat heart. So, based on previous findings, the role of exercise training on collagen concentration and cross-linking in the heart remains to be clarified.
CONCLUSIONS
In summary, aging resulted in increases in extramyocyte space and collagen contents associated with down-regulation of MMPs and up-regulation of TIMP-1 and TGF-β1 in the heart. However, exercise training ameliorated the age-related alterations in pathway signaling (e.g., MMPs-TIMP-1-TGF-β1), suggesting that exercise training protects against fibrosis and ECM remodeling in the aging heart. Further research is necessary to identify therapeutic targets to mitigate fibrosis, cardiovascular disease, and heart failure prevalent with advancing age.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2012R1A1A1042383) and Inha University Research Grant (INHA-47286)
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Ahmed SH, Clark LL, Pennington WR, Webb CS, Bonnema DD, Leonardi AH, McClure CD, Spinale FG, Zile MR. Matrix metalloproteinases/tissue inhibitors of metalloproteinases: relationship between changes in proteolytic determinants of matrix composition and structural, functional, and clinical manifestations of hypertensive heart disease. Circulation. 2006;113:2089–2096. doi: 10.1161/CIRCULATIONAHA.105.573865. [DOI] [PubMed] [Google Scholar]
- Annes JP, Munger JS, Rifkin DB. Making sense of latent TGFβ activation. J Cell Sci. 2003;116:217–224. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- Baudino TA, Carver W, Giles W, Borg T. Cardiac fibroblasts; friends or foe? Am J Physiol Heart Circ Physiol. 2006;291:1015–1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- Benjamin MM, Khalil RA. Matrix metalloproteinase inhibitors as investigative tools in the pathogenesis and management of vascular disease. EXS. 2012;103:209–279. doi: 10.1007/978-3-0348-0364-9_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers SL, Baudino TA. Cardiac myocyte-fibroblast interactions and the coronary vasculature. J Cardiovasc Transl Res. 2012;5:783–793. doi: 10.1007/s12265-012-9407-2. [DOI] [PubMed] [Google Scholar]
- Brooks WW, Conrad CH. Myocardial fibrosis in transforming growth factor β1 heterozygous mice. J Mol Cell Cardiol. 2000;32:187–195. doi: 10.1006/jmcc.1999.1065. [DOI] [PubMed] [Google Scholar]
- Burgess ML, Buggy J, Price RL, Abel FL, Terracio L, Samarel AM, Borg TK. Exercise- and hypertension-induced collagen changes are related to left ventricular function in rat hearts. Am J Physiol Heart Circ Physiol. 1996;39:151–159. doi: 10.1152/ajpheart.1996.270.1.H151. [DOI] [PubMed] [Google Scholar]
- Burgess ML, McCre JC, Hedrick HL. Age-associated changes in cardiac matrix and integrins. Mech Ageing Dev. 2001;122:1739–1756. doi: 10.1016/s0047-6374(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Calderone A, Murphy RL, Lavoie J, Colombo F, Beliveau L. TGF-β1 and prepro-ANP mRNAs are differentially regulated in exercise-induced cardiac hypertrophy. J Appl Physiol. 2001;91:771–776. doi: 10.1152/jappl.2001.91.2.771. [DOI] [PubMed] [Google Scholar]
- Camelliti P, Borg TK, Kohl P. Structural and functional characterization of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- Carvalho Filho E, Ferraz de Carvalho CA, de Souza RR. Age-related changes in elastic fibers of human heart. Gerontology. 1996;42:211–217. doi: 10.1159/000213795. [DOI] [PubMed] [Google Scholar]
- Chapman RE, Scott AA, Deschamps AM, Lowry AS, Stroud RE, Ikonomidis JS, Spinale FG. Matrix metalloproteinase abundance in human myocardial fibroblasts: effects of sustained pharmacologic matrix metalloproteinase inhibition. J Mol Cell Cardiol. 2003;35:539–548. doi: 10.1016/s0022-2828(03)00077-4. [DOI] [PubMed] [Google Scholar]
- Chaponnier C, Gabbiani G. Pathological situations characterized by altered actin isoform expression. J Pathol. 2004;204:386–395. doi: 10.1002/path.1635. [DOI] [PubMed] [Google Scholar]
