Summary
How does coordinated activity between distinct brain regions implement a set of learning rules to sculpt information processing in a given neural circuit? Using interneuron cell-type specific optical activation and pharmacogenetic silencing in vitro, we show that temporally precise pairing of direct entorhinal perforant path (PP) and trisynaptic Schaffer collateral (SC) inputs to CA1 pyramidal cells selectively suppresses SC-associated perisomatic inhibition from cholecystokinin (CCK) expressing interneurons. The CCK interneurons provide a surprisingly strong feed-forward inhibitory drive to effectively control the coincident excitation of CA1 pyramidal neurons by convergent inputs. Thus, in-phase cortico-hippocampal activity provides a powerful heterosynaptic learning rule for long-term gating of information flow through the hippocampal excitatory macrocircuit by the silencing of the CCK inhibitory microcircuit.
Introduction
The output of principal neurons is driven by excitatory input from diverse brain regions while being constrained by local inhibition. Activity dependent forms of plasticity at excitatory and inhibitory synapses, such as long-term potentiation (LTP) and depression (LTD), may provide cellular bases for memory storage within a circuit (Kullmann et al., 2012; Malenka and Bear, 2004; Mayford et al., 2012). Most studies of LTP and LTD have focused on homosynaptic forms of plasticity at excitatory synapses that represent unsupervised synaptic learning rules where activity in a single synaptic pathway alters its own efficacy. Less is known about how convergent inputs from distinct brain regions generate heterosynaptic forms of plasticity where activity in one pathway alters information flow through a second path. Such supervised learning rules are of great theoretical interest as they provide a rich substrate for circuits to perform a wide range of mnemonic computations (Abbott and Regehr, 2004; Spruston, 2008). Here we define how a physiologically relevant, temporally precise pattern of activation of distinct cortical and hippocampal inputs to CA1 pyramidal neurons (PN) implements a heterosynaptic form of plasticity to shape information transfer through the hippocampal macrocircuit by regulating the output of a local inhibitory microcircuit.
In the cortico-hippocampal excitatory circuit, inputs carrying information from distinct layers of the entorhinal cortex (EC) converge on CA1 PNs through two main pathways (Kajiwara et al., 2008). CA1 PNs are excited directly by LIII EC neurons through perforant path (PP) synapses on distal CA1 dendrites in stratum lacunosum moleculare (SLM) and indirectly by LII EC neurons through the trisynaptic path (EC LII→DG→CA3→CA1), in which CA3 Schaffer collaterals (SC) ultimately form synapses on proximal CA1 dendrites in stratum radiatum (SR) (Amaral and Witter, 1989).
This circuit architecture adds a delay line for signals routed through the trisynaptic versus the monosynaptic path (Yeckel and Berger, 1990). Interestingly, although the direct EC inputs only weakly excite CA1 PNs (Jarsky et al., 2005), this sensory information regulates the propagation of signals through the hippocampal trisynaptic loop (Dudman et al., 2007; Golding et al., 2002; Han and Heinemann, 2013; Levy et al., 1998; Remondes and Schuman, 2002; Takahashi and Magee, 2009; Wohrl et al., 2007) and is crucial for spatial (Remondes and Schuman, 2004; Steffenach et al., 2005) and temporal (Suh et al., 2011) memory. One way the weak PP inputs may influence CA1 output is by providing instructive signals for a powerful form of heterosynaptic plasticity at the SC–CA1 synapses termed input-timing-dependent plasticity (ITDP) (Dudman et al., 2007). ITDP is induced by relatively weak, low frequency pairing of PP and SC inputs at a precise 20 ms interval (PP prior to SC) that matches the inherent delay in the hippocampal circuit. This causes a robust enhancement of the SC-evoked depolarization in CA1 PNs, with no change in the PP response. Given that ITDP induction shows high temporal fidelity to the circuit delay and occurs at the ~1 Hz EC-hippocampal firing frequency observed in rodents during exploratory behavior (Csicsvari et al., 1999; Frank et al., 2001), this learning rule is likely to be recruited by behaviorally salient activity.
