Abstract
The respiratory system is immature at birth and significant development occurs postnatally. A critical period of respiratory development occurs in rats around postnatal days 12-13, when enhanced inhibition dominates over suppressed excitation. The mechanisms underlying the heightened inhibition are not fully understood. The present study tested our hypothesis that the inhibition is marked by a switch in glycine receptor subunits from neonatal to adult form around the critical period. An in-depth immunohistochemical and single neuron optical densitometric study was undertaken on four respiratory-related nuclear groups (the pre-Bötzinger complex, nucleus ambiguus, hypoglossal nucleus, and ventrolateral subnucleus of solitary tract nucleus), and a non-respiratory cuneate nucleus in P2-21 rats. Our data revealed that in the respiratory-related nuclear groups: (1) the expressions of GlyRα2 and GlyRα3 were relatively high at P2, but declined after 1-1½ weeks to their lowest levels at P21; (2) the expression of GlyRα1 increased with age and reached significance at P12; and (3) the expression of GlyRβ rose from P2 to P12 followed by a slight decline until P21. No distinct increase in GlyRα1 at P12 was noted in the cuneate nucleus. Thus, there is a switch in dominance of expression from neonatal GlyRα2/α3 to the adult GlyRα1 and a heightened expression of GlyRα1 around the critical period in all respiratory-related nuclear groups, thereby supporting enhanced inhibition at that time. The rise in the expression of GlyRβ around P12 indicates that it plays an important role in forming the mature heteropentameric glycine receptors in these brain stem nuclear groups.
Keywords: critical period, cuneate nucleus, hypoglossal nucleus, nucleus ambiguus, pre-Bötzinger complex, ventrolateral subnucleus of solitary tract nucleus
1. Introduction
In rats, a critical period in respiratory network development exists around postnatal days (P) 12-13, when a striking and transient imbalance between enhanced expression of inhibitory neurochemicals and suppressed expression of excitatory neurochemicals occurs in multiple respiratory-related brain stem nuclear groups (Liu and Wong-Riley, 2002, 2005; Wong-Riley and Liu, 2005, 2008; Wong-Riley et al., 2013). Such an imbalance is also demonstrable electrophysiologically at the synaptic level (Gao et al., 2011). During this narrow window, there is an apparent switch in GABAA receptor subunits from the neonatal α3 to the adult α1 form (Liu and Wong-Riley, 2004, 2006), and a switch from the neonatal Cl−-intruder Na+-K +-2Cl− co-transporter 1 (NKCC1) to the adult Cl−-extruder K+-Cl− co-transporter 2 (KCC2) (Liu and Wong-Riley, 2012), in multiple brain stem respiratory-related nuclear groups. Moreover, the ventilatory and metabolic responses to hypoxia are at their weakest at this time (Liu et al., 2006, 2009).
The enhanced inhibition during the critical period is likely to be mediated by two major inhibitory neurotransmitters, GABA and glycine, and their receptors. Significantly, in the respiratory-related hypoglossal nucleus (XII),a switch in dominance from GABAergic to glycinergic synaptic transmission is evident at the beginning of the second postnatal week, being most prominent during the critical period (Gao et al., 2011). At this time, the expression of glycinergic receptors is significantly increased in multiple respiratory-related nuclear groups of the brain stem (Liu and Wong-Riley, 2002, 2005).
Glycine receptors (GlyR) are pentameric ligand-gated Cl− channels made up of α1, α2, or α3 with or without β subunits (reviewed in Kuhse et al., 1991; Dutertre et al., 2012). α4 subunit is reported thus far mainly in the retina (Heinze et al., 2007). Glycine receptors undergo subunit changes during development in different parts of the nervous system (Malosio et al., 1991; Aroeira et al., 2011; Jonsson et al., 2012). However, the distribution and developmental patterns of the various GlyR subunits are virtually unknown within the brain stem respiratory system.
The present study was undertaken to test our hypothesis that the heightened inhibition during the critical period is marked by a switch in glycine receptor subunits from the neonatal to the adult form. We conducted an in-depth immunohistochemical and single neuron optical densitometric analysis of GlyR α1, α2, α3, and β subunits in four respiratory-related nuclear groups and one nonrespiratory nucleus of P2–21 rats. The respiratory-related nuclear groups are the pre-Bötzinger complex (PBC, a presumed center or kernel for respiratory rhythmogenesis; Smith et al., 1991, 2000; Rekling and Feldman, 1998); nucleus ambiguus (Amb, which receives input from the central respiratory network and innervates airway muscles of the pharynx and larynx to maintain upper airway patency; Jordan, 2001); hypoglossal nucleus (XII, which innervates the tongue and pharyngeal muscles important for maintaining upper airway patency during breathing; Lowe, 1980; Jordan, 2001); and the ventrolateral subnucleus of the solitary tract nucleus (NTSVL, which receives direct projections from pulmonary stretch receptor afferents of the vagus, the superior laryngeal nervers, as well as peripheral chemoafferents; contains inspiratory neurons and is part of the dorsal respiratory group known to project directly or indirectly via the pontine respiratory group to the ventral respiratory column; McCrimmon et al., 1987; Smith et al., 1989; 2013; Ellenberger and Feldman, 1990; Holtman et al., 1990; Finley and Katz, 1992; Bonham, 1995; Subramanian et al., 2007; Yokota et al., 2008; Wong-Riley et al., 2013). The non-respiratory cuneate nucleus (CN) is a relay in the somatosensory system with no known respiratory function, and it served as an internal control.
2. Results
2.1. GlyRα1-immunoreactive (-ir) neurons in the brain stem nuclear groups
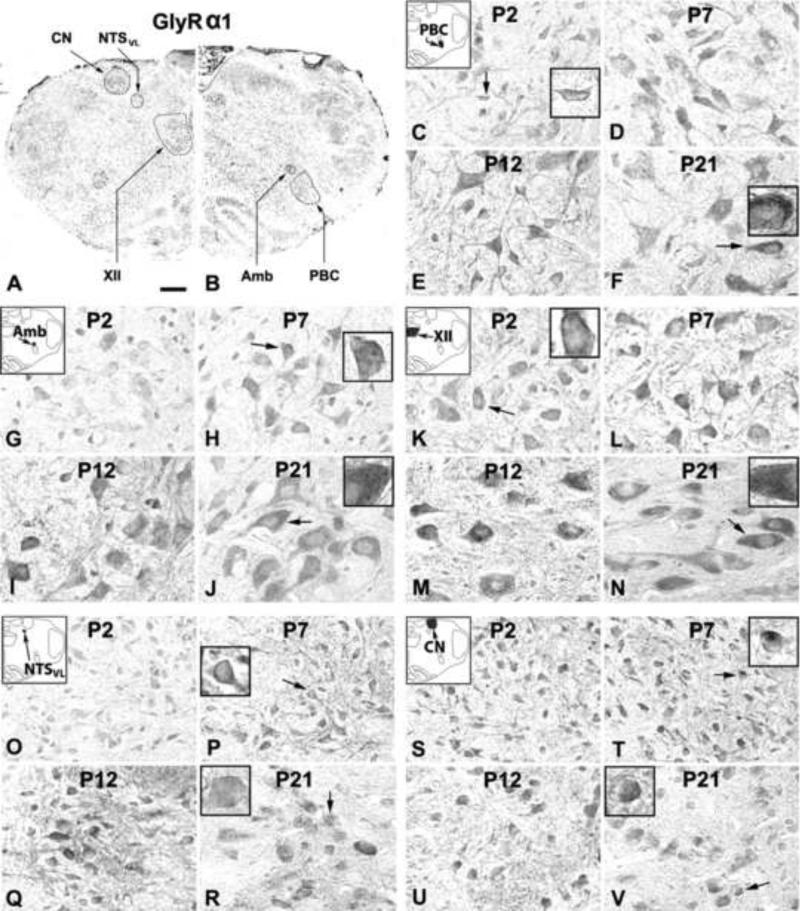
GlyRα1-ir was clearly visible in subpopulations of neurons in all five brain stem nuclear groups examined (Fig. 1A, B). Immunoreaction product was present in cell bodies and proximal dendrites of labeled neurons as well as in the neuropil (including dendritic processes). The plasma membrane of labeled neurons showed clear immunoreaction product (see insets in Fig. 1). The sizes of GlyRα1-ir neurons increased with age and reached a level at P11-P12 approximately 85-95% of that at P21 (Fig. 1). Control sections had no specific immunoreactivity above background (data not shown). One-way ANOVA indicated significant differences (P < 0.01) in GlyRα1-ir among the ages in the PBC, Amb, XII, and NTSVL, but not in the CN. Tukey's Studentized range test that compared one age group with its adjacent younger tested age group revealed a significant rise at P12 for PBC, Amb, and XII (P < 0.01 – P < 0.001, as compared to the values at P11), but not for CN (Fig. 2).
Figure 1.
A and B. Low magnification micrograph of rat brain stem section at P7 immuno-reacted for GlyRα1. GlyRα1-ir neurons and neuropil in the pre-Bötzinger complex (PBC; C-F), nucleus ambiguus (Amb; G-J), hypoglossal nucleus (XII; K-N), ventrolateral subnucleus of the solitary tract nucleus (NTSVL; O-R), and cuneate nucleus (CN; S-V) at postnatal days P2, P7, P12, and P21. Diagrammatic locations of each of the five nuclear groups are shown in the upper left corners of C, G, K, O, and S. In the PBC, Amb, XII, and NTSVL, GlyRα1-ir expression was relatively low at P2, increased slightly at P7, rose significantly at P12, followed by a plateau at P21. Labeling in the neuropil, where neuronal processes and synapses reside, was highest at P12. GlyRα1 immunoreactivity in the CN increased mildly from P2 to P12, then stabilized until P21. In all five nuclear groups and at all ages tested, the plasma membrane of labeled neurons also showed immunoreaction product (see arrows and insets in C, F, H, J, K, N, P, R, T, and V). Scale bar: 345 μm for A and B; 20 μm for the rest (6.66 μm for small insets).