- Cleutjens J, Creemers E. Integration of concepts: cardiac extracellular matrix remodeling after myocardial infarction. J Cardiac Failure. 2002;8:344–348. doi: 10.1054/jcaf.2002.129261. [DOI] [PubMed] [Google Scholar]
- Coker ML, Doscher MA, Thomas CV, Galis ZS, Spinale FG. Matrix metalloproteinase synthesis and expression in isolated LV myocyte preparations. Am J Physiol Heart Circ Physiol. 1999;277:777–787. doi: 10.1152/ajpheart.1999.277.2.H777. [DOI] [PubMed] [Google Scholar]
- Corda S, Samuel JL, Rappaport L. Extracellular matrix and growth factors during heart growth. Heart Fail Rev. 2000;5:119–130. doi: 10.1023/A:1009806403194. [DOI] [PubMed] [Google Scholar]
- Curtis MW, Russell B. Micromechanical regulation in cardiac myocytes and fibroblasts: implications for tissue remodeling. Pflugers Arch. 2011;462:105–117. doi: 10.1007/s00424-011-0931-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debessa CRG, Maifrino LBM, de Sousa RR. Age related changes of collagen network of the human heart. Mech Ageing Dev. 2001;122:1049–1058. doi: 10.1016/s0047-6374(01)00238-x. [DOI] [PubMed] [Google Scholar]
- DeSouza RR. Aging of myocardial collagen. Biogerontology. 2002;3:325–335. doi: 10.1023/a:1021312027486. [DOI] [PubMed] [Google Scholar]
- Ducharme A, Frantz S, Aikawa M, Rabkin E, Lindsey M, Rohde LE, Schoen FJ, Kelly RA, Werb Z, Libby P, Lee RT. Targeted deletion of matrix metalloproteinase-9 attenuates left ventricular enlargement and collagen accumulation after experimental myocardial infarction. J Clin Invest. 2000;106:55–62. doi: 10.1172/JCI8768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G. The myofibroblast in wound healing and fibrocontractive diseases. J Pathol. 2003;200:500–503. doi: 10.1002/path.1427. [DOI] [PubMed] [Google Scholar]
- Gavin TP, Wagner PD. Effect of short-term exercise training on angiogenic growth factor gene responses in rats. J Appl Physiol. 2001;90:1219–1226. doi: 10.1152/jappl.2001.90.4.1219. [DOI] [PubMed] [Google Scholar]
- Goldsmith EC, Borg TK. The dynamic interaction of the extracellular matrix in cardiac remodeling. J Card Fail. 2002;8:314–318. doi: 10.1054/jcaf.2002.129258. [DOI] [PubMed] [Google Scholar]
- Gosselin LE, Adams C, Cotter TA, McCormick RJ, Thomas DP. Effect of exercise training on passive stiffness in locomotor skeletal muscle: role of extracellular matrix. J Appl Physiol. 1998;85:1011–1016. doi: 10.1152/jappl.1998.85.3.1011. [DOI] [PubMed] [Google Scholar]
- Gutierrez JA, Perr HA. Mechanical stretch modulates TGF-β1 and α1 (I) collagen expression in fetal human intestinal smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 1999;277:1074–1080. doi: 10.1152/ajpgi.1999.277.5.G1074. [DOI] [PubMed] [Google Scholar]
- Heinemeier K, Langberg H, Kjaer M. Exercise-induced changes in circulating levels of transforming growth factor-β-1 in humans: methodological considerations. Eur J Appl Physiol. 2003;90:171–177. doi: 10.1007/s00421-003-0881-8. [DOI] [PubMed] [Google Scholar]
- Hinck AP. Structural studies of the TGF-βs and their receptors - insights into evolution of the TGF-β superfamily. FEBS Lett. 2012;586:1860–1870. doi: 10.1016/j.febslet.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Hwang HS, Cirrincione G, Thomas DP, McCormick RJ, Boluyt MO. Aldosterone antagonism fails to attenuate age-associated left ventricular fibrosis. J Gerontol A Biol Sci Med Sci. 2007;62:382–388. doi: 10.1093/gerona/62.4.382. [DOI] [PubMed] [Google Scholar]
- Irwin MW, Mak S, Mann DL, Qu R, Penninger JM, Yan A, Dwood F, Wen WH, Shou Z, Liu P. Tissue expression and immunolocalization of tumor necrosis factor-alpha in postinfarction dysfunctional myocardium. Circulation. 1999;99:1492–1498. doi: 10.1161/01.cir.99.11.1492. [DOI] [PubMed] [Google Scholar]
- Jane-Lise S, Corda S, Chassagne C, Rappaport L. The extracellular matrix and the cytoskeleton in heart hypertrophy and failure. Heart Fail Rev. 2000;5:239–250. doi: 10.1023/A:1009857403356. [DOI] [PubMed] [Google Scholar]