Unlike most forms of activity-dependent LTP that are typically weakened by inhibition (Wigstrom and Gustafsson, 1985), ITDP is robustly induced when inhibition is intact. This raises the question as to whether ITDP results from changes in excitation alone (Dudman et al., 2007; Xu et al., 2012) or from changes in both excitation and inhibition. Because the SC-mediated depolarization of CA1 PNs is normally opposed by strong feed-forward inhibition (FFI) (Buzsaki, 1984; Pouille and Scanziani, 2001), we asked whether the enhancement in the depolarizing synaptic response during ITDP might result, at least in part, from the suppression of FFI. Of the >20 types of inhibitory neurons (INs) in the CA1 region, INs expressing parvalbumin (PV), somatostatin (SOM), or cholecystokinin (CCK) have been implicated in FFI (Klausberger and Somogyi, 2008), but their relative contributions are unknown.
Using cell-specific optogenetic activation and pharmacogenetic silencing we examined how coordinated activity in the entorhinal-hippocampal circuit affects local inhibitory drive onto CA1 PNs from distinct interneuron populations. We found that the ability of SC stimulation to excite CA1 PNs is strongly suppressed by FFI mediated by CCK-expressing INs. Moreover, induction of ITDP enhanced the SC-evoked depolarization in CA1 PNs through both the long-term depression of perisomatic FFI from CCK INs and the long-term enhancement in excitatory transmission. Thus paired activity in the EC and hippocampus acts as a long-term gate of information flow through the hippocampal trisynaptic path by tuning the efficacy of excitation and inhibition in the local CA1 microcircuit.
Results
PP and SC input-timing-dependent plasticity recruits local inhibition
To test the contribution of inhibition to ITDP, we examined the effect of blockade of GABAergic transmission (Figure 1). Intracellular postsynaptic potentials (PSPs) were recorded from CA1 PNs in acute hippocampal slices from adult C57BL/6J mice before and after induction of ITDP using weak paired stimulation of PP and SC inputs at 1 Hz for 90 s (PP 20 ms before SC, Figure 1A). With inhibition intact, this protocol caused a long-lasting enhancement in the depolarizing PSP elicited by SC stimulation (Figures 1B and 1D). Thirty minutes (min) after pairing, the SC-evoked PSP was increased 2.49 ± 0.13 fold relative to the pre-pairing baseline (p<0.0001, n=38); in contrast, the PP PSP was unchanged (0.98 ± 0.14 fold change, data not shown). In contrast, with inhibition blocked in the continuous presence of GABAA and GABAB receptor antagonists (2 μM SR 95531 and 1 μM CGP 55845, respectively), the pairing protocol produced a much smaller enhancement in the SC PSP (1.39 ± 0.06 fold). Although the increase in the excitatory PSP (EPSP) was still significant relative to the baseline SC response (p<0.0001, n=46), the magnitude of ITDP was significantly reduced by GABA receptor (GABAR) blockade (p<0.0001, unpaired t-test) (Figures 1B and 1D).
Figure 1. Full expression of input timing dependent plasticity requires inhibition.

(A) CA1 circuit diagram of the experimental configuration. Stimulating electrodes at the perforant path (PP, dark blue) and Schaffer collateral (SC, black) inputs excite distal and proximal dendrites of a CA1 pyramidal neuron (PNs, light blue with recording electrode) and feed-forward inhibitory neurons (INs, red and purple) that provide compartmentalized somatic and dendritic inhibition to the PNs.
(B) Time course of ITDP, plotted as mean normalized peak of SC PSP before and after PP+SC pairing induction (arrow) in the absence (Control, blue) and continuous presence of GABAA and GABABR antagonists (+ SR 95531 (2 μM), CGP 55845(1 μM), red). Traces show SC PSP pre (black) and post (control blue, +SR, CGP red) ITDP induction, dashed lines indicate the corresponding X-axis time points for averaging the PSPs (normalized to pre pairing baseline).
(C) Blockade of inhibition only during the pairing protocol (+SR (4μM), CGP (2μM), red bar corresponds to application time) did not affect expression of ITDP. As GABAR blockade enhances PSP amplitude, stimulus strength was decreased in SR, CGP to maintain the PSP at its baseline value during ITDP pairing (arrow). Immediately after pairing, the antagonists were removed from the bath solution and the stimulus strength was returned to its initial value.
(D) Role of inhibition in ITDP induction and expression. Individual experiments (symbols) and mean (± SEM, bars) of normalized SC PSPs averaged during 30-35 mins after ITDP induction with inhibition intact (Control), inhibition blocked throughout experiment (+SR, CGP throughout), or inhibition blocked only during pairing (+SR, CGP during pairing). Traces show PSPs before (pre) and after (post) induction of ITDP under different conditions.
