Abstract
Histone deacetylase inhibitors (HDACi) are still the focus of epigenetic modulator development due to their effective intervention in many pathological processes. In our previous research, a potent HDACi was designed, synthesized and validated as a promising antitumor candidate named ZYJ-34c. Enlarged scale synthesis of ZYJ-34c for further detailed research was hindered by the occurrence of a by-product, which was identified as an isomer of ZYJ-34c by HRMS and 1H NMR. Subsequent synthesis route modification and optimization revealed that these two isomers were a pair of epimers and their absolute configurations could be directly determined by our optimized synthesis routes, through which each optically pure epimer could be stereoselectively synthesized, respectively. Based on these results, we concluded that our previously reported absolute configuration of ZYJ-34c was incorrect. It is worth noting that the epimer of ZYJ-34c exhibited more potent HDACs inhibition and both in vitro and in vivo antitumor activities, and moreover, their different HDACs inhibitory activities could be rationalized by computational simulations of their binding modes in HDAC2.
Introduction
An epigenetic trait is a stably heritable phenotype caused by changes in a chromosome without DNA sequence alterations.1 Aberrant epigenetic covalent modifications of DNA or chromatin histones will cause disordered gene expression and cellular functions, and consequently many diseases, of which cancer is the most dreadful.2,3 Hitherto many kinds of epigenetic modifying enzymes have been revealed as drug intervention targets, such as histone deacetylases (HDACs), which are responsible for histone lysine residues deacetylation resulting in chromosomal DNA condensation and gene transcriptional repression.4 Histone deacetylases inhibitors (HDACi) account for the largest proportion in epigenetic drug research and development.5 Currently, three HDACi, Vorinostat (SAHA), Romidepsin (FK228) and Resminostat (4SC-201) have been approved by the FDA as anticancer agents, meanwhile over twenty other HDACi are in clinical trials.6
Through our previous several rounds of structural optimization and activity evaluation,7–9 we obtained a potent tetrahydroisoquinoline-based HDACi, ZYJ-34c with marker in vitro and in vivo antitumor potency.9 Because ZYJ-34c was initially synthesized according to the methods described in Scheme 1 and its 1H NMR (Fig. S1†) and HRMS data (Fig. S2†) appeared reasonable, we took it for granted that the structure of ZYJ-34c should be the one shown in Scheme 1 as previously reported.9
Scheme 1.
Previously reported synthesis route and structure of ZYJ-34c
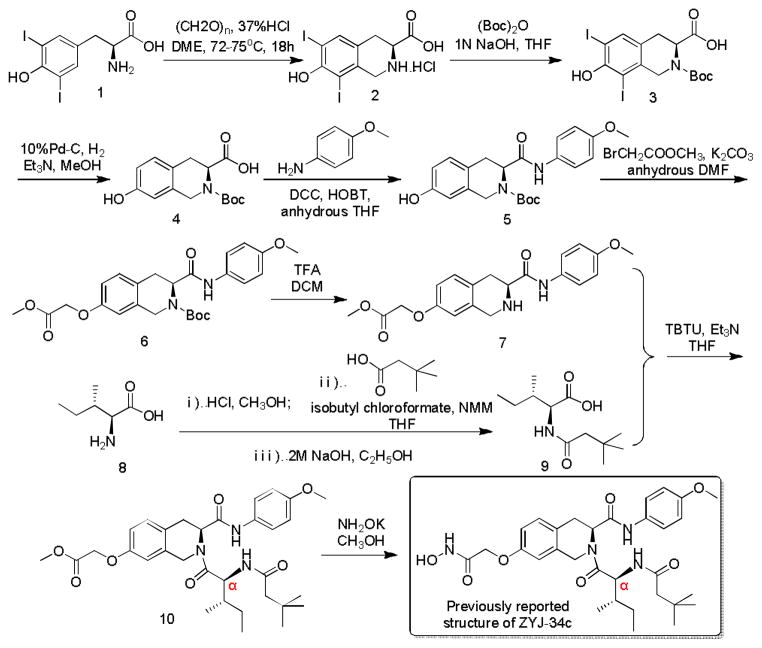
However, enlarged scale synthesis of ZYJ-34c for further detailed research was hindered by the occurrence of a by-product. In fact, this impurity has already been detected in our milligram scale synthesis. According to the peak areas (Fig. 1a), the ratio of the two components is about 3:1. At that time, we took it for granted that the major component at retention time (RT) 6.4 min was our desired compound ZYJ-34c and that the minor component at RT 7.2 min was some useless by-product. We tried recrystallization using almost all common laboratory solvents and mixed solvent but it did not work. Because the RT of the byproduct was too close to that of our main product (Fig. 1a), we could only collect the main product by preparative C18 column for further activity evaluation. This dramatically hindered the further research and development of ZYJ-34c.