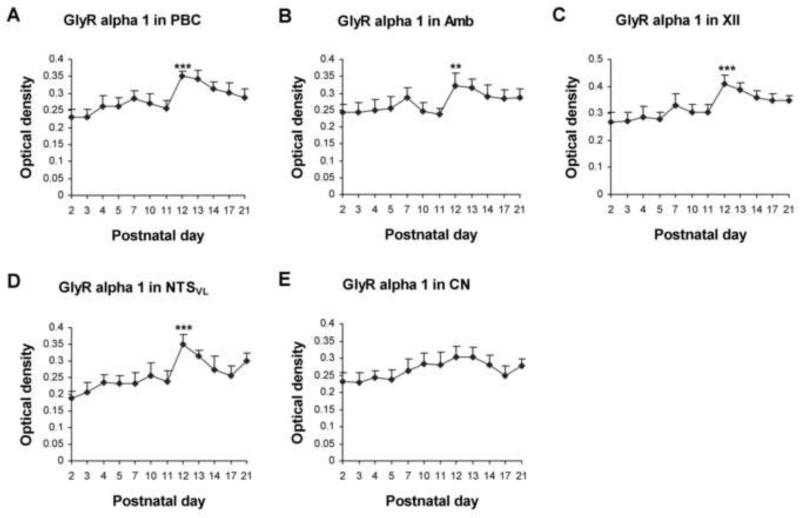
Figure 2.
Optical densitometric measurements of immunoreaction product for GlyRα1 in individual neurons of the PBC (A), Amb (B), XII (C), NTSVL (D), and CN (E) from P2 to P21. Data points were presented as mean ± SEM. In the first four nuclear groups, GlyRα1-ir was lowest at P2, increased slightly until P7-10, then rose significantly at P12, followed by either a gradual decline or a plateau until P21. The expression in the CN also increased slightly from P2 to P12-13, but there was not a significant rise at P12. ANOVA yielded significant differences in the expression of GlyRα1-ir among the ages in the PBC, Amb, XII, and NTSVL (P < 0.01; not shown), but not in the CN. Tukey's Studentized tests that compared one age group with its immediately adjacent younger age group showed significance only at P12. **, P < 0.01; ***, P < 0.001.
2.1.1. GlyRα1-immunoreactive neurons in the PBC
GlyRα1-ir was observed in ~ 45% - 60% of the PBC neurons. They were multipolar, granular, or fusiform in shape and small or medium in size (Fig. 1C-F). The size of small neurons ranged from 5 to 8 μm in diameter at P2 to 7-9 μm at P21, and medium-sized neurons ranged from 9.5 - 14 μm at P2 to 12 - 20.5 μm at P21. The expression of GlyRα1 increased gradually from P2 to P7 (P < 0.05 for Tukey's test between P2 and P7), but significantly from P11 to P12 (P < 0.001), followed by a plateau until P21 (Fig. 2A). P12 was the only time point in the first 3 postnatal weeks when a day-to-day significance was found. Tukey's test also revealed that the values at P12, P13, and P14 were significantly higher than those of each tested day from P2 to P11 (P < 0.05 – P < 0.001), and the values at P17 and P21 were significantly higher than those of P2 and P3 (P < 0.05 – P < 0.001).
2.1.2. GlyRα1-immunoreactive neurons in the Amb
About 50% - 65% of Amb neurons demonstrated GlyRα1-ir. These neurons were multipolar or oval in shape and mainly medium or small in size (Fig. 1G-J). The size of small neurons ranged from 6 to 8.5 μm in diameter at P2 to 7 - 11 μm at P21, and medium-sized neurons ranged from 11 - 15 μm at P2 to 16.5-21 μm at P21. Occasionally, a few large labeled neurons (24 - 28 μm in diameter) were observed at P21. GlyRα1 immunoreactivity exhibited a trend similar to that of the PBC, with a significant increase at P12 (P < 0.01) (Fig. 2B). Tukey's test also yielded significant differences between values at P12-13 and those of each individual days from P2 to P11 (P < 0.05 – P < 0.01), except for P7.
2.1.3. GlyRα1-immunoreactive neurons in the XII
GlyRα1-ir was present in ~ 80% - 90% of XII neurons. They were multipolar, oval, or fusiform in shape and mainly medium or large in size (Fig. 1K-N). Medium-sized neurons ranged from 11 to 15 μm in diameter at P2 to 14 - 20.5 μm at P21, and large neurons ranged from 17 - 19.5 μm at P2 to 24 - 28.5 μm at P21. The developmental trend of GlyRα1-ir was comparable to those of the PBC and Amb, with a gradual increase from P2 to P7 (P < 0.01 for Tukey's test between the two time points) and a significant rise at P12 (P < 0.001) (Fig. 2C). Tukey's test also showed that the value at each individual days from P12 to P21 was significantly higher than those of each tested day from P2 to P11 (P < 0.01 – P < 0.001), except for P7.
2.1.4. GlyRα1-immunoreactive neurons in the NTSVL
GlyRα1-ir was observed in about 35% - 50% of the NTSVL neurons. They were multipolar, granular, oval, or fusiform in shape and mainly small in size (Fig. 1O-R). The small neurons ranged from 5 - 8 μm in diameter at P2 to 7 - 11.5 μm at P21. Occasionally, a few of medium-sized neurons (14.5 - 19.5 μm in diameter) were observed at P17-21. The expression of GlyRα1 increased gradually from P2 to P10 (P < 0.05 for Tukey's test between the two time points), followed by a significant rise at P12 (P < 0.001) and a gradual decline at P13-14, then plateaued until P21 (Fig. 2D). Tukey's test also yielded significance in values between each individual days at P12, P13, P21 and each day from P2 to P11 (P < 0.05 – P < 0.001, except for a lack of significance between P10 and P21), between P2 or P3 and P14 or P17 (P < 0.05 – P < 0.01), as well as between P12 and P14, P17, or P21 (P < 0.05 – P < 0.01).
2.1.5. GlyRα1-immunoreactive neurons in the CN
About 30% - 50% of neurons in the CN demonstrated GlyRα1-ir (Fig. 1S-V). These labeled neurons were oval, multipolar, or granular in shape and mainly small in size. Small neurons ranged from 5 - 7.5 μm in diameter at P2 to 6 - 12.5 μm at P21, and medium-sized labeled neurons ranged from 8.5 - 10 μm at P2 to 14 - 19 μm at P21. The expression of GlyRα1 showed a much gentler rise from P2 to P21 (Fig. 2E). However, Tukey's test did not yield significant differences between any two age groups.
Two-way ANOVA indicated significant differences in GlyRα1 expression among the five nuclear groups (P < 0.01). Subsequent Tukey's tests revealed that the values were significantly higher in XII than in CN between P12 and P17 (P < 0.05 – P < 0.01). They were also higher in XII than in Amb at P12 (P < 0.05).
2.2. GlyRα2-immunoreactive neurons in the brain stem nuclear groups
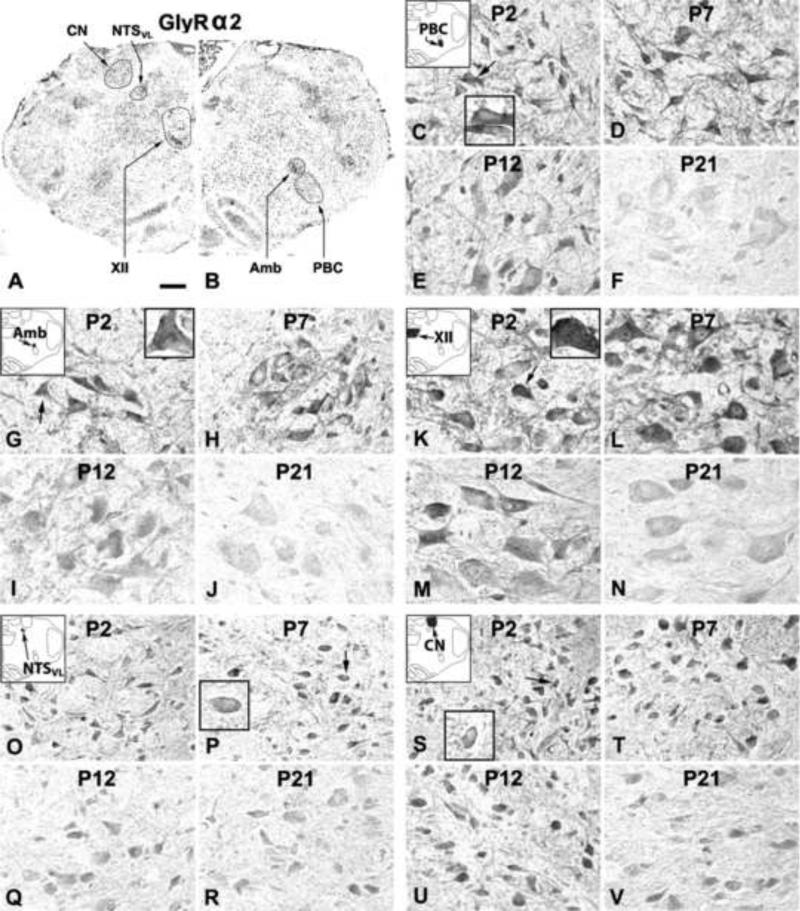
In general, GlyRα2-ir product was clearly visible in subpopulations of neurons in each of the brain stem nuclear group examined (Fig. 3A, B). Labeling was present in cell bodies and proximal dendrites of neurons as well as in the neuropil. Labeling could also be observed along the plasma membrane (see insets in Fig. 3). Developmental changes in sizes and shapes of GlyRα2-ir neurons were comparable to those of GlyRα1-ir neurons, and will not be described in detail below. Control sections demonstrated no specific immunoreactive product above background (data not shown). One-way ANOVA indicated significant differences (P < 0.01) in GlyRα2-ir among the ages in the PBC, Amb, XII, and NTSVL, but Tukey's test failed to yield significant differences between any two adjacent tested age groups. Significant differences between non-adjacent age groups are detailed below.
Figure 3.
A and B. Low magnification micrograph of rat brain stem section at P7 immuno-reacted for GlyRα2. GlyRα2-ir neurons and neuropil in the PBC (C-F), Amb (G-J), XII (K-N), NTSVL (O-R), and CN (S-V) at P2, P7, P12, and P21. In the first four nuclear groups, GlyRα2-ir expression was highest at P2 and P7, slightly reduced at P12, and further reduced at P21. The pattern in the CN was more constant from P2 to P21. The intensity of neuropil labeling in all nuclear groups was similar to those of neuronal cell bodies. Plasma membrane labeling was observable at P2, P7, and P12 (see arrows and insets in C, G, K, P, and S), but was not clearly detectable at P21. Scale bar: 345 μm for A and B; 20 μm for the rest (6.66 μm for small insets).