These results confirm the importance of inhibition for ITDP (Xu et al., 2012, but cf. Dudman et al., 2007; see Discussion), but do not distinguish whether it is required for ITDP induction or expression. To address this point, we blocked inhibition only during ITDP induction (Figure 1C). First, we measured PP and SC PSPs with inhibition intact and then rapidly applied GABAR antagonists (4 μM SR, 2 μM CGP). As removal of inhibition increased the magnitude of the PSPs, we adjusted the stimulation strength of the two pathways to match the initial PSP amplitude and then delivered the ITDP induction protocol. Next we washed out the antagonists using a fast perfusion system to restore inhibition within 5 mins after pairing and we reset the stimulation strengths to their initial values. In this paradigm, the pairing protocol produced a large potentiation of the SC PSP (2.78 ± 0.19 change; p<0.0001, n=16), similar to the size of ITDP with inhibition intact (p=0.2125, unpaired t-test) (Figures 1C and 1D). This implies that inhibition contributes to the expression but not induction of ITDP.
Potentiation of the SC-evoked PSP during ITDP involves long-term depression of feed-forward inhibition
The decrease in ITDP with GABAR blockade suggests that the increase in the SC-evoked PSP during ITDP results, at least in part, from a long lasting decrease in FFI. To test this idea, we measured the PP- and SC-evoked inhibitory postsynaptic currents (IPSCs) before and after ITDP induction by voltage clamping the soma membrane at +10 mV, near the EPSC reversal potential (Figure 2A). Stimulation of the PP or SC inputs evoked a large outward IPSC in the CA1 PN soma that was fully blocked by GABAR antagonists (data not shown, but see Figure 6D1). Glutamate receptor blockers also reduced the IPSC to <5% of its initial value, indicating that the IPSC results from feed-forward excitation of local INs, rather than direct activation of inhibitory axons (Figure S1A-C). The SC-evoked IPSC was strongly reduced following induction of ITDP (under current clamp) by 58.8 ± 3.7% (from 1.01 ± 0.09 nA to 0.42 ± 0.06 nA; p<0.0001, paired t-test, n=9) (Figure 2A), whereas the PP IPSC was unchanged (0.24 ± 0.03 nA before vs. 0.25 ± 0.2 nA after ITDP; p=0.4034, paired t-test, n=9). This supports the view that the expression of ITDP involves a reduction in FFI activated specifically by the SC inputs.
Figure 2. ITDP induction recruits a long-term depression of inhibition (iLTD) that helps boost the SC-evoked PSP.

(A) SC-evoked IPSC (A3) but not PP IPSC (A2) is depressed following induction of ITDP. A1 shows protocol in which PP- and SC-evoked IPSCs were monitored in a CA1 PN under whole cell voltage clamp (VC) at Vm +10 mV before (Pre) and after (Post) induction of ITDP (at arrow). Membrane was at resting potential under current clamp (IC) at all other times. (A4) Individual (symbols) and mean (bars, ± SEM) IPSC amplitudes 2 mins before (Pre) and 25-35 mins after (Post) ITDP induction. Lines connect data points from same cells.
(B) Effect of ITDP on net PSP, pure EPSP and inferred IPSP. Top traces: Synaptic responses evoked by stimulating PP (B1) or SC (B2) inputs in absence (PSP, black) and presence (EPSP, red) of GABAR antagonists. Responses shown before (Pre) and after (Post) induction of ITDP. (PSP – EPSP) difference trace shown below (Inferred IPSP, blue). GABAR antagonists were washed out after measurement of EPSP. (B3) Inferred PP and SC IPSP amplitude before (Pre) and after (Post) induction of ITDP (symbols show individual and bars show mean ± SEM values). Dashed lines show peaks of synaptic potentials.
(C) Plots of SC-evoked PSP amplitude (mean ± SEM) as a function of time before and after induction of ITDP (black, +Pairing) or in control slices where ITDP was not induced (blue, -Pairing). Towards the end of experiment inhibition was assessed by application of GABAR antagonists (+SR, CGP). Solid and dashed lines intersecting the Y-axis show PSP amplitude before and after GABAR blockade. Arrow and dotted line intersecting the X-axis shows time of ITDP induction.