Fig. 1.
a) HPLC chromatogram of Scheme 1 crude product and the hypothetic structures of ZYJ-34c and its epimer. b) HPLC chromatogram of Scheme 2 product and the actual structure of ZYJ-34c epimer. c) HPLC chromatogram of Scheme 3 product and the actual structure of ZYJ-34c.
Results and Discussion
In order to synthesize ZYJ-34c without formation of this impurity by optimizing reaction conditions or synthesis route, we firstly collected this impurity using preparative HPLC to analyze what exactly it was. 1H NMR (Fig. S3†) and HRMS data (Fig. S4†) revealed that this by-product was an isomer of ZYJ-34c. Based on the analysis of our synthesis route shown in Scheme 1 we hypothesized that the isomer should be an epimer of ZYJ-34c and the racemization most probably happened in the Cα of ZYJ-34c during the condensation of intermediates 7 and 9. So we performed HPLC analysis of the methyl ester 10 and the result that intermediate 10 contained two adjacent peaks (Fig. S5†) confirmed our hypothesis. There was another possibility that intermediate 9 was obtained as a mixture of two epimers because its synthesis methods involved esterification, condensation and saponification, which might cause racemization of 9. Due to no available reported specific rotation of 9, we derivatized our synthesized 9 by condensation with other amines having ultraviolet absorption so that we could easily use HPLC to detect the optical purity of 9. The HPLC analysis results of these condensation products (Fig. S6 †) indirectly demonstrated that intermediate 9 obtained in Scheme 1 was optical pure. Above mentioned information further confirmed our hypothesis that the racemization of Cα of ZYJ-34c occurred during the amide bond formation between 7 and 9. So we took it for granted that the structures of ZYJ-34c and its epimer should be the ones shown in Fig. 1a.
Subsequently, we tried to eliminate the racemization in the condensation of 7 and 9 by controlling reaction temperature and using some other coupling reagents such as DCC and DEPBT, however, no satisfying results were obtained according to the HPLC analysis results (Fig. S7†).
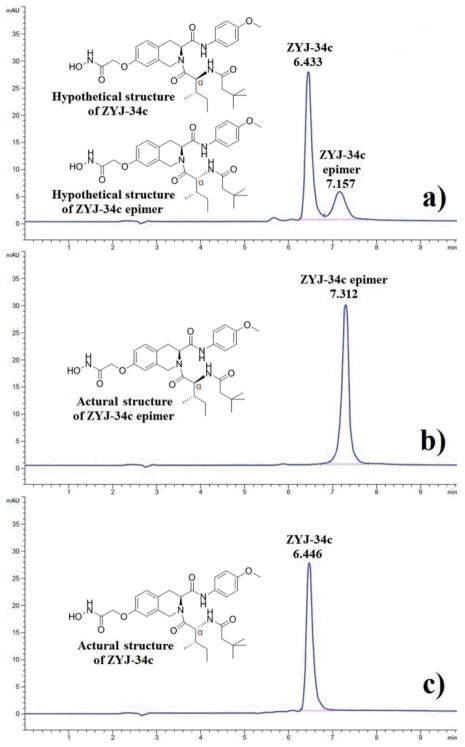
Considering the most important mechanism of racemization involving the oxazolone intermediate formation (Scheme S1†), which is not so facile when the acyl substituent on the α-amine group is an alkoxycarbonyl protecting group such as tert-butoxycarbonyl (Boc) group,10,11 we established a modified synthesis route (Scheme 2) in which compound 7 was coupled with Boc-L-isoleucine 11. Then Boc group cleavage of 12 and subsequent coupling with 3,3-dimethylbutyric acid afforded the intermediate 10, which was finally transformed into the corresponding hydroxamic acid. HPLC analysis result revealed that this product was optically pure (Fig. 1b), however, its RT was 7.312 min, which seemed close to that of the ZYJ-34c epimer (7.157 min, Fig. 1a). NMR spectrums confirmed that the target compound synthesized in Scheme 2 was exactly ZYJ-34c epimer separated from the crude product of Scheme 1. This result indicated that our previously reported structure of ZYJ-34c was incorrect.
