Abstract
Stress alters brain cell properties and then disturbs cognitive processes, such as learning and memory. In this study, we investigated the effect of postnatal treadmill exercise on hippocampal neurogenesis and spatial learning ability of rat pups following prenatal noise stress. The impact of exercise intensity (mild-intensity exercise vs heavy-intensity exercise) was also compared. The pregnant rats in the stress-applied group were exposed to a 95 dB supersonic machine sound for 1 h once a day from the 15th day after mating until delivery. After birth, the rat pups in the exercise groups were made to run on a treadmill for 30 min once a day for 7 consecutive days, starting 4 weeks after birth. The spatial learning ability was tested using radial-arm maze task and hippocampal neurogenesis was determined by 5-bromo-2′-deoxyuridine (BrdU) immunohistochemistry. The rat pups born from the stress-applied maternal rats spent more time for the seeking of water and showed higher number of error in the radial-arm maze task compared to the control group. These rat pups showed suppressed neurogenesis in the hippocampus. In contrast, the rat pups performed postnatal treadmill exercise saved time for seeking of water and showed lower number of error compared to the stress-applied group. Postnatal treadmill exercise also enhanced neurogenesis in the hippocampus. The mild-intensity exercise showed more potent impact compared to the heavy-intensity exercise. The present results reveal that postnatal treadmill exercise lessens prenatal stress-induced deterioration of brain function in offspring.
Keywords: Prenatal noise stress, Postnatal treadmill exercise, Spatial learning ability, Neurogenrsis
INTRODUCTION
Stress is a biologically significant factor altering brain cell properties, and then disturbs cognitive processes, such as learning and memory, and consequently limits the quality of human life (Kim and Diamond, 2002). Maternal stress during pregnancy alters adult behavior of offspring (Lemaire et al., 2000). Fetal responses (heart rate, heart rate variability, and motor activity) were increased by maternal sympathetic activation evoked by a benign cognitive stressor, and these results suggest that fetal neurobehavioral regulation is routinely disrupted by maternal environmental intrusions (DiPietro et al., 2003). Maternal prenatal stress is the etiology of prematurity-related outcomes, and these effects are mediated, in part, by the maternal-placental-fetal neuroendocrine axis, and specifically by placental corticotropin-releasing hormone (Wadhwa, 2005). Prenatal stress impaired spatial learning and memory in the Morris water maze in the young rat offspring (Yang et al., 2006).
Hippocampus is the brain area implicated in memory acquisition and neuroendocrine regulation of stress hormones (Coe et al., 2003; Fuchs et al., 2001; Kim and Diamond, 2002). Hippocampal neurogenesis is closely associated with learning ability and memory capability (Cho et al., 2013; Kim et al., 2010; Shin et al., 2013; Shors et al., 2001). Prenatal stress, both early and late stage during pregnancy, reduced hippocampal volume and inhibited neurogenesis in the hippocampal dentate gyrus (Coe et al., 2003). Noise stress to pregnant rats caused growth retardation and decreased neurogenesis in the hippocampus, and resulted in impaired spatial learning ability in rat pups (Kim et al., 2006).
Exercise is known to enhance cell proliferation and/or neurogenesis in the hippocampal dentate gyrus (Holmes et al., 2004; Trejo et al., 2001) and improves recovery from stress-oriented diseases (Anderson et al., 2000; Seo et al., 2013a). Exercise is not harmful to mother and fetus (Lokey et al., 1991), and exercise can be beneficial to the pregnant woman in the absence of obstetric or medical complications (Ezmerli et al., 2000).
In the present study, we investigated whether maternal stress during the late pregnancy exerts influence on hippocampal neurogenesis and spatial learning ability of offspring. And then, the effect of postnatal treadmill exercise on hippocampal neurogenesis and spatial learning ability of rat pups following prenatal noise stress were also investigated. In addition, the impact of exercise intensity (mild-intensity exercise vs heavy-intensity exercise) was also compared.
MATERIALS AND METHODS
Animals and treatments
Male Sprague-Dawley rats (250±10 g, 12 weeks old, n=15) and female Sprague-Dawley rats (180±10 g, 8 weeks old, n=15) were used for this study. The experimental procedures were performed in accordance with the guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. The female rats were allowed to mate with the male rats for 1 day. After mating, the female rats were housed individually in a plastic home cage with a controlled temperature (20±2°C) and light-dark cycles consisting of 12 h light and 12 h darkness (lights on from 07:00 h to 19:00 h). Food and water were made available ad libitum.