2.2.1. GlyRα2-immunoreactive neurons in the PBC
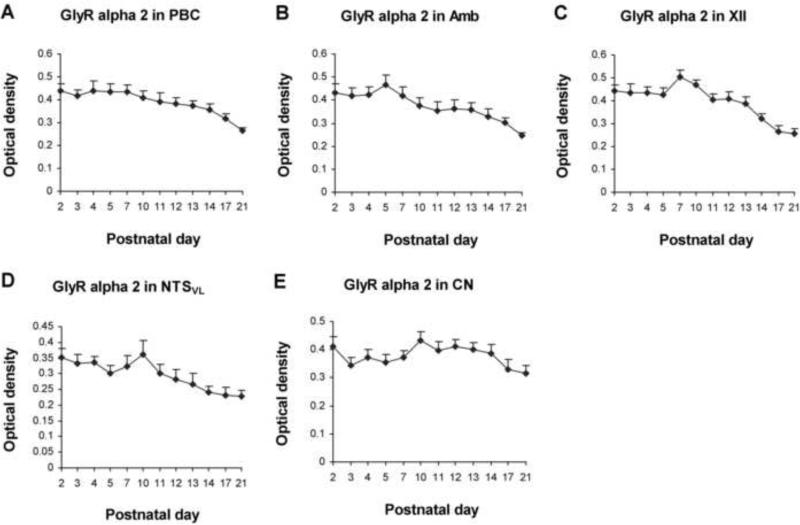
GlyRα2-ir was observed in ~ 45% - 55% of the PBC neurons (Fig. 3C-F). The expression of GlyRα2 was relatively high from P2 to P7, followed by a gradual decline until P21 (Fig. 4A). Although no significant differences were found between any two adjacent tested age groups, Tukey's test revealed that the value at P21 was significantly lower than those of each individual days from P2 to P12 (P < 0.05 – P < 0.001). The value at P17 was also significantly lower than those of P2 and individual days from P4 to P7 (P < 0.05).
Figure 4.
Optical densitometric measurements of immunoreaction product for GlyRα2-ir in individual neurons of the PBC (A), Amb (B), XII (C), NTSVL (D), and CN (E) from P2 to P21. Data points were presented as mean ± SEM. In the first four nuclear groups, GlyRα2-ir was relatively high from P2 to P7-10, followed by a gradual decline until P21. Labeling in the CN was relatively stable from P2 to P21, with the lowest value at P21. ANOVA yielded significant differences in the expression of GlyRα2-ir among the ages in the first four nuclear groups (P < 0.01; not shown), but not in the CN. Tukey's tests did not show significant differences between any two adjacent age groups.
2.2.2. GlyRα2-immunoreactive neurons in the Amb
GlyRα2-ir was present in ~ 55% - 65% of Amb neurons (Fig. 3G-J). The expression was relatively high from P2 to P5, but declined gradually thereafter until P21 (Fig. 4B). Tukey's test indicated that the levels at P17 and P21 were significantly lower than those at each day tested from P2 to P10 (P < 0.05 – P < 0.001). The value at P21 was also lower than those at P11, P12, or P13 (P < 0.05). The levels at P13-14 were also lower than that at P5 (P < 0.01 and P < 0.001, respectively).
2.2.3. GlyRα2-immunoreactive neurons in the XII
About 75% - 85% of the XII neurons exhibited GlyRα2-ir (Fig. 3K-N). The level was relatively high from P2 to P7, but declined gradually thereafter until P21 (Fig. 4C). Tukey's test revealed significant differences between P17 or P21 and each tested day from P2 to P11 (P < 0.05 - P < 0.001, except for a lack of significance between P11 and P17), and between P7 and P14.
2.2.4. GlyRα2-immunoreactive neurons in the NTSVL
GlyRα2-ir was present in ~ 35% - 45% of the NTSVL neurons (Fig. 3O-R). The expression followed a trend similar to those of the other three nuclear groups, with relatively high levels from P2 to P10 and a gradual decline until P21 (Fig. 4D). Tukey's test yielded significant differences between P10 and P14, P17, and P21 (P < 0.05).
2.2.5. GlyRα2-immunoreactive neurons in the CN
GlyRα2-ir was observed in ~ 30% - 35% of the CN neurons (Fig. 3S-V). The expression was relatively constant with minor fluctuations from P2 to P21, with the lowest levels at P17-21 (Fig. 4E). Tukey's test did not show significant differences between any two age groups.
Two-way ANOVA indicated significant differences in GlyRα2 expression among the five nuclear groups (P < 0.01). Subsequent Tukey's tests revealed that the values were significantly higher in Amb than in NTSVL at P5 (P < 0.05 – P < 0.01), and higher in XII than in NTSVL at P7 (P < 0.05 for both).
2.3. GlyRα3-immunoreactive neurons in the brain stem nuclear groups
GlyRα3-ir product was clearly observed in subpopulations of neurons in each of the brain stem nuclear group examined (Fig. 5A, B). Labeling was present in cell bodies and proximal dendrites of neurons as well as in the neuropil. The plasma membrane of many labeled neurons showed clear immunoreaction product (see insets in Fig. 5). Control sections demonstrated no specific immunoreactive product above background (data not shown). One-way ANOVA indicated significant differences in GlyRα3-ir among the ages in the PBC, Amb, XII, and NTSVL (P < 0.01). However, Tukey's test did not yield significant differences between any two adjacent tested age groups.
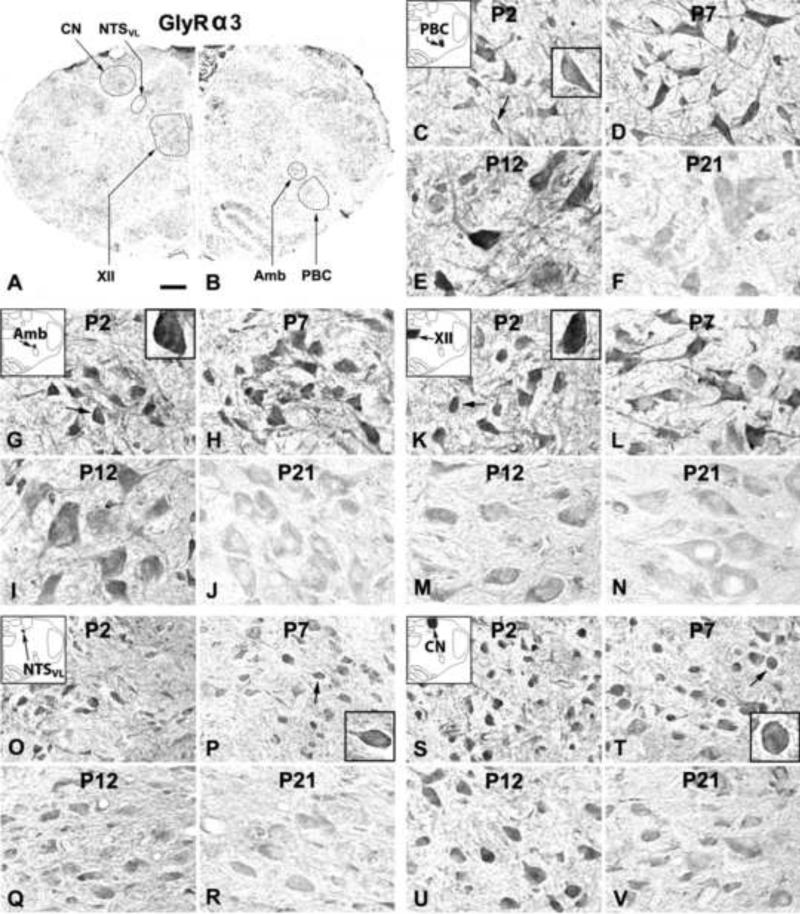
Figure 5.
A and B. Low magnification micrograph of rat brain stem section at P7 immuno-reacted for GlyRα3. GlyRα3-ir neurons and neuropil in the PBC (C-F), Amb (G-J), XII (K-N), NTSVL (O-R), and CN (S-V) at P2, P7, P12, and P21. In the first four nuclear groups, GlyRα3-ir expression was highest at P2, P7, and P12, but lowest at P21. The expression in the CN was more constant from P2 to P21. The neuropil labeling was relatively constant at P2, P7, and P12, but was reduced at P21. The plasma membrane showed immunoreactions product at P2, P7, and P12 (see arrows and insets in C, G, K, P, and T), but was much fainter at P21. Scale bar: 345 μm for A and B; 20 μm for the rest (6.66 μm for small insets).
2.3.1. GlyRα3-immunoreactive neurons in the PBC
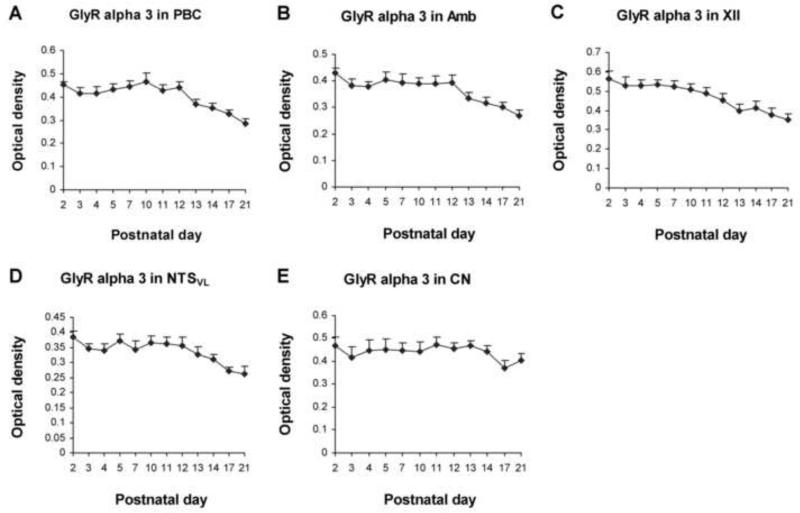
GlyRα3-ir was seen in ~ 50% - 65% of the PBC neurons (Fig. 5C-F). The expression of GlyRα3 was relatively high from P2 to P12, followed by a gradual decline until P21 (Fig. 6A). Tukey's test revealed a significant difference between the much lower value at P21 and those at each of the tested days from P2 to P12 (P < 0.01 – P < 0.001), and between the much lower value at P17 and those at P2, P7, and P10 (P < 0.05 for all).
Figure 6.
Optical densitometric measurements of immunoreaction product for GlyRα3-ir in individual neurons of the PBC (A), Amb (B), XII (C), NTSVL (D), and CN (E) from P2 to P21. Data points were presented as mean ± SEM. In the first four nuclear groups, GlyRα3-ir was relatively high from P2 to P10-12, then declined gradually until P21. The expression in the CN was relatively constant from P2 to P21. ANOVA yielded significant differences in the expression of GlyRα3-ir among the ages in the first four nuclear groups (P < 0.01; not shown), but Tukey's test did not show significant differences between any two adjacent age groups.