Scheme 2.
Modified synthesis route
In order to determine the real structure of ZYJ-34c, we used the same reaction conditions of Scheme 2 to establish Scheme 3, in which D-alloisoleucine 13 was substituted for L-isoleucine 8 in Scheme 2. As expected, HPLC analysis result revealed that the product of Scheme 3 was also optically pure (Fig. 1c) and its RT (6.446 min) and NMR spectrums all demonstrated that it was exactly ZYJ-34c published in our previous work.9
Scheme 3.
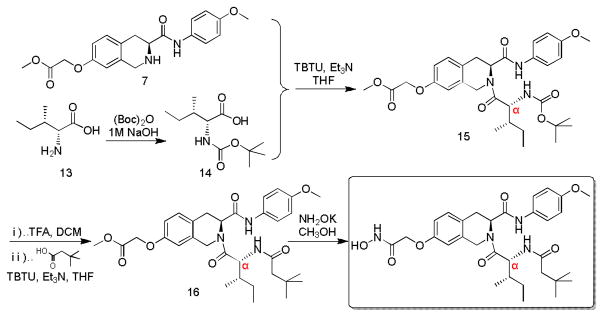
Modified synthesis route
Compound ZYJ-34c was validated as a promising antitumor candidate with superior in vivo antitumor potency compared with the approved drug SAHA.9 Through above mentioned Scheme 3, we could obtain optically pure ZYJ-34c on a large scale for further preclinical research. However, the starting material D-alloisoleucine 13 is a very expensive unnatural amino acid, which makes the production cost of ZYJ-34c unacceptable. Therefore, we focused our attention on ZYJ-34c epimer because of its much more available starting material L-isoleucine 11.
It was exciting that ZYJ-34c epimer exhibited more potent inhibitory activities than both ZYJ-34c and SAHA against HDAC1, HDAC2 and HDAC3. Although ZYJ-34c epimer was inferior to SAHA against HDAC6, it was still superior to ZYJ-34c. All tested compounds exhibited no obvious inhibition against class IIa HDACs using MDA-MB-231 cell lysate as enzyme source (Table 1).
Table 1.
HDACs inhibitory activities of tested compounds
| Compds | IC50 (nM)α | ||||
|---|---|---|---|---|---|
| Class I | Class IIa | Class IIb | |||
| HDAC1 | HDAC2 | HDAC3 | Cell lysate | HDAC6 | |
| ZYJ-34c epimer | 8.1 | 117.1 | 11.0 | NAβ | 570 |
| ZYJ-34c | 39.3 | 428.4 | 58.3 | NAβ | 1320 |
| SAHA | 75.6 | 256.4 | 28.4 | NAβ | 118 |
Values are the means of at least two independent experiments.
NA: Not active at 10 μM.
To further compare their antiproliferative activities, this pair of diastereomers was evaluated against several tumor cell lines. Results in Table 2 showed that ZYJ-34c epimer exhibited more potent in vitro antitumor activities than ZYJ-34c and SAHA against all tested tumor cell lines. Meanwhile, it was notable that ZYJ-34c epimer and ZYJ-34c possessed lower toxicity to normal human lung fibroblast cell line (WI38) compared with SAHA.