Two weeks after mating, the pregnant rats were divided into two groups: the control group and the stress-applied group (n=6 in each group). From the 15th day after mating, the pregnant rats were subcutaneously injected with 100 mg/kg 5-bromo-2′-deoxyuridine (BrdU: Sigma Chemical Co., St. Louis, MO, USA), once a day at 30 min before the starting of experimental treatment until delivery. The pregnant rats in the stress-applied group were exposed to a 95 dB supersonic machine sound for 1 h once a day from the 15th day after mating until delivery, while the rats in the control group were left undisturbed. After birth, the offspring were left together with the respective maternal rats.
And then, the effects of intensity of postnatal treadmill exercise on hippocampal neurogenesis were investigated. The rat pups born from the control group were divided into three groups: the control group, the mild-intensity exercise group, and the heavy-intensity exercise group. The rat pups born from the stress-applied group were divided into three groups: the stress-applied group, the stress-applied and mild-intensity exercise group, and the stress-applied and heavy-intensity exercise group. The rat pups in the all groups were also subcutaneously injected with 50 mg/kg BrdU once a day at 30 min before the starting of treadmill running for 7 consecutive days.
Treadmill exercise protocol
The rat pups in the exercise groups were made to run on a treadmill for 30 min once a day for 7 consecutive days starting 4 weeks after birth, according to the previously described method (Kim et al., 2003). The exercise load for the mild-intensity exercise group consisted of running at a speed of 5 meters/min for the first 5 min, at a speed of 8 meters/min for the next 5 min, and at a speed of 10 meters/min for the last 20 min, with 0 degree of inclination. The exercise load for the heavy-intensity exercise group consisted of running at a speed of 10 meters/min for the first 5 min, at a speed of 13 meters/min for the next 5 min, and at a speed of 16 meters/min for the last 20 min, at 0 degree of inclination.
Radial-arm maze task
The spatial learning ability was tested using radial-arm maze task, as the previously described method (Kim et al., 2010; Seo et al., 2013b). The radial-arm maze apparatus consisted of central octagonal plate (30 cm in diameter) with radiating eight arms (50 cm in length and 10 cm in width). The apparatus was seated 1 m above the floor. A small receptacle filled with water (3 cm in diameter and 1 cm in depth) was located at the end of each arm. On the 34 days after birth, the rat pups received shaping sessions before the spatial learning test. On the 35 days after birth, test for spatial learning ability was performed. The rat pups deprived of water for 24 h allowed to explore for water and to drink during 5 min. The time spent for the seeking of water in each arm was counted. The trial was finished when the rat found water in all eight arms or over 5 min elapsed. Re-entering into the arm where rat had previously visited was counted as the error number.
Tissue preparation
For brain tissue preparation, the animals were fully anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and then fixed with a freshly prepared solution consisting of 4% paraformaldehyde (PFA) in 100 mM phosphate buffer (PB, pH 7.4). The brains were then removed, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40 μm thickness were made using a freezing microtome (Leica, Nussloch, Germany).
BrdU immunhistochemistry
For detection of newly generated cells in the hippocampus, BrdU incorporation was visualized via the previously described immunohistochemical method (Cho et al., 2013; Kim et al., 2006). The sections were first permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, then pretreated in 50% formamide-2 ×standard saline citrate (SSC) at 65°C for 2 h, denaturated in 2 N HCl at 37°C for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). Afterwards, the sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed three times with PBS and incubated for 1 h with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). Then, the sections were incubated for another 1 h with avidin-peroxidase complex (1:100; Vector Laboratories). For BrdU visualization, the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.02% 3,3′-diaminobenzidine tetrahydrochloride containing nickel chloride (40 mg/mL) and 0.03% hydrogen peroxide for 5 min, and the sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific, New Jersey, NJ, USA).
Data analysis
The area in the selected region of the hippocampus was measured using Image-Pro® Plus software (Media Cybernetics, Silver Spring, MD, USA). The number of BruU-positive cells in each area in the hippocampus was counted hemilaterally through a light microscope (Olympus, Tokyo, Japan). The data were expressed as the number of cells per mm2 of the area of the hippocampus. Statistical analysis was performed using t-test and one-way ANOVA followed by Duncan post-hoc test. Results are presented as the mean±standard error of the mean (SEM). Differences were considered significant at P<0.05.