- Jin H, Yang R, Li W, Lu H, Ryan AM, Ogasawara AK, Peborgh JV, Paoni NF. Effects of exercise training on cardiac function, gene expression, and apoptosis in rats. Am J Physiol Heart Circ Physiol. 2000;279:2994–3002. doi: 10.1152/ajpheart.2000.279.6.H2994. [DOI] [PubMed] [Google Scholar]
- Jugdutt BI. Remodeling of the myocardium and potential targets in the collagen degradation and synthesis pathways. Curr Drug Targets Cardiovasc Haematol Disord. 2003;3:1–30. doi: 10.2174/1568006033337276. [DOI] [PubMed] [Google Scholar]
- Kajstura J, Cheng W, Sarangarajan R, Li P, Li B, Nitahara JA, Chapnick S, Reiss K, Olivetti G, Anversa P. Necrotic and apoptotic myocyte cell death in the aging heart of Fischer 344 rats. Am J Physiol Heart Circ Physiol. 1996;271:1215–1228. doi: 10.1152/ajpheart.1996.271.3.H1215. [DOI] [PubMed] [Google Scholar]
- Kassiri Z, Khokha R. Myocardial extracellular matrix and its regulation by metalloproteinases and their inhibitors. Thromb Haemost. 2005;93:212–219. doi: 10.1160/TH04-08-0522. [DOI] [PubMed] [Google Scholar]
- Kjaer M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol Rev. 2004;84:649–698. doi: 10.1152/physrev.00031.2003. [DOI] [PubMed] [Google Scholar]
- Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg T, LeRoy EC. Diseases of the extracellular mtrix. J Mol Med. 1998;76:224–225. doi: 10.1007/s001090050212. [DOI] [PubMed] [Google Scholar]
- Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming grow factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overload rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- Kwak HB, Kim JH, Joshi K, Yeh A, Martinez DA, Lawler JM. Exercise training reduces fibrosis and matrix metalloproteinase dysregulation in the aging rat heart. FASEB J. 2011;25:1106–1117. doi: 10.1096/fj.10-172924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak HB, Song W, Lawler JM. Exercise training attenuates age-induced elevation in Bax/Bcl-2 ratio, apoptosis, and remodeling in the rat heart. FASEB J. 2006;20:791–3. doi: 10.1096/fj.05-5116fje. [DOI] [PubMed] [Google Scholar]
- Lafleur MA, Tester AM, Thompson EW. Selective involvement of TIMP-2 in the second activational cleavage of pro-MMP-2: refinement of the pro-MMP-2 activation mechanism. FEBS Lett. 2003;553:457–463. doi: 10.1016/s0014-5793(03)01094-9. [DOI] [PubMed] [Google Scholar]
- Li YY, Feng YQ, Kadokami T, McTiernan CF, Draviam R, Watkins SC, Feldman AM. Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor α can be modulated by anti-tumor necrosis factor α therapy. Proc Natl Acad Sci USA. 2000;97:12746–12751. doi: 10.1073/pnas.97.23.12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsey ML, Goshorn DK, Squires CE, Escobar GP, Hendrick JW, Mingoia JT, Sweterlitsch SE, Spinale FG. Age-dependent changes in myocardial matrix metalloproteinase/tissue inhibitor of metalloproteinase profiles and fibroblast function. Cardiovasc Res. 2005;66:410–419. doi: 10.1016/j.cardiores.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Lindsey ML, Mann DL, Entman ML, Spinale FG. Extracellular matrix remodeling following myocardial injury. Ann Med. 2003;35:316–326. doi: 10.1080/07853890310001285. [DOI] [PubMed] [Google Scholar]
- Lovelock JD, Baker AH, Dong JF, Bergeron AL, McPheat W, Sivasubrananian N, Mann DL. Heterogeneous effects of tissue inhibitors of matrix metalloproteinases on cardiac fibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:461–468. doi: 10.1152/ajpheart.00402.2004. [DOI] [PubMed] [Google Scholar]
- Mann DL. Tumor necrosis factor-induced signal transduction and left ventricular remodeling. J Card Fail. 2002;8:379–386. doi: 10.1054/jcaf.2002.129253. [DOI] [PubMed] [Google Scholar]
- Mays P, Bishop JE, Laurent GJ. Age-related changes in the proportion of types I and III collagen. Mech Ageing Dev. 1988;45:203–212. doi: 10.1016/0047-6374(88)90002-4. [DOI] [PubMed] [Google Scholar]