Table 2.
IC50 values (μM)α of tested compounds in MTT assays
| ZYJ-34c epimer | ZYJ-34c | SAHA | ||
|---|---|---|---|---|
| Breast Cancer | MDA-MB-231 | 2.29 | 4.67 | 4.70 |
| MDA-MB-468 | 1.70 | 6.10 | 3.74 | |
| MDA-MB-435 | 1.00 | 4.14 | 1.81 | |
| BT549 | 0.96 | 3.46 | 3.35 | |
| BT474 | 0.50 | 3.02 | 1.20 | |
|
| ||||
| Colon Cancer | HCT116 | 0.95 | 4.09 | 1.45 |
| HT29 | 0.86 | 3.33 | 1.25 | |
| SW620 | 1.52 | 5.73 | 1.86 | |
|
| ||||
| Ovarian Cancer | SK-OV-3 | 1.66 | 6.58 | 1.88 |
|
| ||||
| Prostate Cancer | PC-3 | 0.91 | 3.15 | 4.20 |
|
| ||||
| Lung Cancer | A549 | 1.25 | 6.00 | 3.00 |
|
| ||||
| Cervical Cancer | HeLa | 2.24 | 9.27 | 3.22 |
|
| ||||
| Melanoma | SK-MEL-28 | 0.87 | 3.41 | 1.70 |
|
| ||||
| Leukemia | K562 | 0.84 | 2.54 | 1.25 |
|
| ||||
| Lymphoma | U937 | 0.46 | 1.62 | 1.23 |
|
| ||||
| Normal Cell | WI38 | 16.0 | 52.0 | 4.59 |
Values are the means of three independent experiments.
Encouraged by its excellent in vitro activity, ZYJ-34c epimer was progressed to an in vivo experiment. We applied the same MDA-MB-231 xenograft mouse model as in our previous research8,9 with ZYJ-34c and SAHA as positive control. The final dissected tumor volume, tumor growth inhibition (TGI) and relative increment ration (T/C) shown in Fig. 2 all indicated that ZYJ-34c epimer was the most potent compound, which was in line with its HDACs inhibitory activities and in vitro antiproliferative activities.
Fig. 2.

In vivo antitumor activity comparison of tested compounds
The proposed binding modes of ZYJ-34c epimer and ZYJ-34c in the active site of HDAC2 were respectively navigated by molecular dynamic (MD) simulations to probe the reason why ZYJ-34c epimer was more potent than its diastereomer. We chose HDAC2 for the following three reasons. First, all Zn2+ dependant HDACs, especially isoforms belonging to the same class bear a highly conserved active site. Second, Class I HDACs, especially HDAC1, HDAC2 and HDAC3 are the most tumor-related HDACs isoforms.12 Third, the HDAC2 crystal structure has been reported (PDB ID: 3MAX). After 200 ps of simulation, both the complexes had converged and reached equilibrium (Fig. S8†). After MD simulation, MM-GBSA method was used to calculate the Gibbs free energy associated with the binding of inhibitors to HDAC2. The total binding energy (ΔGb) of ZYJ-34c epimer (−63.44 kJ/mol) was slightly lower than that of ZYJ-34c (-61.58 kJ/mol), which was in accordance with their HDACs inhibitory activity. In order to investigate the influence of different chirality on protein-ligand interaction, MM-GBSA decomposition calculation was performed. Calculation results of two key residues (PRO-23 and ASP-93, Table S1†), which interacted with the chiral side chains of the two epimers, and the binding modes in HDAC2 (Fig. 3) indicated that compared with ZYJ-34c, its epimer could not only form an additional −0.503 kcal/mol of hydrophobic interaction with PRO-23 (Fig. 3b) but also reduce 3.579 kcal/mol of repulsive force against ASP-93 (Fig. 3a).
Fig. 3.