RESULTS
Effect of postnatal exercise on spatial learning ability in rat pups
The time taken to complete eight successful performances was 98.57±17.05 sec in the control group, 59.28±9.73 sec in the mild-intensity exercise group, 64.00±6.67 sec in the heavy-intensity exercise group, 294.33±54.41 sec in the stress-applied group, 76.22±8.53 sec in the stress-applied and mild-intensity exercise group, and 108.11±13.09 sec in the stress-applied and heavy-intensity exercise group.
The number of error made before eight successful performances was 6.57±1.70 in the control group, 2.42±0.76 in the mild-intensity exercise group, 4.42±0.91 in the heavy-intensity exercise group, 12.11±1.53 in the stress-applied group, 5.77±1.20 the stress-applied and mild-intensity exercise group, and 11.22±1.73 in the stress-applied and heavy-intensity exercise group.
In the present results, the rat pups born from the stress-applied maternal rats spent more time for the seeking of water and showed higher number of error in the radial-arm maze task compared to the control group (P<0.05). In contrast, the rat pups performed postnatal treadmill exercise saved time for seeking of water and showed lower number of error (P<0.05). The more potent improving effect of treadmill exercise on spatial learning ability was shown in the mild-intensity exercise group (P<0.05).
Effect of postnatal exercise on hippocampal neurogenesis in rat pups
Fig. 1 shows BrdU-positive cells in the hippocampus of rat pups. The number of BrdU-positive cells in the hippocampal dentate gyrus was 3,419.04±151.38/mm2 in the control group, 4,501.32±175.50/mm2 in the mild-intensity exercise group, 4,039.07± 101.84/mm2 in the heavy-intensity exercise group, 2,985.80± 150.76/mm2 in the stress-applied group, 3,808.29±117.21/mm2 in the stress-applied and mild-intensity exercise group, and 3,643.87 ±196.19/mm2 the stress-applied and heavy-intensity exercise group.
Fig. 1.
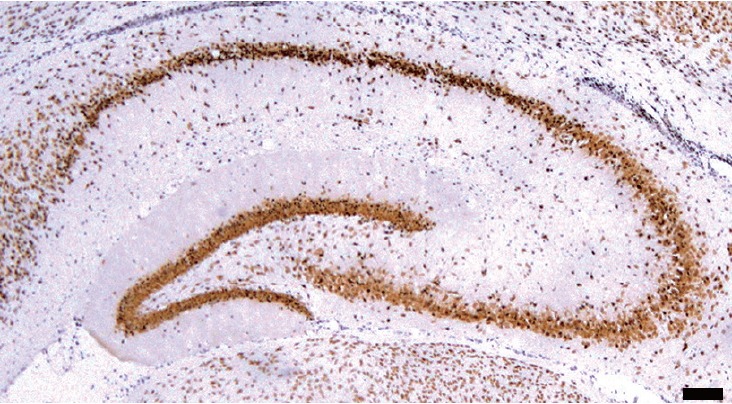
Photomicrographs of 5-bromo-2′-deoxyuridine (BrdU)-positive cells in the hippocampus. Black dots represent BrdU-positive cells. The scale bar represents 400 μm.
The number of BrdU-positive cells the hippocampal CA1 region was 2,495.04±93.67/mm2 in the control group, 2,954.26±98.06/mm2 in the mild-intensity exercise group, 2,689.29±107.59/mm2 in the heavy-intensity exercise group, 2,200.28±132.77/mm2 in the stress-applied group, 2,617.42±72.86/mm2 in the stress-applied and mild-intensity exercise group, and 2,479.19±41.18/mm2 in the stress-applied and heavy-intensity exercise group.
The number of BrdU-positive cells in the hippocampal CA2 and CA3 regions was 1,187.69±28.84/mm2 in the control group, 1,644.55±121.70/mm2 the mild-intensity exercise group, 1,402.80 ±74.07/mm2 in the heavy-intensity exercise group, 1,096.42±60.82/mm2 in the stress-applied group, 1,303.50±76.68/mm2 in the stress-applied and mild-intensity exercise group, and 1,256.76±45.91/mm2 in the stress-applied and heavy-intensity exercise group.
In the present results, the rat pups born from the stress-applied maternal rats showed suppressed neurogenesis in the hippocampus compared to the control group (P<0.05). In contrast, the rat pups performed postnatal treadmill exercise showed enhanced neurogenesis in the hippocampus compared to the stress-applied group (P<0.05). The more potent enhancing effect of treadmill exercise on neurogenesis was observed in the mild-intensity exercise group (P<0.05).
