Abstract
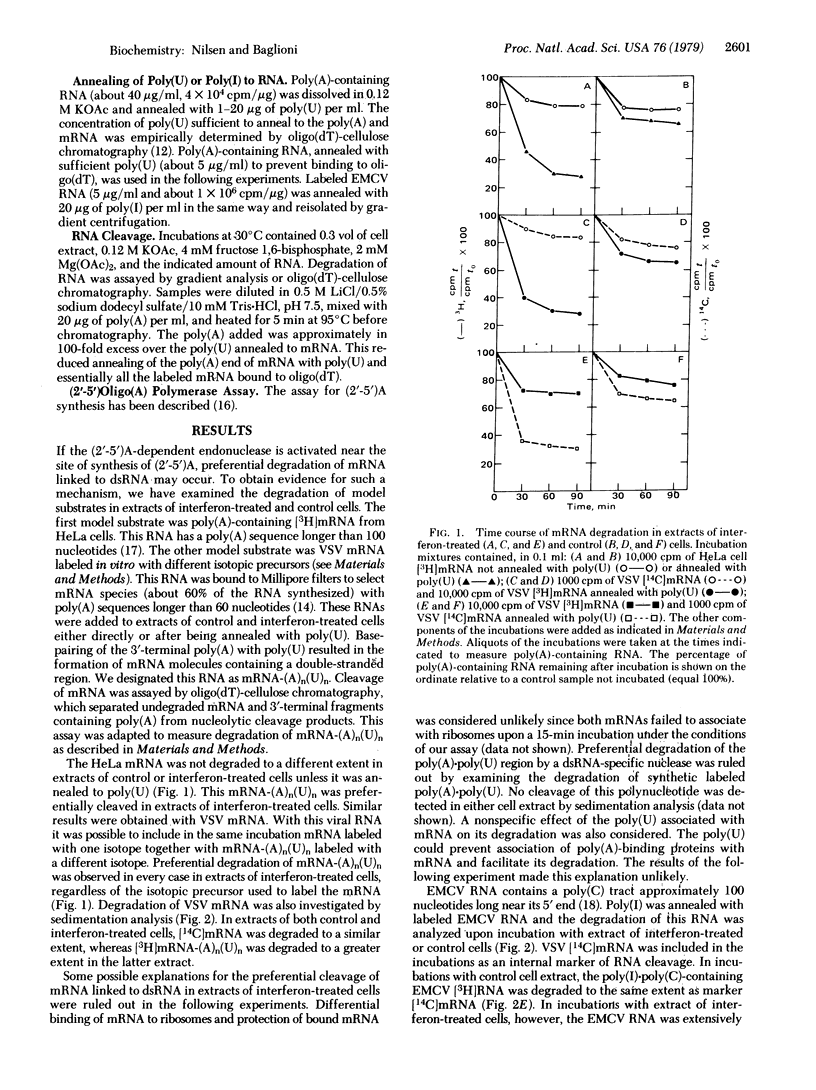
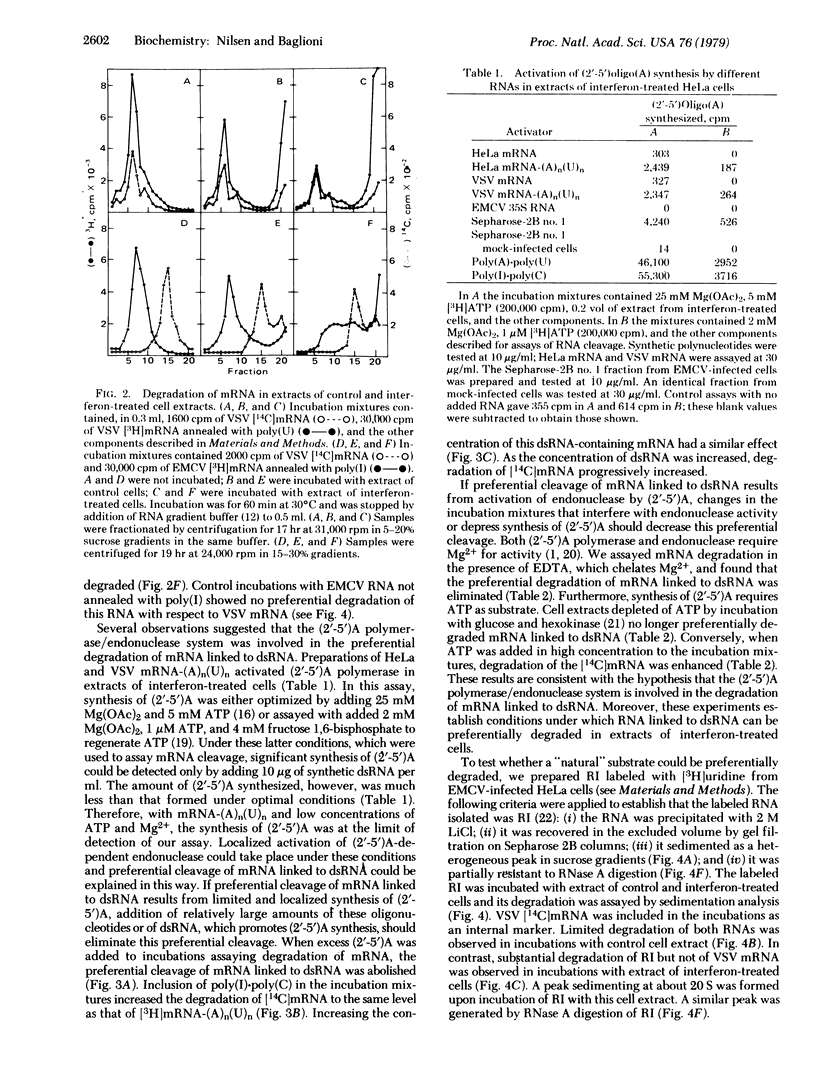
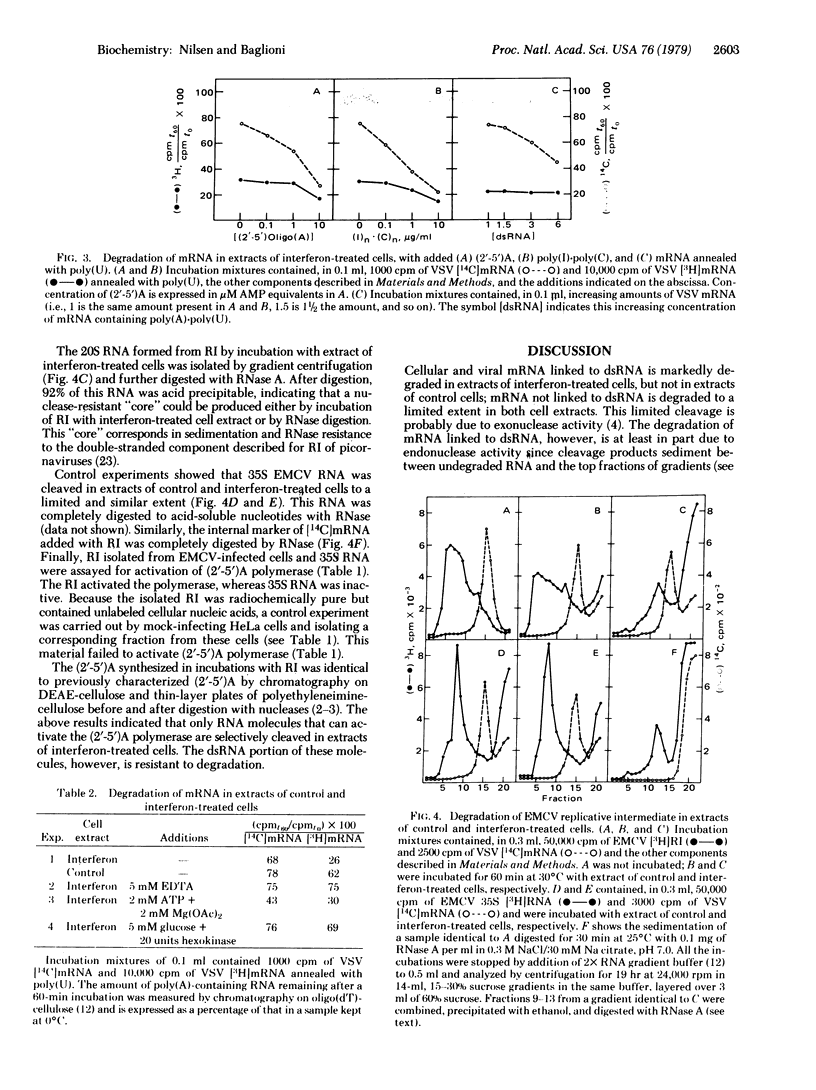
In extracts of interferon-treated HeLa cells, RNA covalently linked to double-stranded RNA (dsRNA) is preferentially degraded compared with mRNA not linked in dsRNA. This was established by following the degradation of poly(A)-containing mRNA annealed with poly(U), of poly(C)-containing encephalomyocarditis virus RNA annealed with poly(I), and of the replicative intermediate of the virus isolated from infected cells. In extracts of interferon-treated cells, dsRNA promotes the synthesis of a series of oligonucleotides, designated (2'-5')oligo(A), which in turn activate an endonuclease. Several lines of evidence suggest that the (2'-5')oligo(A) polymerase/endonuclease system is involved in the preferential degradation of mRNA linked to dsRNA. Conditions that prevent synthesis of (2'-5')oligo(A) prevent this preferential degradation, whereas addition of (2'-5')oligo(A) or conditions that favor its synthesis result in degradation of mRNA both linked and not linked to dsRNA. These results are best explained by a localized activation of the endonuclease near the dsRNA region of our model substrates. We propose that in infected cells activation of the endonuclease takes place near the replicative intermediates of RNA viruses. The replicative intermediates of encephalomyocarditis virus promote synthesis of (2'-5')-oligo(A) in extracts of interferon-treated cells and are degraded to a 20S "core" resistant to digestion with RNase A. This mechanism may be responsible for discrimination between viral and cellular mRNA in interferon-treated cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baglioni C., Minks M. A., Maroney P. A. Interferon action may be mediated by activation of a nuclease by pppA2'p5'A2'p5'A. Nature. 1978 Jun 22;273(5664):684–687. doi: 10.1038/273684a0. [DOI] [PubMed] [Google Scholar]