2.3.2. GlyRα3-immunoreactive neurons in the Amb
About 60% - 75% of the Amb neurons were GlyRα3-ir (Fig. 5G-J). The expression was relatively high from P2 to P12 but declined gradually until P21 (Fig. 6B). Tukey's test yielded significant differences between the much lower value at P21 and each of the tested days from P2 to P12 (P < 0.05 – P < 0.01), and P17's value was much lower than that at P2 (P < 0.05).
2.3.3. GlyRα3-immunoreactive neurons in the XII
GlyRα3-ir was present in ~ 70% - 85% of the XII neurons (Fig. 5K-N). Labeling was relatively high from P2 to P12, but declined gradually until P21 (Fig. 6C). Tukey's test indicated that the expression at P21 was significantly lower than those at each tested days from P2 to P10 (P < 0.05 – P < 0.01), and between the much lower values at P13 or P17 and that at P2 (P < 0.05).
2.3.4. GlyRα3-immunoreactive neurons in the NTSVL
GlyRα3-ir was observed in ~ 40% - 55% of the NTSVL neurons (Fig. 5O-R). The expression followed a trend similar to those of the other three nuclear groups, with a relatively high level from P2 to P12, followed by a gradual decline until P21 (Fig. 6D). Tukey's test revealed that the value at P21 was significantly lower than those at each of the tested days at P2, P5, P10, P11, and P12 (P < 0.05), and the value at P17 was lower than that at P2 (P < 0.05).
2.3.5. GlyRα3-immunoreactive neurons in the CN
GlyRα3-ir was present in ~ 50% - 60% of the CN neurons (Fig. 5S-V). The expression was relatively stable between P2 and P21, with the lowest levels at P17-21 (Fig. 6E). Tukey's test did not yield significant differences between any two age groups.
Two-way ANOVA indicated significant differences in GlyRα3 expression among the five nuclear groups (P < 0.01). Subsequent Tukey's tests revealed that the values were significantly higher in XII than in NTSVL at P2, P3, P4, and P7 (P < 0.05 – P < 0.01).
2.4. GlyRβ-immunoreactive neurons in the brain stem nuclear groups
GlyRβ-ir product was clearly demonstrable in subpopulations of neurons in each of the brain stem nuclear group examined (Fig. 7A, B). Labeling was present in cell bodies and proximal dendrites of neurons as well as in the neuropil. The plasma membrane of many labeled neurons showed immunoreactions product (see insets in Fig. 7). Control sections had no specific labeling above background (data not shown). One-way ANOVA indicated significant differences in GlyRβ-ir among the ages in all five nuclear groups examined (P < 0.01). However, Tukey's test did not yield significant differences between any two adjacent tested age groups. Significances between non-adjacent age groups are detailed below.
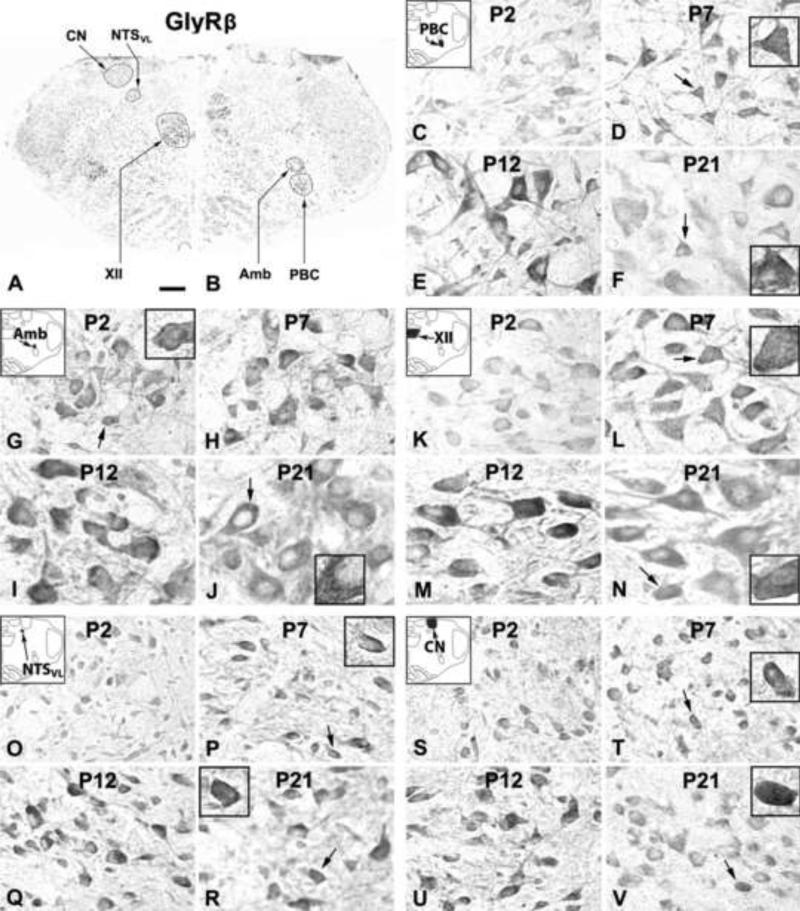
Figure 7.
A and B. Low magnification micrograph of rat brain stem section at P7 immuno-reacted for GlyRβ. GlyRβ-ir neurons and neuropil in the PBC (C-F), Amb (G-J), XII (K-N), NTSVL (O-R), and CN (S-V) at P2, P7, P12, and P21. In all five nuclear groups, the expression was relatively low at P2, increased at P7 and peaked at P12, followed by a slight decline at P21. Labeling intensity in the neuropil was similar to that in neuronal cell bodies. Reaction product on the plasma membrane of labeled neurons was observable at all ages in all five nuclear groups (see arrows and insets in D, F, G, J, L, N, P, R, T, and V). Scale bar: 345 μm for A and B; 20 μm for the rest (6.66 μm for small insets).
2.4.1. GlyRβ-immunoreactive neurons in the PBC
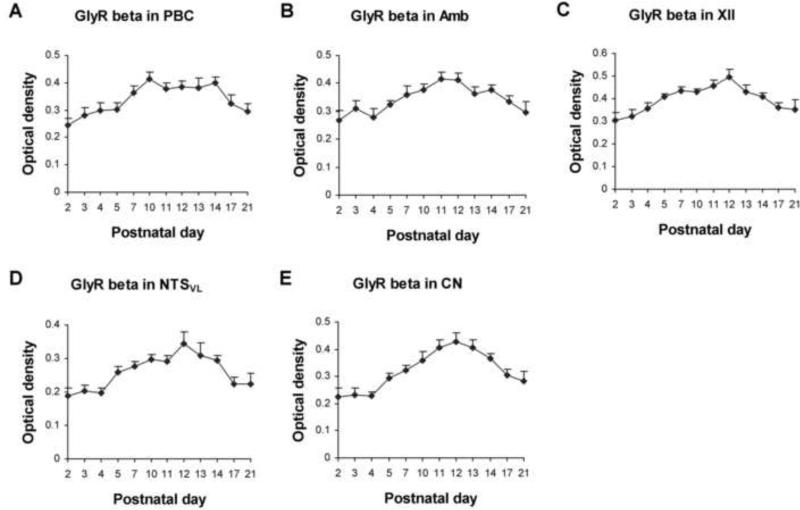
GlyRβ-ir was present in ~ 50% - 70% of the PBC neurons (Fig. 7C-F). The expression was lowest at P2 but rose gradually to reach a peak at P10, followed by a plateau until P21 (Fig. 8A). Tukey's test revealed that the values at each tested day from P7 to P14 was significantly higher than that at P2 (P < 0.05 - P < 0.01), and that the value at P10 was significantly higher than that at P3 (P < 0.05).
Figure 8.
Optical densitometric measurements of immunoreaction product for GlyRβ-ir in individual neurons of the PBC (A), Amb (B), XII (C), NTSVL (D), and CN (E) from P2 to P21. In all five nuclear groups, GlyRβ-ir was lowest at P2, increased thereafter to reach a peak around P12, followed by a gradual decline until P21. ANOVA revealed significant differences in the expression of GlyRβ-ir among the ages in all five nuclear groups (P < 0.01; not shown), but Tukey's test did not yield significant differences between any two adjacent age groups.
2.4.2. GlyRβ-immunoreactive neurons in the Amb
GlyRβ-ir was observed in ~ 55% - 75% of the Amb neurons (Fig. 7G-J). The expression was the lowest at P2, but rose gradually to peak at P11-12, followed by a gradual decline until P21 (Fig. 8B). Tukey's test showed that the expression at P11 and P12 was significantly higher than that at P2 (P < 0.05).
2.4.3. GlyRβ-immunoreactive neurons in the XII
About 75% - 85% of the XII neurons exhibited GlyRβ-ir (Fig. 7K-N). The expression was again the lowest at P2, rose gradually to peak at P12, followed by a gradual decline until P21 (Fig. 8C). Tukey's test indicated that the expression at P12 was significantly higher than those at each tested days at P2, P3, P4, P17, and P21 (P < 0.05 - P < 0.001), and the value at P11 was much higher than those at P2 and P3 (P < 0.01 or P < 0.05, respectively).
2.4.4. GlyRβ-immunoreactive neurons in the NTSVL
GlyRβ-ir was present in ~ 40% - 50% of the XII neurons (Fig. 7O-R). The trend of expression was similar to those of the other three nuclear groups, in that the level was lowest at P2, then rose gradually to reach a peak at P12, followed by a gradual decline until P21 (Fig. 8D). Tukey's test showed that the expression at P12 was significantly higher than those at P2, P3, P4, P17, or P21 (P < 0.05 - P < 0.001), and that the value at P13 was significantly higher than those at P2 and P4 (P < 0.05).
2.4.5. GlyRβ-immunoreactive neurons in the CN
Approximately 35% - 45% of the CN neurons demonstrated GlyRβ-ir (Fig. 7S-V). The expression followed a trend similar to those in the Amb, XII, and NTSVL, with the lowest level at P2, a gradual rise to peak at P12, followed by a gradual decline until P21 (Fig. 8E). Tukey's test revealed that the expression at each tested day from P11 to P14 was significantly higher than those at P2, P3, P4, or P21 (P < 0.05 – P < 0.001, except for a lack of significance between P14 and P21), and the value at P12 was significantly higher than those at P5 or P17 (P < 0.05).
Two-way ANOVA indicated significant differences in GlyRβ expression among the five nuclear groups (P < 0.01). Subsequent Tukey's tests revealed that the values were significantly higher in XII than in NTSVL at P4-12, P17, and P21 (P < 0.05 – P < 0.01).