DISCUSSION
Prenatal stress in rats induced lifespan reduction of neurogenesis in the hippocampal dentate gyrus and produced impairment in hippocampal-related spatial tasks (Coe et al., 2003; Lemaire et al., 2000). Reduction in neurogenesis is closely associated with deterioration of hippocampal-dependent learning ability (Madsen et al., 2003; Shors et al., 2001). Stressful events are closely associated with decreased neurogenesis in the hippocampal dentate gyrus as well as with impaired performance on learning tasks (Fuchs et al., 2001; Trejo et al., 2001). Nishio et al. (2001) suggested that prenatal stress might have sex-dependent effects on emotional behavior and learning ability of neonatal rats. Gue et al. (2004) reported the relation of psychological factors with the etiology of irritable bowel syndrome often associated with abdominal pain. They presented that partial restraint stress enhanced abdominal contractions in response to rectal distension in rats (Gue et al., 2004). Exposure to predator stress during pregnancy induced anxiety-like behaviors of maternal rats (Seo et al., 2013a). Exposure of noise stress to pregnant rats caused growth retardation of rat pups, and these rat pups showed decrement of neurogenesis in the hippocampus and impairment of spatial learning ability (Kim et al., 2006).
In the present results, the rat pups born form the maternal rats exposed to the noise stress during pregnancy showed decrement in hippocampal neurogenesis. These rat pups also showed impairment of spatial learning ability.
Exercise has been recommended as one of the non-pharmacologic means for treating neuropsychiatric diseases (Cho et al., 2013; Kim et al., 2011; Lang et al., 2010; Seo et al., 2013b). Exercise increases neuronal activity in the hippocampus, motor cortex, and striatum (McCloskey et al., 2001). Enhancing effect of exercise on hippocampal neurogenesis induces long-term potentiation, which improves spatial learning ability and memory function (Farmer et al., 2004; Kim et al., 2013). Treadmill exercise during pregnancy ameliorates anxiety-like behaviors of maternal rats caused by predator stress during pregnancy (Seo et al., 2013a).
In this study, we focused the effects of postnatal treadmill exercise of rat pups after receiving noise-induced stress during pregnancy. In the present results, postnatal treadmill exercise of rat pups born form the noise-exposed maternal rats during pregnancy showed increment in hippocampal neurogenesis. These rat pups also showed improved spatial learning ability in spite of noise exposure during pregnancy. In item of the exercise intensity, the mild-intensity exercise showed more potent impact compared to the heavy-intensity exercise. The present results reveal that post-natal treadmill exercise lessens prenatal stress-induced deterioration of brain function in offspring.
Acknowledgments
This work was supported by the National Research Foundation of Korea funded by the Korean Government (NRF-2010-327-G00123).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- Anderson BJ, Rapp DN, Baek DH, McCloskey DP, Coburn-Litvak PS, Robinson JK. Exercise influences spatial learning in the radial arm maze. Physiol Behav. 2000;70:425–429. doi: 10.1016/s0031-9384(00)00282-1. [DOI] [PubMed] [Google Scholar]
- Cho HS, Shin MS, Song W, Jun TW, Lim BV, Kim YP, Kim CJ. Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopa-mine-induced Parkinson’s rats. J Exer Rehabil. 2013;9:354–361. doi: 10.12965/jer.130048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coe CL, Kramer M, Czeh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- DiPietro JA, Costigan KA, Gurewitsch ED. Fetal response to induced maternal stress. Early Hum Dev. 2003;74:125–138. doi: 10.1016/j.earlhumdev.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Ezmerli NM. Exercise in pregnancy. Prim Care Update Ob Gyns. 2000;7:260–265. doi: 10.1016/s1068-607x(00)00056-1. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Flugge G, Ohl F, Lucassen P, Vollmann-Honsdorf GK, Michaelis T. Psychosocial stress, glucocorticoids, and structural alterations in the tree shrew hippocampus. Physiol Behav. 2001;73:285–291. doi: 10.1016/s0031-9384(01)00497-8. [DOI] [PubMed] [Google Scholar]