- Baglioni C., Weber L. A. The use of phosphorylated sugars to support protein synthesis with some mammalian cell extracts. FEBS Lett. 1978 Apr 1;88(1):37–40. doi: 10.1016/0014-5793(78)80601-2. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Oligonucleotide inhibitor of protein synthesis made in extracts of interferon-treated chick embryo cells: comparison with the mouse low molecular weight inhibitor. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1167–1171. doi: 10.1073/pnas.75.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltimore D. Structure of the poliovirus replicative intermediate RNA. J Mol Biol. 1968 Mar 14;32(2):359–368. doi: 10.1016/0022-2836(68)90015-6. [DOI] [PubMed] [Google Scholar]
- Chumakov K. M., Agol V. I. Poly(C) sequence is located near the 5'-end of encephalomyocarditis virus RNA. Biochem Biophys Res Commun. 1976 Jul 26;71(2):551–557. doi: 10.1016/0006-291x(76)90822-6. [DOI] [PubMed] [Google Scholar]
- Clemens M. J., Williams B. R. Inhibition of cell-free protein synthesis by pppA2'p5'A2'p5'A: a novel oligonucleotide synthesized by interferon-treated L cell extracts. Cell. 1978 Mar;13(3):565–572. doi: 10.1016/0092-8674(78)90329-x. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Sen G. C., Dubois M. F., Ratner L., Slattery E., Lengyel P. Interferon action: two distinct pathways for inhibition of protein synthesis by double-stranded RNA. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5893–5897. doi: 10.1073/pnas.75.12.5893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman R. M. Antiviral activity of interferons. Bacteriol Rev. 1977 Sep;41(3):543–567. doi: 10.1128/br.41.3.543-567.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Morrison M. R., Merkel C. G., Lingrel J. B. Size heterogeneity of polyadenylate sequences in mouse globin messenger RNA. J Mol Biol. 1974 Jun 25;86(2):363–371. doi: 10.1016/0022-2836(74)90025-4. [DOI] [PubMed] [Google Scholar]