3. Discussion
The present large-scale, in-depth developmental study revealed for the first time that: 1) the expression of GlyRα1 increased with age in four respiratory-related nuclear groups, and reached the only day-to-day significant increase at P12, the height of the critical period; 2) the expressions of GlyRα2 and GlyRα3 in the four nuclear groups were relatively high at P2 but declined after the first 1 to 1 ½ weeks to reach the lowest level at P21; 3) the expression of GlyRβ followed closely that of GlyRα1 in that the level increased with age; however, there was not a day-to-day significant increase at P12; and 4) in the non-respiratory cuneate nucleus, the expressions of GlyRα1-α3 had a much gentler rise and fall with age, whereas that of GlyRβ was comparable to those in the other four nuclear groups. Thus, our results strongly support our hypothesis that a developmental switch in GlyR subunits (from GlyRα2 and/or GlyRα3 to GlyRα1) occurs in respiratory-related nuclear groups around the critical period (P12). The developmental increase in the expression of GlyRβ suggests that it contributes to a heteromeric αβ subunit composition in mature glycine receptors.
3.1. Functions of glycine receptors
Glycine is one of the two major inhibitory neurotransmitters in the central nervous system (Aprison and Daly, 1978). Like GABA, glycine is critical for fast synaptic inhibition in the brain stem respiratory system and is essential for normal respiratory pattern generation by controlling respiratory phase transitions during pre- and postinspiration in the neonate and in the adult (Schmid et al., 1991; Paton and Richter, 1995; Shao and Feldman, 1997; Büsselberg et al., 2001; Dutschmann and Paton, 2002). Although glycinergic pacemaker neurons have been found in the PBC (Morgado-Valle et al., 2010), they are few in number, and inhibition is considered not necessary for inspiratory rhythmogenesis in the PBC (Feldman et al., 2013). Glycinergic inhibition is essential for coordinating the activity of breathing and upper airway patency that involves Amb and XII motoneurons (Singer et al., 1998; Dutschmann and Paton, 2002). Inhibitory pump cells in the NTSVL of rats adjust respiratory control in response to lung volume changes, including the Breuer-Hering reflex (Ezure and Tanaka, 2004; Kubin et al., 2006; Janczewski et al., 2013). Rat pump cells also receive a phasic inhibitory input at the transition from inspiration to expiration, and glycinergic inhibition is involved during early inspiration (Miyazaki et al., 1999). With hypoxia, glycine is thought to be involved in the late, depressive phase of the hypoxic ventilatory response (Kato et al., 2000).
In many regions of the brain, both glycine and GABA transition postnatally from an initial depolarizing, excitatory phase to a mature hyperpolarizing inhibitory one (Fulton et al., 1980; Ben-Ari et al., 1989; Owens et al., 1996; Bakus et al., 1998). In the rat respiratory system, such transition occurs during the first two postnatal weeks in hypoglossal motoneurons, coincidental with a switch in GlyR subunit mRNAs from α2 to α1 (Singer et al., 1998). However, in the PBC, the transition reportedly occurs at embryonic day 19 (Ren and Greer, 2006). A postnatal switch from the neonatal GABAA receptor α3 to the mature α1 (Liu and Wong-Riley, 2004, 2006) and from GlyRα2/α3 to GlyRα1 (present study) are consistent with postnatal maturation of Cl−-mediated synaptic transmission in multiple respiratory-related nuclear groups in the brain stem. However, they do not directly address the question of depolarization versus hyperpolarization events.
The action of glycine is mediated through its pentameric ligand-gated Cl− channel receptors (Betz and Becker, 1988). These receptors belong to the Cys loop receptor family, which includes nicotinic acetylcholine receptors (nAChR), GABAA receptors (GABAAR), and serotonergic type 3 receptors (5-HT3R) (Dutertre et al., 2012). Five genes have been identified for GlyRs: α1-α4 and a single β gene (reviewed in Kuhse et al., 1991). All subunits share high sequence identity (> 80%), are ~ 48 kDa in molecular weight, provide the ligand-binding motif, and can form homomeric receptors. The β subunit, on the other hand, is ~58 kDa, can only form heteromers with α subunits, contributes to agonist binding, and interacts with gephyrin to enhance GlyR clustering at the synapse (Meyer et al., 1995; Kneussel and Betz, 2000; Maas et al., 2006).
3.2. Developmental changes in glycine receptor subunit expression
3.2.1. GlyRα2
The higher expression of GlyRα2 during the first postnatal week and its decline with age in all four respiratory-related nuclear groups is consistent with this subunit being regarded as the embryonic or neonatal form, whose level declines rapidly by the 3rd postnatal week (Becker et al., 1988; Malosio et al., 1991; Watanabe and Akagi, 1995; Lynch, 2009). GlyRα2s mediate inhibitory postsynaptic currents (IPSCs) that have slow decay kinetics (Dutertre et al., 2012). This is reminiscent of the longer decay time of the glycine-mediated miniature IPSCs during the first postnatal week in hypoglossal motoneurons (Gao et al., 2011). GlyRα2s form homomeric receptors that are localized to extrasynaptic sites and rely on paracrine glycine and taurine signaling to provide a slow, tonic effect on neurons (Aroeira et al., 2011; Jonsson et al., 2012). As the level of intracellular Cl− is kept high by the greater expression of the Cl−-intruder Na+-K+-2Cl− co-transporter 1 (NKCC1) in many brain stem respiratory-related neurons (Liu and Wong-Riley, 2012), the homomeric GlyRα2s may mediate depolarization that stimulates calcium influx essential for the development of many neuronal properties, including glycinergic synapses (Lynch, 2009). Interestingly enough, GlyRα2 knock-out mice are phenotypically normal (Young-Pearse et al., 2006).
3.2.2. GlyRα3
Although GlyRα3, like GlyRα2, exhibited an age-dependent decrease in its expression in the four respiratory-related nuclear groups, its reduction did not commence until after P12, i.e., about 2-5 days later than that of GlyRα2. This discrepancy in timing implies that α2 and α3 are not regulated in synchrony and that α3 continues to play an important role into the second postnatal week and perhaps later. GlyRα3 confers medium-fast kinetics that is intermediate between the neonatal slow α2 and the mature fast α1 (Weiss et al., 2008). In the respiratory network, the rhythmic activity is affected by the phosphorylation state of GlyRα3, which is controlled by serotonergic receptors, specifically 5-HT1AR (Manzke et al., 2010; Shevtsova et al., 2011). The 5-HT1AR- GlyRα3 signaling can rescue opioid-induced respiratory depression by glycinergic inhibition of inhibitory neurons, thereby inducing disinhibition (Manzke et al., 2010; Shevtsova et al., 2011). Between P2 and P11, such modulation may be more efficient, as the expressions of both 5-HT 1A and GlyRα3 are at their highest in several respiratory-related nuclear groups (Liu and Wong-Riley, 2010a, and the present study).
3.2.3. GlyRα1
The expression of GlyRα1 increased with age in all four respiratory-related nuclear groups examined. Thus, this is the predominant α isoform that contributes to the mature αβ GlyRs (Lynch, 2009). Remarkably, the expression of GlyRα1 peaked significantly at P12, at the height of the critical period, in all four respiratory-related nuclear groups. GlyRα1-containing receptors mediate inhibitory postsynaptic currents that have short mean open times and fast decay kinetics (Singer et al., 1998). The peaking of GlyRα1 at P12 implies that at the height of the critical period, glycinergic inhibition is strong within the respiratory network. Consistent with this idea are our findings in hypoglossal motoneurons that the amplitude, mean frequency and charge transfer of miniature IPSCs are significantly increased at P12-13, that the amplitude and frequency of spontaneous IPSCs are significantly increased at P12-13, and that glycinergic transmission dominates over GABAergic ones after the first postnatal week (Gao et al., 2011).
A point mutation in the Glra1 gene leads to spasticity (Saul et al., 1994), and Glra1 null mutation causes fine motor tremor and muscle spasms that begin at the second postnatal week and worsens until death by three weeks of age in mice (Buckwalter et al., 1994). These findings imply that none of the other GlyR subunits can compensate for the loss of GlyRα1 function.
3.2.4. GlyRβ
The expression of the β subunit in all five brain stem nuclear groups examined exhibited an age-dependent increase from P2 to P12, peaking at the height of the critical period before down-regulating slightly until P21. This trend is similar though not identical to that of GlyRα1. As β subunits cannot form homomers by themselves, they need α subunits to generate heteropentamers. Recent experiments indicate that the mature pentamer is made up of 2α and 3β subunits rather than the 3α2β model proposed previously (Langosch et al., 1988; Deutertre et al., 2012). As the level of GlyRα3 remained quite high until P12 and that of GlyRα1 did not peak until P12 in the four respiratory-related nuclear groups, it is possible that β subunits form GlyRs with α3 in the few days before the critical period, then with α1 and possibly α3 during the critical period before transitioning to mainly α1. The β subunit determines the ligand-binding property of GlyRs and is essential in anchoring GlyR at synaptic sites (Grudzinska et al., 2005).
3.3. Critical period of respiratory development
Glycine is likely to play an important role during the critical period, when heightened inhibition and suppressed excitation demonstrable both neurochemically and electrophysiologically render the respiratory system less capable of responding adequately to hypoxia (Liu and Wong-Riley, 2002, 2005, Liu et al., 2006, 2009; Gao et al., 2011). In addition to a switch in dominance from the neonatal GlyRα2 and/or α3 to the mature GlyRα1 (the present study) that signals a more robust inhibition, the transient rise in inhibition during the critical period is further contributed by several factors occurring concurrently: a) a switch in the dominance of expression from NKCC1 to KCC2, enabling either a transition from depolarizing to hyperpolarizing, or a strengthening of the hyperpolarizing potentials mediated by GABA and glycine receptors (Liu and Wong-Riley, 2012); b) a switch in dominance from the neonatal α3 to the mature α1 for the GABAA receptors (Liu and Wong-Riley, 2004, 2006); c) a suppressed expression of multiple serotonergic receptors, serotonin transporter, and serotonin synthesizing enzyme in multiple brain stem respiratory-related nuclear groups (Liu and Wong-Riley, 2008; 2010a,b), which would attenuate the “net stimulatory effect” of serotonin on respiratory output (Lindsay and Feldman, 1993; Hodges and Richerson, 2008); reduced 5-HT1AR expression would also imply that its disinhibitory effect on respiration via GlyRα3 (Manzke et al., 2010; Shevtsova et al., 2011) would be diminished; and d) a significant reduction in the expression of brain-derived neurotrophic factor (BDNF) and its high-affinity receptor (tyrosine protein kinase B, TrkB) in multiple respiratory-related nuclear groups (Liu and Wong-Riley, 2013) indicates that their normal enhancement of excitation and suppression of inhibition (Wardle and Poo, 2003) would be reduced. These cumulative effects culminate in a transient state of augmented inhibition during the critical period that eventually subsides into a more balanced state of excitation and inhibition. During this time, the animals’ ventilatory and metabolic responses to acute hypoxia are at their weakest (Liu et al., 2006, 2009). It is also noteworthy that rats challenged with a primary immune system-altering infection and a secondary sublethal dose of endotoxin die mainly on day 12 (Blood-Siegfried et al., 2002). These findings have special implication for Sudden Infant Death Syndrome (SIDS), as a critical period constitutes one of its three risk factors (Filiano and Kinney, 1994).