(D) Timing-dependence of induction of ITDP and iLTD. Tuning curve, plotting magnitude of ITDP of SC PSP (blue) and iLTD (red) (mean ± SEM) after pairing PP and SC stimulation at indicated timing intervals (negative sign indicates PP before SC). Plasticity measured 30-35 mins after pairing protocol. iLTD measured by increase in SC PSP upon GABAR block. Traces show SC PSPs before (Pre, blue) and after (Post, black) ITDP induction at 0, -10 and -40 ms pairing intervals and PSP elicited upon subsequent GABAR block (+SR, CGP, red). See also Figure S1.
Figure 6. Pharmacogenetic silencing of CCK INs suppresses FFI in CA1 PNs evoked by PP and SC stimulation.
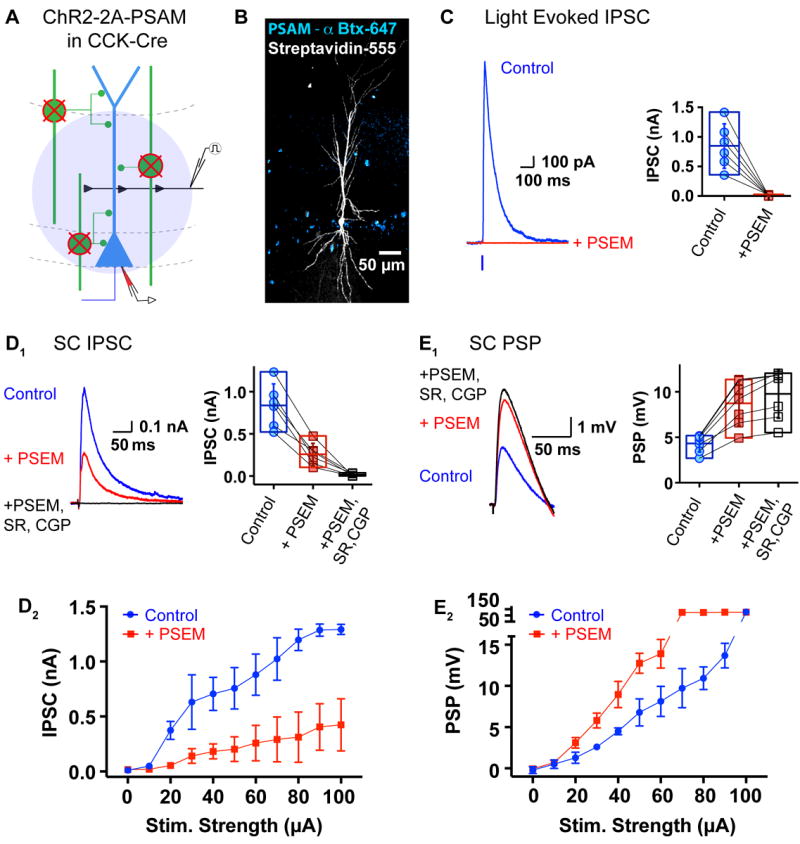
(A) Experimental scheme for pharmacogenetic silencing of CCK INs. CCK-Cre INs (green) coexpressing ChR2 and PSAM (ChR2-2A-PSAM) were excited with light or SC electrical stimulation. IPSCs or PSPs were recorded in a CA1 PN (blue) before and after silencing of CCK INs with PSEM.
(B) Confocal projection image (20X) of Cre-dependent expression of PSAM (blue, αBtx-Alexa 647) and an intracellularly filled CA1 PN (white, Streptavidin-Alexa 555) in hippocampal CA1 of a virally transduced CCK-Cre mouse.
(C) Example trace (left) and summary plot of individual and mean (bars, ± SEM) (right) light-evoked IPSCs in CA1 PN in absence (Control, blue circles) and presence of 3 μM PSEM (+PSEM, red squares).
(D-E) Silencing of CCK INs with PSEM reduces SC-evoked IPSCs (D) and PSPs (E) in CA1 PNs. Example trace and summary plot of individual and mean ± SEM of IPSCs (D1, Vm = +10 mV) and PSPs (E1, RMP, same cell at identical stimulation intensity) in response to electrical stimulation of the SC input with inhibition intact (Control, blue closed circles), after 10 min in presence of PSEM to silence CCK INs (+PSEM, red closed squares) and with all inhibition blocked (+PSEM, +SR, CGP, black open squares). Input-output relations for IPSC (D2) and PSP (E2) (mean ± SEM) as a function of SC stimulation intensity before (control, blue circles) and after 15 mins in PSEM (+PSEM, red squares). See also Figure S4.