- Gue M, Del Rio-Lacheze C, Eutamene H, Theodorou V, Fioramonti J, Bueno L. Sex differences in learning deficits induced by prenatal stress in juvenile rats. Behav Brain Res. 2004;150:149–157. doi: 10.1016/S0166-4328(03)00250-X. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Galea LA, Mistlberger RE, Kempermann G. Adult hippocampal neurogenesis and voluntary running activity: circadian and dose-dependent effects. J Neurosci. 2004;76:216–222. doi: 10.1002/jnr.20039. [DOI] [PubMed] [Google Scholar]
- Kim H, Lee MH, Chang HK, Lee TH, Lee HH, Shin MC, Shin MS, Won R, Shin HS, Kim CJ. Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain Dev. 2006;28:109–114. doi: 10.1016/j.braindev.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim SE, Kim BK, Kim TW, Ji ES, Kim JD, Shin MS, Choi YW, Kim CJ. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci Lett. 2011;504:35–39. doi: 10.1016/j.neulet.2011.08.052. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Diamond DM. The stressed hippocampus, synaptic plasticity and lost memories. Nat Rev Neurosci. 2002;3:453–462. doi: 10.1038/nrn849. [DOI] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365. doi: 10.1016/j.exger.2010.02.005. [DOI] [PubMed] [Google Scholar]
- Kim SE, Ko IG, Shin MS, Kim CJ, Jin BK, Hong HP, Jee YS. Treadmill exercise and wheel exercise enhance expressions of neutrophic factors in the hippocampus of lipopolysaccharide-injected rats. Neurosci Lett. 2013;538:54–59. doi: 10.1016/j.neulet.2013.01.039. [DOI] [PubMed] [Google Scholar]
- Kim YP, Kim HB, Jang MH, Lim BV, Kim YJ, Kim H, Kim SS, Kim EH, Kim CJ. Magnitude- and time-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Int J Sports Med. 2003;24:114–117. doi: 10.1055/s-2003-38202. [DOI] [PubMed] [Google Scholar]
- Lang R, Koegel LK, Ashbaugh K, Regester A, Ence W, Smith W. Physical exercise and children with autism spectrum disorders: A systematic review. Res Autism Spectr Disord. 2010;4:565–576. [Google Scholar]
- Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci U S A. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokey EA, Tran ZV, Wells CL, Myers BC, Tran AC. Effects of physical exercise on pregnancy outcomes: A meta-analytic review. Med Sci Sports Exerc. 1991;23:1234–1239. [PubMed] [Google Scholar]
- Madsen TM, Kristjansen PEG, Bolwig TG, Wortwein G. Arrested neuronal proliferation and impaired hippocampal function following fractionated brain irradiation in the adult rat. Neuroscience. 2003;119:635–642. doi: 10.1016/s0306-4522(03)00199-4. [DOI] [PubMed] [Google Scholar]
- McCloskey D, Adamo D, Anderson B. Exercise increases metabolic capacity in the motor cortex and striatum, but not in the hippocampus. Brain Res. 2001;891:168–175. doi: 10.1016/s0006-8993(00)03200-5. [DOI] [PubMed] [Google Scholar]
- Nishio H, Kasuga S, Ushijima M, Harada Y. Prenatal stress and postnatal development of neonatal rats--sex-dependent effects on emotional behavior and learning ability of neonatal rats. Int J Dev Neurosc. 2001;19:37–45. doi: 10.1016/s0736-5748(00)00070-8. [DOI] [PubMed] [Google Scholar]
- Seo JH, Kim TW, Kim CJ, Sung YH, Lee SJ. Treadmill exercise during pregnancy ameliorates post traumatic stress disorder induced anxiety like responses in maternal rats. Mol Med Rep. 2013a;7:389–395. doi: 10.3892/mmr.2012.1197. [DOI] [PubMed] [Google Scholar]
- Seo TB, Cho HS, Shin MS, Kim CJ, Ji ES, Baek SS. Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats. J Exerc Rehabil. 2013b;9:220–229. doi: 10.12965/jer.130003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin MS, Ko IG, Kim SE, Kim BK, Kim TS, Lee SH, Hwang DS, Kim CJ, Park JK, Lim BV. Treadmill exercise ameliorates symptoms of methimazole-induced hypothyroidism through enhancing neurogenesis and suppressing apoptosis in the hippocampus of rat pups. Int J Dev Neurosci. 2013;31:214–223. doi: 10.1016/j.ijdevneu.2013.01.003. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult. J Neurosci. 2001;21:1628–1634. doi: 10.1523/JNEUROSCI.21-05-01628.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadhwa PD. Psychoneuroendocrine processes in human pregnancy influence fetal development and health. Psychoneuroendocrinology. 2005;30:724–743. doi: 10.1016/j.psyneuen.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Yang J, Han H, Cao J, Li L, Xu L. Prenatal stress modifies hippocampal synaptic plasticity and spatial learning in young rat offspring. Hippocampus. 2006;16:431–436. doi: 10.1002/hipo.20181. [DOI] [PubMed] [Google Scholar]