4. Experimental procedures
4.1. Tissue preparation
All experiments and animal procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health Publications No. 80-23, revised 1996), and all protocols were approved by the Medical College of Wisconsin Animal Care and Use Committee (approval can be provided upon request). All efforts were made to minimize the number of animals used and their suffering.
A total of 96 Sprague-Dawley rats, both male and female, from 8 litters were used. Rat pups were sacrificed at each of postnatal days P2, 3, 4, 5, 7, 10, 11, 12, 13, 14, 17, and 21. They were deeply anesthetized with 0.6% sodium pentobarbital (60 mg/kg IP; Diamondback drugs, Scottsdale, AZ) and perfused through the aorta with 4% paraformaldehyde-4% sucrose in 0.1 M sodium phosphate buffered saline (PBS), pH 7.4. Brain stems were then removed, postfixed in the same fixative for 3 h at 4°C, cryoprotected by immersion in increasing concentrations of sucrose (10, 20, and 30%) in 0.1 M PBS at 4°C, then frozen on dry ice and stored at −80°C until use.
4.2. Characterization of antibodies
Table 1 shows a summary of the antibodies used in the present study. The anti-GlyR α1 was an affinity purified rabbit polyclonal antibody raised against the human synthetic GlyRα1 peptide of 24 amino acids (between aa 190 and 239), and it yielded a single band of ~ 48 kDa by western blot analysis (manufacture's datasheet). The anti-GlyR α2 was a rabbit polyclonal antibody raised against the human C-terminus of GlyRα2 (between aa 371 and 420), and it yielded a single band at ~ 48 kDa by western blot analysis (manufacture's datasheet). Previous immunohistochemical study showed specific labeling of GlyR α2 (García-Alcocer et al., 2008). The anti-GlyR α3 was an affinity-purified goat polyclonal antibody raised against a 15 amino acid synthetic peptide between aa 400-450 of the C-terminus of human GlyRα3, and it yielded a single band at ~ 48 kDa by western blot analysis (manufacture's datasheet). In transfected HEK293T cells, the GlyRα3 antibody recognizes only the recombinant rat GlyRα3 protein and not GlyRα1, α2, or α4 subunits (Weltzien et al., 2012). Immunohistochemical studies showed specific labeling of GlyRα3 with this antibody (Majumdar et al., 2007; García-Alcocer et al., 2008; Weiss et al., 2008), and the staining was abolished in tissues of GlyRα3 knockout mice (Heinze et al., 2007). The anti-GlyRβ was an affinity-purified goat polyclonal antibody raised against a 20 amino acid synthetic peptide between aa 400-450 of the C-terminus of human GlyRβ , and it yielded a single band at ~ 58 kDa by western blot analysis (manufacture's datasheet and Laterza et al., 2006). Its specificity was also shown in immunohistochemical studies (García-Alcocer et al., 2008). The genes encoding human GlyR α1, α2, α3, and β have been mapped to chromosomes 5q31.3, Xp21.2-22.1, 4q32, and 4q33-34, respectively (Grenningloh et al., 1990; Handford et al., 1996; Nikolic et al., 1998).
Table 1.
Primary Antibodies Used
| Antigen | Immunogen | Manufacturer, species, type Catalog number | Dilution used |
|---|---|---|---|
| Glycine receptor α1 subunit (GlyR α1) | Human synthetic GlyR α1 peptide, 24 amino acid between aa 190-239 | Santa Cruz Biotech (Santa Cruz, CA), rabbit polyclonal IgG, sc-133629 (Q-24) | 1:150 |
| Glycine receptor α2 subunit (GlyR α2) | Human C-terminus of GlyR α2 (aa 371-420) | Santa Cruz Biotech (Santa Cruz, CA), rabbit polyclonal IgG, sc-20133 (H-50) | 1:400 |
| Glycine receptor α3 subunit (GlyR α3) | 15 amino acid synthetic peptide between aa 400-450 of the C-terminus of human GlyRα3 | Santa Cruz Biotech (Santa Cruz, CA), goat polyclonal IgG, sc-17282 (C-15) | 1:100 |
| Glycine receptor β subunit (GlyR β) | 20 amino acid synthetic peptide between aa 400-450 of the C-terminus of human GlyR β | Santa Cruz Biotech (Santa Cruz, CA), goat polyclonal IgG, sc-17285 (C-20) | 1:300 |
4.3. Immunohistochemistry
Coronal sections (12-μm thickness) of frozen brain stems were cut with a Leica CM1900 cryostat (Leica Microsystems, Heidelberger, Nussloch, Germany). Individual sets of serial sections were mounted on gelatin-coated slides. In the same litter, sections from 3 rats at different ages were mounted on the same slides and processed together. Ages were grouped typically as follows: P2-10-21, P3-4-17, P5-7-14, and P11-12-13. All sections from all rats were processed under identical conditions (i.e., time, temperature, and concentration of reagents). They were blocked overnight at 4°C with 5% nonfat dry milk-5% normal goat serum-1% Triton X-100 in 0.1 M PBS (pH 7.4). Alternating sets of sections were then incubated at 4°C for 36 h in one of the primary antibodies diluted at the proper concentration in the same solution as used for blocking (see Table 1 for antibodies’ concentration). Sections were rinsed 3 times, 5 min each, in PBS, then incubated in the secondary antibodies: 1:100 goat anti-rabbit IgG-HRP (Bio-Rad, Hercules, CA) for GlyRα1, GlyRα2, and 1:100 rabbit anti-goat IgG-HRP (Millipore Corp, Temecula, CA) for GlyRα3 and GlyRβ, diluted in the modified blocking solution (without Triton X-100) for 4 h at room temperature. After rinsing twice with PBS and once with 0.1 M ammonium phosphate buffer (APB), pH 7.0, immunoreactivity was detected with 0.05% DAB-0.004% H2O2 in APB for 5 min. The reaction was then stopped with APB for 5 min, rinsed in PBS three times, dehydrated, and coverslipped. Control sections were processed without primary antibodies or with a non-immune serum in place of the primary antibodies.
4.4. Semi-quantitative optical densitometry
The immunoreactivity of GlyR α1, α2, α3, and β in the cell bodies of individual neurons in various nuclear groups was semi-quantitatively analyzed by optical densitometry performed with a Zeiss Zonax MPM 03 photometer, a ×25 objective, and a 2-μm-diameter measuring spot. White (tungsten) light was used for illumination, and all lighting conditions were held constant for all of the measurements. Since light intensity can directly affect optical densitometric values, a stepped density filter (Edmund Industrial Optics, Barrington, NJ) with 10-step increments of 0.1 (from 0.1 to 1) was used to precisely adjust the intensity of the light source to a standard value identical for all samples.
The boundary of each brain stem nuclear group studied was determined with the aid of the Paxinos and Watson's “The Rat Brain Atlas” (Academic Press, New York, 1986). The PBC was identified with the neurokinin-1 receptor labeling (Gray et al., 1999), its rostral and caudal boundary was determined according to the detailed descriptions of Smith et al. (1991), and as described in our previous papers (Liu and Wong-Riley, 2002, 2005). The part of the nucleus ambiguus chosen for the present study was the semicompact formation and the rostral loose formation innervating upper airway muscles with pharyngolaryngomotor functions (Bieger and Hopkins, 1987). For the remaining nuclear groups, measurements were taken from the central main portion of each nuclear group. The optical densitometric value of each neuron measured was an average of two to four spots (depending on the cell size) within the cytoplasm (avoiding the nucleus). About 100 neurons in each brain stem nuclear group were measured for each marker in each rat, and a total of about 800 neurons at each age for each marker were measured. For statistical analyses, each sample's optical density value for each nuclear group of each rat was the average of about 100 labeled neurons. A total of 192,000 neurons were measured for the present study. Mean optical density values, standard deviations, and standard errors of the mean in each nuclear group at each age were then obtained. Statistical comparisons were made among the age groups by using one-way analysis of variance (ANOVA) (to control for the type I comparisonwise error rate) and, when significant differences were found, comparisons were made between successive age groups (e.g., P2 vs. P3, P3 vs. P4, and P5 vs. P7) by using Tukey's Studentized range test (a post hoc multiple comparisons, to control for the type I experimentwise error rate). Additional Tukey's tests were conducted between two groups that were not immediately adjacent to each other, and significant differences, if any, were presented in the Results section (but not shown in the graphs to minimize confusion). Two-way ANOVA was also done to determine if there was differential expression across nuclear groups, and when significance was found, Tukey's test was then done to determine when and where such differences were found. Significance was set at P < 0.01 for one- or two-way ANOVA and P < 0.05 for Tukey's test.
The percentage of labeled neurons for each glycinergic subunit in each nuclear group was calculated based on the number of labeled neurons divided by the total number of neurons shown in adjacent Nissl stained sections through the entire extent of each nuclear group. Only neurons sectioned through their centers (i.e., with clear nucleus) were counted. The range of percentages during development was based on values obtained at P2, P7, P12, and P21.
Highlights.
Four glycine receptor subunits were studied in four respiratory-related nuclear groups.
Glycine receptor 2 and 3 were relatively high at P2, but declined after 1-1½ weeks.
Glycine receptor 1 significantly increased at postnatal day (P) 12.
Glycine receptor rose from P2 to P12, followed by a slight decline thereafter.
A switch in dominance of glycine receptor subunits at P12 implies enhanced inhibition.
Acknowledgements
This study was supported by a grant from National Institutes of Health, grant number: R01 HD048954.
Role of authors
Both authors have full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Abbreviations
- 5-HT
serotonin
- XII
hypoglossal nucleus
- Amb
nucleus ambiguus
- ANOVA
analysis of variance
- APB
ammonium phosphate buffer
- BDNF
brain-derived neurotrophic factor
- CN
cuneate nucleus
- CNS
central nervous system
- GABA
gamma aminobutyric acid
- GlyR
glycine receptor
- IgG-HRP
immunoglobulin conjugated to horseradish peroxidase
- IPSC
inhibitory postsynaptic currents
- ir
immunoreactive
- KCC2
K+-Cl- co-transporter 2
- NK1R
neurokinin 1 receptor
- NKCC1
Na+-K +-2Cl− co-transporter 1
- NMDAR
N-methyl-D-aspartate receptor
- NTSVL
ventrolateral subnucleus of the solitary tract nucleus
- P
postnatal day
- PBC
pre-Bötzinger complex
- PBS
phosphate buffered saline
- TrkB
tyrosine protein kinase B
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
Both authors declare no competing financial interests.