- Hovanessian A. G., Kerr I. M. Synthesis of an oligonucleotide inhibitor of protein synthesis in rabbit reticulocyte lysates analogous to that formed in extracts from interferon-treated cells. Eur J Biochem. 1978 Mar;84(1):149–159. doi: 10.1111/j.1432-1033.1978.tb12151.x. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E., Hovanessian A. G. Nature of inhibitor of cell-free protein synthesis formed in response to interferon and double-stranded RNA. Nature. 1977 Aug 11;268(5620):540–542. doi: 10.1038/268540a0. [DOI] [PubMed] [Google Scholar]
- Kerr I. M., Brown R. E. pppA2'p5'A2'p5'A: an inhibitor of protein synthesis synthesized with an enzyme fraction from interferon-treated cells. Proc Natl Acad Sci U S A. 1978 Jan;75(1):256–260. doi: 10.1073/pnas.75.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer G., Henderson A. B., Pinphanichakarn P., Wallis M. H., Hardesty B. Partial reaction of peptide initiation inhibited by phosphorylation of either initiation factor eIF-2 or 40S ribosomal proteins. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1445–1449. doi: 10.1073/pnas.74.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minks M. A., Benvin S., Maroney P. A., Baglioni C. Metabolic stability of 2' 5'oligo (A) and activity of 2' 5'oligo (A)-dependent endonuclease in extracts of interferon-treated and control HeLa cells. Nucleic Acids Res. 1979 Feb;6(2):767–780. doi: 10.1093/nar/6.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter A., Carey N., Fellner P. Presence of a large poly(rC) tract within the RNA of encephalomyocarditis virus. Nature. 1974 Apr 19;248(5450):675–678. doi: 10.1038/248675a0. [DOI] [PubMed] [Google Scholar]