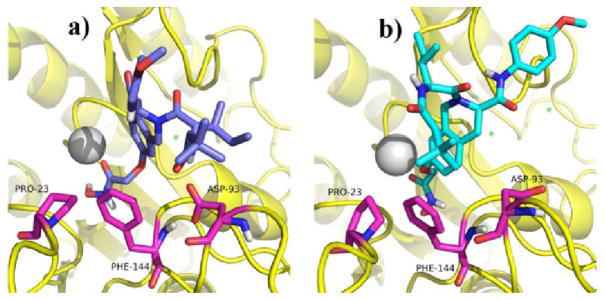
Proposed binding modes of ZYJ-34c (blue stick, a) and its epimer (cyan stick, b) in HDAC2. Protein is represented in ribbon diagrams with amino acid residues labeled in purple sticks. The Zn2+ is shown as a gray sphere.
Conclusions
In conclusion, we successfully determined the exact absolute configurations of the previous HDACi ZYJ-34c and its newly discovered epimer by a facile asymmetric synthetic method. It is interesting that ZYJ-34c epimer exhibited more potent HDACs inhibition and antitumor activities than ZYJ-34c. More importantly, both diastereomers could be obtained on large scale using our asymmetric synthetic method, which laid a solid foundation for further research and development of ZYJ-34c epimer as a promising antitumor candidate. Moreover, the different HDACs inhibitory activities of the two epimers could be rationalized by computational study, validating MD simulations and MM-GBSA as reliable methods for HDACi discovery, at least for rational design and screening of our tetrahydroisoquinoline-based HDACi.
Supplementary Material
Acknowledgments
This work was supported by National Scientific and Technological Major Project of Ministry of Science and Technology of China (Grant No.2011ZX09401-015), National Natural Science Foundation of China (Grant No.21302111, Grant No.21172134), Independent Innovation Foundation of Shandong University, IIFSDU (Grant No.2013GN013) and National Cancer Institute of the National Institute of Health (Award No.R01CA163452).
Footnotes
Electronic Supplementary Information (ESI) available: [details of any supplementary information available should be included here]. See DOI: 10.1039/b000000x/
Notes and references
- 1.Berger SL, Kouzarides T, Shiekhattar R, Shilarifard A. Genes Dev. 2009;23:781–783. doi: 10.1101/gad.1787609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones PA, Baylin SB. Cell. 2007;128:683–692. doi: 10.1016/j.cell.2007.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsankova N, Renthal W, Kumar A, Nestler EJ. Nat Rev Neurosci. 2007;8:355–367. doi: 10.1038/nrn2132. [DOI] [PubMed] [Google Scholar]
- 4.Wolffe AP. Science. 1996;272:371–372. doi: 10.1126/science.272.5260.371. [DOI] [PubMed] [Google Scholar]
- 5.Best JD, Carey N. Drug Discov Today. 2010;15:65–70. doi: 10.1016/j.drudis.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 6.http://www.clinicaltrials.gov/
- 7.Zhang Y, Feng J, Liu C, Zhang L, Jiao J, Fang H, Su L, Zhang X, Zhang J, Li M, Wang B, Xu W. Bioorg Med Chem. 2010;18:1761–1772. doi: 10.1016/j.bmc.2010.01.060. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Y, Feng J, Jia Y, Wang X, Zhang L, Liu C, Fang H, Xu W. J Med Chem. 2011;54:2823–2838. doi: 10.1021/jm101605z. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Y, Fang H, Feng J, Jia Y, Wang X, Xu W. J Med Chem. 2011;54:5532–5539. doi: 10.1021/jm200577a. [DOI] [PubMed] [Google Scholar]
- 10.Jones J. Amino Acid and Peptide Synthesis. Chapter 5 Oxford University Press; New York: 2002. [Google Scholar]
- 11.Joullié MM, Lassen KM. Arkivoc. 2010;8:189–250. [Google Scholar]
- 12.Venugopal B, Evans TR. Curr Med Chem. 2011;18:1658–1671. doi: 10.2174/092986711795471284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.