LITERATURE CITED
- Aprison MH, Daly EC. Biochemical aspects of transmission at inhibitory synapses: the role of glycine. Adv. Neurochem. 1978;3:203–294. [Google Scholar]
- Aroeira RI, Ribeiro JA, Sebastião AM, Valente CA. Age-related changes of glycine receptor at the rat hippocampus: from the embryo to the adult. J. Neurochem. 2011;118:339–353. doi: 10.1111/j.1471-4159.2011.07197.x. [DOI] [PubMed] [Google Scholar]
- Bakus KH, Deitmer JW, Friauf E. Glycine-activated currents are changed by coincident membrane depolarization in developing rat auditory brainstem neurons. J. Physiol. (Lond) 1998;507:783–794. doi: 10.1111/j.1469-7793.1998.783bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker CM, Hoch W, Betz H. Glycine receptor heterogeneity in rat spinal cord during postnatal development. EMBO J. 1988;7:3717–3726. doi: 10.1002/j.1460-2075.1988.tb03255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synapse potentials in immature rat CA3 hippocampal neurons. J. Physiol. (Lond) 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betz H, Becker CM. The mammalian glycine receptor: biology and structure of a neuronal chloride channel protein. Neurochem. Int. 1988;13:137–146. doi: 10.1016/0197-0186(88)90048-4. [DOI] [PubMed] [Google Scholar]
- Bieger D, Hopkins DA. Viscerotopic representation of the upper alimentary tract in the medulla oblongata in the rat: the nucleus ambiguus. J. Comp. Neurol. 1987;262:546–562. doi: 10.1002/cne.902620408. [DOI] [PubMed] [Google Scholar]
- Blood-Siegfried J, Nyska A, Lieder H, Joe M, Vega L, Patterson R, Germolec D. Synergistic effect of influenza a virus on endotoxin-induced mortality in rat pups: a potential model for sudden infant death syndrome. Pediatr. Res. 2002;52:481–490. doi: 10.1203/00006450-200210000-00005. [DOI] [PubMed] [Google Scholar]
- Bonham AC. Neurotransmitters in the CNS control of breathing. Respir. Physiol. 1995;101:219–230. doi: 10.1016/0034-5687(95)00045-f. [DOI] [PubMed] [Google Scholar]
- Buckwalter MS, Cook SA, Davisson MT, White WF, Camper SA. A frameshift mutation in the mouse alpha 1 glycine receptor gene (Glra1) results in progressive neurological symptoms and juvenile death. Hum. Mol. Genet. 1994;3:2025–2030. doi: 10.1093/hmg/3.11.2025. [DOI] [PubMed] [Google Scholar]
- Büsselberg D, Bischoff AM, Paton JF, Richter DW. Reorganisation of respiratory network activity after loss of glycinergic inhibition. Pflugers Arch. 2001;441:444–449. doi: 10.1007/s004240000453. [DOI] [PubMed] [Google Scholar]
- Dutertre S, Becker CM, Betz H. Inhibitory glycine receptors: an update. J. Biol. Chem. 2012;287:40216–40223. doi: 10.1074/jbc.R112.408229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutschmann M, Paton JF. Glycinergic inhibition is essential for co-ordinating cranial and spinal respiratory motor outputs in the neonatal rat. J. Physiol. 2002;543:643–653. doi: 10.1113/jphysiol.2001.013466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain. Res. 1990;513:35–42. doi: 10.1016/0006-8993(90)91086-v. [DOI] [PubMed] [Google Scholar]
- Ezure K, Tanaka I. GABA, in some cases together with glycine, is used as the inhibitory transmitter by pump cells in the Hering-Breuer reflex pathway of the rat. Neurosci. 2004;127:409–417. doi: 10.1016/j.neuroscience.2004.05.032. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA, Gray PA. Understanding the rhythm of breathing: so near, yet so far. Annu. Rev. Physiol. 2013;75:423–452. doi: 10.1146/annurev-physiol-040510-130049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filiano JJ, Kinney HC. A perspective on neuropathologic findings in victims of the sudden infant death syndrome: the triple-risk model. Biol. Neonate. 1994;65:194–197. doi: 10.1159/000244052. [DOI] [PubMed] [Google Scholar]
- Finley JCW, Katz DM. The central organization of carotid body afferent projection to the brain stem of the rat. Brain. Res. 1992;572:108–116. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Fulton BP, Miledi R, Takahashi T. Electrical synapses between motoneurons in the spinal cord of the newborn rat. Proc. R. Soc. Lond. B. Biol. Sci. 1980;208:115–120. doi: 10.1098/rspb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Gao XP, Liu QS, Liu Q, Wong-Riley MTT. Excitatory-inhibitory imbalance in hypoglossal neurons during the critical period of postnatal development in the rat. J, Physiol. 2011;589:1991–2006. doi: 10.1113/jphysiol.2010.198945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Alcocer G, Mejía C, Berumen LC, Miledi R, Martínez-Torres A. Developmental expression of glycine receptor subunits in rat cerebellum. Int. J. Dev. Neurosci. 2008;26:319–322. doi: 10.1016/j.ijdevneu.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the pre-Bötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grenningloh G, Schmieden V, Schofield PR, Seeburg PH, Siddique T, Mohandas TK, Becker CM, Betz H. Alpha subunit variants of the human glycine receptor: primary structures, functional expression and chromosomal localization of the corresponding genes. EMBO J. 1990;9:771–776. doi: 10.1002/j.1460-2075.1990.tb08172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grudzinska J, Schemm R, Haeger S, Nicke A, Schmalzing G, Betz H, Laube B. The beta subunit determines the ligand binding properties of synaptic glycine receptors. Neuron. 2005;45:727–739. doi: 10.1016/j.neuron.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Handford CA, Lynch JW, Baker E, Webb GC, Ford JH, Sutherland GR, Schofield PR. The human glycine receptor beta subunit: primary structure, functional characterisation and chromosomal localisation of the human and murine genes. Brain Res. Mol. Brain Res. 1996;35:211–219. [PubMed] [Google Scholar]
- Heinze L, Harvey RJ, Haverkamp S, Wässle H. Diversity of glycine receptors in the mouse retina: localization of the alpha4 subunit. J. Comp. Neurol. 2007;500:693–707. doi: 10.1002/cne.21201. [DOI] [PubMed] [Google Scholar]
- Hodges MR, Richerson GB. Contributions of 5-HT neurons to respiratory control: neuromodulatory and trophic effects. Respir. Physiol. Neurobiol. 2008;164:222–232. doi: 10.1016/j.resp.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtman JR, Jr., Marion LJ, Speck DF. Origin of serotonin-containing projections to the ventral respiratory group in the rat. Neurosci. 1990;37:541–552. doi: 10.1016/0306-4522(90)90422-z. [DOI] [PubMed] [Google Scholar]
- Janczewski WA, Tashima A, Hsu P, Cui Y, Feldman JL. Role of inhibition in respiratory pattern generation. J. Neurosci. 2013;33:5454–5465. doi: 10.1523/JNEUROSCI.1595-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson S, Morud J, Pickering C, Adermark L, Ericson M, Söderpalm B. Changes in glycine receptor subunit expression in forebrain regions of the Wistar rat over development. Brain Res. 2012;1446:12–21. doi: 10.1016/j.brainres.2012.01.050. [DOI] [PubMed] [Google Scholar]
- Jordan D. Central nervous pathways and control of the airways. Respir. Physiol. 2001;125:67–81. doi: 10.1016/s0034-5687(00)00205-x. [DOI] [PubMed] [Google Scholar]
- Kato T, Hayashi F, Tatsumi K, Kuriyama T, Fukuda Y. Inhibitory mechanisms in hypoxic respiratory depression studied in an in vitro preparation. Neurosci. Res. 2000;38:281–288. doi: 10.1016/s0168-0102(00)00171-1. [DOI] [PubMed] [Google Scholar]
- Kneussel M, Betz H. Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: the membrane activation model. Trends Neurosci. 2000;23:429–435. doi: 10.1016/s0166-2236(00)01627-1. [DOI] [PubMed] [Google Scholar]
- Kubin L, Alheid GF, Zuperku EJ, McCrimmon DR. Central pathways of pulmonary and lower airway vagal afferents. J. Appl. Physiol. 2006;101:618–627. doi: 10.1152/japplphysiol.00252.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhse J, Becker CM, Schmieden V, Hoch W, Pribilia I, Langosch D, Malosio ML, Muntz M, Betz H. Heterogeneity of the inhibitory glycine receptor. Ann. N Y Acad. Sci. 1991;625:129–135. doi: 10.1111/j.1749-6632.1991.tb33836.x. [DOI] [PubMed] [Google Scholar]
- Langosch D, Thomas L, Betz H. Conserved quaternary structure of ligand-gated ion channels: the postsynaptic glycine receptor is a pentamer. Proc. Natl. Acad. Sci. USA. 1988;85:7394–7398. doi: 10.1073/pnas.85.19.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laterza OF, Modur VR, Crimmins DL, Olander JV, Landt Y, Lee JM, Ladenson JH. Identification of novel brain biomarkers. Clin. Chem. 2006;52:1713–1721. doi: 10.1373/clinchem.2006.070912. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Feldman JL. Modulation of respiratory activity of neonatal rat phrenic motoneurones by serotonin. J. Physiol. 1993;461:213–233. doi: 10.1113/jphysiol.1993.sp019510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal expression of neurotransmitters, receptors, and cytochrome oxidase in the rat pre-Bötzinger complex. J. Appl. Physiol. 2002;92:923–934. doi: 10.1152/japplphysiol.00977.2001. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in the rat pre-Botzinger complex. J. Appl. Physiol. 2004;96:1825–1831. doi: 10.1152/japplphysiol.01264.2003. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal developmental expressions of neurotransmitters and receptors in various brain stem nuclei of rats. J. Appl. Physiol. 2005;98:1442–1457. doi: 10.1152/japplphysiol.01301.2004. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Developmental changes in the expression of GABAA receptor subunits alpha1, alpha2, and alpha3 in brain stem nuclei of rats. Brain Res. 2006;1098:129–138. doi: 10.1016/j.brainres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal changes in the expression of serotonin 2A receptors in various brain stem nuclei of the rat. J. Appl. Physiol. 2008;104:1801–1808. doi: 10.1152/japplphysiol.00057.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal changes in the expressions of serotonin 1A, 1B, and 2A receptors in ten brain stem nuclei of the rat: implication for a sensitive period. Neurosci. 