- Ratner L., Wiegand R. C., Farrell P. J., Sen G. C., Cabrer B., Lengyel P. Interferon, double-stranded RNA and RNA degradation. Fractionation of the endonucleaseINT system into two macromolecular components; role of a small molecule in nuclease activation. Biochem Biophys Res Commun. 1978 Apr 14;81(3):947–954. doi: 10.1016/0006-291x(78)91443-2. [DOI] [PubMed] [Google Scholar]
- Sheiness D., Darnell J. E. Polyadenylic acid segment in mRNA becomes shorter with age. Nat New Biol. 1973 Feb 28;241(113):265–268. doi: 10.1038/newbio241265a0. [DOI] [PubMed] [Google Scholar]
- Spector D. H., Baltimore D. Polyadenylic acid on poliovirus RNA. II. poly(A) on intracellular RNAs. J Virol. 1975 Jun;15(6):1418–1431. doi: 10.1128/jvi.15.6.1418-1431.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toneguzzo F., Ghosh H. P. Characterization and translation of methylated and unmethylated vesicular stomatitis virus mRNA synthesized in vitro by ribonucleoprotein particles from vesicular stomatitis virus-infected L cells. J Virol. 1976 Feb;17(2):477–491. doi: 10.1128/jvi.17.2.477-491.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber L. A., Feman E. R., Baglioni C. A cell free system from HeLa cells active in initiation of protein synthesis. Biochemistry. 1975 Dec 2;14(24):5315–5321. doi: 10.1021/bi00695a015. [DOI] [PubMed] [Google Scholar]
- Weber L. A., Simili M., Baglioni C. Binding of viral and cellular messenger RNAs to ribosomes in eukaryotic cell extracts. Methods Enzymol. 1979;60:351–360. doi: 10.1016/s0076-6879(79)60033-2. [DOI] [PubMed] [Google Scholar]
- Zilberstein A., Kimchi A., Schmidt A., Revel M. Isolation of two interferon-induced translational inhibitors: a protein kinase and an oligo-isoadenylate synthetase. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4734–4738. doi: 10.1073/pnas.75.10.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]