- Mendes AB, Ferro M, Rodrigues B, Souza MR, Araujo RC, Souza RR. Quantification of left ventricular myocardial collagen system in children, young adults, and the elderly. Medicina (B Aires) 2012;72:216–220. [PubMed] [Google Scholar]
- Murray DB, Levick SP, Brower GL, Janicki JS. Inhibition of matrix metal-loproteinase activity prevents increases in myocardial tumor necrosis factor-alpha. J Mol Cell Cardiol. 2010;49:245–250. doi: 10.1016/j.yjmcc.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen CT, Hall CS, Scott MJ, Zhu Q, Marsh J, Wickline SA. Age-related alterations in cardiac tissue microstructure and material properties in Fischer 344 rats. Ultrasound Med Biol. 2001;27:611–619. doi: 10.1016/s0301-5629(01)00343-x. [DOI] [PubMed] [Google Scholar]
- Oral H, Fisher SG, Fay WP, Singh SN, Fletcher RD, Morady F. Effects of amiodrone on tumor necrosis factor-alpha levels in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:388–391. doi: 10.1016/s0002-9149(98)00874-1. [DOI] [PubMed] [Google Scholar]
- Pauschinger M, Chandrasekharan K, Li J, Schwimmbeck PL, Noutsias M, Schultheiss HP. Mechanisms of extracellular matrix remodeling in dilated cardiomyopathy. Herz. 2002;27:677–682. doi: 10.1007/s00059-002-2413-4. [DOI] [PubMed] [Google Scholar]
- Peterson JT. The importance of estimating the therapeutic index in the development of matrix metalloproteinase inhibitors. Cardiovasc Res. 2006;69:677–687. doi: 10.1016/j.cardiores.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Poobalarahi F, Baicu CF, Bradshaw AD. Cardiac myofibroblasts differentiated in 3-D culture exhibit distinct changes in collagen I production, processing, and matrix deposition. Am J Physiol Heart Circ Physiol. 2006;291:2924–2932. doi: 10.1152/ajpheart.00153.2006. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA, O’Regan DJ, Ball SG. Tumor necrosis factor α induces human atrial myofibroblast proliferation, invasion, and MMP-9 secretion: inhibition by simvastatin. Cardiovasc Res. 2004;64:507–515. doi: 10.1016/j.cardiores.2004.07.020. [DOI] [PubMed] [Google Scholar]
- Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling. Pharmacol Ther. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Sada JI, West AB. Myofibroblasts. I. Paracrine cells important I health and disease. Am J Physiol Cell Physiol. 1999;277:1–9. doi: 10.1152/ajpcell.1999.277.1.C1. [DOI] [PubMed] [Google Scholar]
- Robert R, Besse S, Sabri A, Silvestre JS, Assayag P, Thiem NV, Swynghedauw B, Delcayre C. Differential regulation of matrix metalloproteinases associated with aging and hypertension in the rat heart. Lab Invest. 1997;76:729–738. [PubMed] [Google Scholar]
- Rohde LE, Ducharme A, Arroyo LH, Aikaw M, Sukhova GH, Lopez-Anaya A, McClure KF, Mitchell PG, Libby P, Lee RT. Matrix metalloproteinase inhibition attenuates early left ventricular enlargement after experimental myocardial infarction in mice. Circulation. 1999;99:3063–3070. doi: 10.1161/01.cir.99.23.3063. [DOI] [PubMed] [Google Scholar]
- Rosenkranz S, Flesch M, Amann K, Haeuseler C, Kilter H, Seeland U, Schluter KD, Bohm M. Alterations of β-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpression of TGF-β1. Am J Physiol Heart Circ Physiol. 2002;283:1253–1262. doi: 10.1152/ajpheart.00578.2001. [DOI] [PubMed] [Google Scholar]
- Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004;199:174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- Sales VL, Engelmayr GC, Mettler BA, Johnson JA, Sacks MS, Mayer JE. Transforming growth factor-β1 modulates extracellular matrix production, proliferation, and apoptosis of endothelial progenitor cells in tissue-engineering scaffolds. Circulation. 2006;114:I193–I199. doi: 10.1161/CIRCULATIONAHA.105.001628. [DOI] [PubMed] [Google Scholar]