2010a;165:61–78. doi: 10.1016/j.neuroscience.2009.09.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal changes in tryptophan hydroxylase and serotonin transporter immunoreactivity in multiple brainstem nuclei of the rat: implications for a sensitive period. J. Comp. Neurol. 2010b;518:1082–1097. doi: 10.1002/cne.22265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal development of Na+-K+-2Cl− co-transporter 1 and K+-Cl− co-transporter 2 immunoreactivity in multiple brain stem respiratory nuclei of the rat. Neurosci. 2012;210:1–20. doi: 10.1016/j.neuroscience.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wong-Riley MTT. Postnatal development of brain-derived neurotrophic factor (BDNF) and tyrosine protein kinase B (TrkB) receptor immunoreactivity in multiple brain stem respiratory-related nuclei of the rat. J. Comp. Neurol. 2013;521:109–129. doi: 10.1002/cne.23164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Lowry TF, Wong-Riley MTT. Postnatal changes in ventilation during normoxia and acute hypoxia in the rat: implication for a sensitive period. J. Physiol. 2006;577:957–970. doi: 10.1113/jphysiol.2006.121970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Fehring C, Lowry TF, Wong-Riley MTT. Postnatal development of metabolic rate during normoxia and acute hypoxia in rats: implication for a sensitive period. J. Appl. Physiol. 2009;106:1212–1222. doi: 10.1152/japplphysiol.90949.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe AA. The neural regulation of tongue movements. Prog. Neurobiol. 1980;15:295–344. doi: 10.1016/0301-0082(80)90008-8. [DOI] [PubMed] [Google Scholar]
- Lynch JW. Native glycine receptor subtypes and their physiological roles. Neuropharmacol. 2009;56:303–309. doi: 10.1016/j.neuropharm.2008.07.034. [DOI] [PubMed] [Google Scholar]
- Maas C, Tagnaouti N, Loebrich S, Behrend B, Lappe-Siefke C, Kneussel M. Neuronal cotransport of glycine receptor and the scaffold protein gephyrin. J. Cell Biol. 2006;172:441–451. doi: 10.1083/jcb.200506066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar S, Heinze L, Haverkamp S, Ivanova E, Wässle H. Glycine receptors of A-type ganglion cells of the mouse retina. Vis. Neurosci. 2007;24:471–487. doi: 10.1017/S0952523807070174. [DOI] [PubMed] [Google Scholar]
- Malosio ML, Marquèze-Pouey B, Kuhse J, Betz H. Widespread expression of glycine receptor subunit mRNAs in the adult and developing rat brain. EMBO J. 1991;10:2401–2409. doi: 10.1002/j.1460-2075.1991.tb07779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Niebert M, Koch UR, Caley A, Vogelgesang S, Hülsmann S, Ponimaskin E, Müller U, Smart TG, Harvey RJ, Richter DW. Serotonin receptor 1A-modulated phosphorylation of glycine receptor α3 controls breathing in mice J. Clin. Invest. 2010;120:4118–4128. doi: 10.1172/JCI43029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Speck DF, Feldman JL. Role of the ventrolateral region of the nucleus of the tractus solitarius in processing respiratory afferent input from vagus and superior laryngeal nerves. Exp. Brain Res. 1987;67:449–459. doi: 10.1007/BF00247278. [DOI] [PubMed] [Google Scholar]
- Meyer G, Kirsch J, Betz H, Langosch D. Identification of a gephyrin binding motif on the glycine receptor beta subunit. Neuron. 1995;15:563–572. doi: 10.1016/0896-6273(95)90145-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Tanaka I, Ezure K. Excitatory and inhibitory synaptic inputs shape the discharge pattern of pump neurons of the nucleus tractus solitarii in the rat. Exp. Brain Res. 1999;129:191–200. doi: 10.1007/s002210050889. [DOI] [PubMed] [Google Scholar]
- Morgado-Valle C, Baca SM, Feldman JL. Glycinergic pacemaker neurons in preBötzinger complex of neonatal mouse. J. Neurosci. 2010;30:3634–3639. doi: 10.1523/JNEUROSCI.3040-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolic Z, Laube B, Weber RG, Lichter P, Kioschis P, Poustka A, Mulhardt C, Becker CM. The human glycine receptor subunit alpha3. Glra3 gene structure, chromosomal localization, and functional characterization of alternative transcripts. J. Biol. Chem. 1998;273:19708–19714. doi: 10.1074/jbc.273.31.19708. [DOI] [PubMed] [Google Scholar]
- Owens DF, Boyce LH, David MBE, Kriegstein AR. Excitatory GABA responses in embryonic and neonatal cortical slices demonstrated by gramicidin perforated-patch recordings and calcium imaging. J. Neurosci. 1996;16:6414–6423. doi: 10.1523/JNEUROSCI.16-20-06414.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton JF, Richter DW. Role of fast inhibitory synaptic mechanisms in respiratory rhythm generation in the maturing mouse. J. Physiol. 1995;484:505–521. doi: 10.1113/jphysiol.1995.sp020682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain Atlas. Academic Press; San Diego: 1986. [Google Scholar]
- Rekling JC, Feldman JL. Pre-Bötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu. Rev. Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Ren J, Greer JJ. Modulation of respiratory rhythmogenesis by chloride-mediated conductances during the perinatal period. J. Neurosci. 2006;26:3721–3730. doi: 10.1523/JNEUROSCI.0026-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul B, Schmieden V, Kling C, Mulhardt C, Gass P, Kuhse J, Becker CM. Point mutation of glycine receptor alpha 1 subunit in the spasmodic mouse affects agonist responses. FEBS Lett. 1994;350:71–76. doi: 10.1016/0014-5793(94)00736-5. [DOI] [PubMed] [Google Scholar]
- Schmid K, Bohmer G, Gebauer K. Glycine receptor-mediated fast synaptic inhibition in the brainstem respiratory system. Respir. Physiol. 1991;84:351–361. doi: 10.1016/0034-5687(91)90129-7. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Respiratory rhythm generation and synaptic inhibition of expiratory neurons in pre-Bötzinger complex: differential roles of glycinergic and GABAergic neural transmission. J. Neurophysiol. 1997;77:1853–1860. doi: 10.1152/jn.1997.77.4.1853. [DOI] [PubMed] [Google Scholar]
- Shevtsova NA, Manzke T, Molkov YI, Bischoff A, Smith JC, Rybak IA, Richter DW. Computational modelling of 5-HT receptor-mediated reorganization of the brainstem respiratory network. Eur. J. Neurosci. 2011;34:1276–1291. doi: 10.1111/j.1460-9568.2011.07825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JH, Talley EM, Bayliss DA, Berger AJ. Development of glycinergic synaptic transmission to rat brain stem motoneurons. J. Neurophysiol. 1998;80:2608–2620. doi: 10.1152/jn.1998.80.5.2608. [DOI] [PubMed] [Google Scholar]
- Smith JC, Morrison DE, Ellenberger HH, Otto MR, Feldman JL. Brainstem projections to the major respiratory neuron populations in the medulla of the cat. J. Comp. Neurol. 1989;281:69–96. doi: 10.1002/cne.902810107. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brain stem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Butera RJ, Koshiya N, Del Negro C, Wilson CG, Johnson SM. Respiratory rhythm generation in neonatal and adult mammals: the hybrid pacemaker-network model. Respir. Physiol. 2000;122:131–147. doi: 10.1016/s0034-5687(00)00155-9. [DOI] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF. Brainstem respiratory networks: building blocks and microcircuits. Trends Neurosci. 2013;36:152–162. doi: 10.1016/j.tins.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian HH, Chow CM, Balnave RJ. Identification of different types of respiratory neurons in the dorsal brainstem nucleus tractus solitarius of the rat. Brain Res. 2007;1141:119–132. doi: 10.1016/j.brainres.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Wardle RA, Poo MM. Brain-derived neurotrophic factor modulation of GABAergic synapses by postsynaptic regulation of chloride transport. J. Neurosci. 2003;23:8722–8732. doi: 10.1523/JNEUROSCI.23-25-08722.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe E, Akagi H. Distribution patterns of mRNAs encoding glycine receptor channels in the developing rat spinal cord. Neurosci. Res. 1995;23:377–382. doi: 10.1016/0168-0102(95)00972-V. [DOI] [PubMed] [Google Scholar]
- Weiss J, O'Sullivan GA, Heinze L, Chen HX, Betz H, Wässle H. Glycinergic input of small-field amacrine cells in the retinas of wildtype and glycine receptor deficient mice. Mol. Cell Neurosci. 2008;37:40–55. doi: 10.1016/j.mcn.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Weltzien F, Puller C, O'Sullivan GA, Paarmann I, Betz H. Distribution of the glycine receptor β-subunit in the mouse CNS as revealed by a novel monoclonal antibody. J. Comp. Neurol. 2012;520:3962–3981. doi: 10.1002/cne.23139. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q. Neurochemical development of brain stem nuclei involved in the control of respiration. Respir. Physiol. Neurobiol. 2005;149:83–98. doi: 10.1016/j.resp.2005.01.011. [DOI] [PubMed] [Google Scholar]
- Wong-Riley MTT, Liu Q. Neurochemical and physiological correlates of a critical period of respiratory development in the rat. Respir. Physiol. Neurobiol. 2008;164:28–37. doi: 10.1016/j.resp.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Riley MT, Liu Q, Gao XP. Peripheral-central chemoreceptor interaction and the significance of a critical period in the development of respiratory control. Respir. Physiol. Neurobiol. 2013;185:156–169. doi: 10.1016/j.resp.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Oka T, Nakamura S, Yasui Y. GABAergic neurons in the ventrolateral subnucleus of the nucleus tractus solitarius are in contact with Kölliker-Fuse nucleus neurons projecting to the rostral ventral respiratory group and phrenic nucleus in the rat. Brain Res. 2008;1228:113–126. doi: 10.1016/j.brainres.2008.06.089. [DOI] [PubMed] [Google Scholar]
- Young-Pearse TL, Ivic L, Kriegstein AR, Cepko CL. Characterization of mice with targeted deletion of glycine receptor alpha 2. Mol. Cell Biol. 2006;26:5728–5734. doi: 10.1128/MCB.00237-06. [DOI] [PMC free article] [PubMed] [Google Scholar]