- Sarasa-Renedo A, Chiquet M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand J Med Sci Sports. 2005;15:223–230. doi: 10.1111/j.1600-0838.2005.00461.x. [DOI] [PubMed] [Google Scholar]
- Schupp DJ, Huck BP, Sykora J, Flechtenmacher C, Gorenflo M, Koch A, Sack FU, Haass M, Katus HA, Ulmer HE, Hagl S, Otto HF, Schnabel PA. Right ventricular expression of extracellular matrix proteins, matrix-metalloproteinases, and their inhibitors over a period of 3 years after heart transplantation. Virchows Arch. 2006;448:184–194. doi: 10.1007/s00428-005-0050-z. [DOI] [PubMed] [Google Scholar]
- Seeland U, Haeuseler C, Hinrichs R, Rpsenkranz S, Pfitzner T, Scharffetter-Kochanek K, Bohm M. Myocardial fibrosis in transforming growth factor-β1 (TGF-β1) transgenic mice is associated with inhibition of interstitial collagenase. Eur J Clin Invest. 2002;32:295–303. doi: 10.1046/j.1365-2362.2002.00985.x. [DOI] [PubMed] [Google Scholar]
- Sivasubramanian N, Coker ML, Kurrelmeyer KM, MacLellan WR, De-Mayo FJ, Spinale FG, Mann DL. Left ventricular remodeling in transgenic mice with cardiac restricted overexpression of tumor necrosis factor. Circulation. 2001;104:826–831. doi: 10.1161/hc3401.093154. [DOI] [PubMed] [Google Scholar]
- Siwik DA, Chang DL, Colucci WS. Interleukin-1 beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ Res. 2000;86:1259–1265. doi: 10.1161/01.res.86.12.1259. [DOI] [PubMed] [Google Scholar]
- Siwik DA, Colucci WS. Regulation of Matrix metalloproteinases by cytokines and reactive oxygen/nitrogen species in the myocardium. Heart Fail Rev. 2004;9:43–51. doi: 10.1023/B:HREV.0000011393.40674.13. [DOI] [PubMed] [Google Scholar]
- Souders CA, Bowers SL, Baudino TA. Cardiac fibroblast: the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swynghedauw B. Molecular mechanisms of myocardial remodeling. Physiol Rev. 1999;79:215–262. doi: 10.1152/physrev.1999.79.1.215. [DOI] [PubMed] [Google Scholar]
- Thomas CV, Coker ML, Zellner JL, Handy JR, Crumbley AJ, Spinale FG. Increased matrix metalloproteinase activity and selective upregulation in LV myocardium from patients with end-stage dilated cardiomyopathy. Circulation. 1998;97:1708–1715. doi: 10.1161/01.cir.97.17.1708. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Cotter TA, Li X, McCormick J, Gosselin LE. Exercise training attenuates aging-associated increases in collagen and collagen cross-linking of the left but not the right ventricle in the rat. Eur J Appl Physiol. 2001;85:164–169. doi: 10.1007/s004210100447. [DOI] [PubMed] [Google Scholar]
- Thomas DP, Zimmerman SD, Hansen TR, Martin DT, McCormick RJ. Collagen gene expression in rat left ventricle: interactive effect of age and exercise training. J Appl Physiol. 2000;89:1462–1468. doi: 10.1152/jappl.2000.89.4.1462. [DOI] [PubMed] [Google Scholar]
- Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodeling. Nat Rev Mol Cell Biol. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- Tsuruda T, Costello-Boerrigter LC, Burnett JC. Matrix metalloproteinases: pathways of induction by bioactive molecules. Heart Fail Rev. 2004;9:53–61. doi: 10.1023/B:HREV.0000011394.34355.bb. [DOI] [PubMed] [Google Scholar]
- Tyagi SC, Kumar S, Voelker DJ, Reddy HK, Janicki JS, Curtis JJ. Differential gene expression of extracellular matrix components in dilated cardiomyopathy. J Cell Biochem. 1996;63:185–198. doi: 10.1002/(sici)1097-4644(19961101)63:2<185::aid-jcb6>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Woodiwiss AJ, Oosthuyse T, Norton GR. Reduced cardiac stiffness following exercise is associated with preserved myocardial collagen characteristics in the rat. Eur J Appl Physiol. 1998;78:148–154. doi: 10.1007/s004210050400. [DOI] [PubMed] [Google Scholar]
- Yu WH, Yu S, Meng Q, Brew K, Woessner JF. TIMP-3 binds to sulfated glycosaminoglycans of the extracellular matrix. J Biol Chem. 2000;275:31226–31232. doi: 10.1074/jbc.M000907200. [DOI] [PubMed] [Google Scholar]