How does FFI normally sculpt the depolarization of the CA1 PN by its SC input? And how does the suppression of inhibition during ITDP contribute to the increase in the net PSP? We addressed these questions using brief applications of GABAR antagonists before and after ITDP induction to directly measure the EPSP and estimate the underlying IPSP component of the PSP (Figure 2B). Prior to induction of ITDP, blockade of inhibition increased the amplitude of the SC-evoked net PSP by 120.7 ± 12.6% (p<0.001, paired t-test, n=4; Figure 2B). Because it is not possible to directly measure the pure IPSP from FFI (due in part to the overlapping EPSP), we inferred the IPSP size by subtracting the EPSP measured upon GABAR blockade from the net PSP (EPSP+IPSP) with inhibition intact (an approach we validated with a computational model, Figure S1D; see also Pouille and Scanziani, 2001). Next, we washed out the GABAR blockers and applied the pairing protocol to induce ITDP. Reapplication of GABAR blockers 30 mins later produced only a small 12.7 ± 1.2% (p<0.01, paired t-test) increase in the SC PSP, indicating a large reduction in the size of the inferred IPSP (-5.02 ± 0.39 mV before ITDP versus -2.54 ± 0.12 mV after ITDP, p<0.005, paired t-test; Figure 2B). In contrast, the pairing protocol caused no change in the inferred IPSP elicited by PP stimulation (-1.51 ± 0.2 mV before versus -1.52 ± 0.2 mV after pairing, p=0.7955, paired t-test), consistent with the lack of PP ITDP. The suppressive effect of ITDP on GABAergic transmission was further evaluated by comparing the effect of GABAR blockers on the SC-evoked PSP in control slices versus slices in which ITDP was induced. Whereas the GABAR antagonists (applied after 30-40 mins of stable recording) increased the PSP in control slices by 116.7 ± 5.2% (p<0.0001, n=16), there was only a 15.1 ± 6.7% increase (p<0.001, n=12) in the PSP recorded slices in which ITDP was induced (Figure 2C; also seen by the input-output curve of Figure S1E).
The above results indicate that the large enhancement of the net depolarizing SC PSP following induction of ITDP likely results from the sum of two complementary processes: a long-term potentiation of the EPSP (eLTP), which accounts for the ~40% potentiation when ITDP is induced in the presence of GABAR blockers, and a long-term depression of the IPSP (iLTD), which accounts for the additional ~100% increase in the PSP observed when inhibition is intact.
As the net ITDP is finely tuned to the -20 ms pairing interval (Dudman et al., 2007), we next asked whether the iLTD component of ITDP is similarly tuned to this delay. We monitored changes in SC-evoked FFI following pairing of the PP and SC inputs at variable delays (+20 to -40 ms; negative numbers correspond to stimulation of PP before SC). In agreement with Dudman et al., we found that ITDP was selectively induced at the -20 ms pairing interval (Figure 2D). As shown above (Figure 2C), application of GABAR blockers 30-40 min after induction of ITDP at this pairing interval produced only a small increase in the SC PSP because of the suppression of inhibition. In contrast, the GABAR antagonists produced a significantly larger increase in the SC PSP following pairing at other intervals (p<0.0005, one way ANOVA compared to the -20 ms data set, n=5-8 per pairing interval), similar to the ~2 fold increase in PSP size when GABAR antagonists were applied under baseline conditions. This indicates that the timing dependence for the suppression of inhibition is closely tuned to the optimal -20 ms pairing interval that elicits ITDP.
A selective reduction in perisomatic inhibition causes differential expression of ITDP in the CA1 PN soma and dendrites
The specificity with which ITDP reduces the SC-evoked IPSP versus the PP-evoked IPSP suggests that ITDP does not depress INs indiscriminately. Given that inhibition is highly compartmentalized with non-overlapping populations of INs targeting the CA1 PN soma and dendrites (Klausberger and Somogyi, 2008), we next asked whether soma- or dendrite-targeting INs were targeted by ITDP. Whole cell current-clamp recordings obtained separately from CA1 PN soma and apical dendrites (~250 μm from the soma in SR) showed that induction of ITDP caused a much smaller increase in the dendritic PSP (1.44 ± 0.04 fold change; p<0.001, n=5) than in the somatic PSP (2.61 ± 0.22 fold change; p<0.001, n=7; p<0.005, dendrite vs. soma, t-test; Figures 3B, 3C). The PP-evoked dendritic PSP was unaltered during ITDP (p=0.5083), similar to the somatic PP PSP.
Figure 3. ITDP selectively depresses perisomatic inhibition.
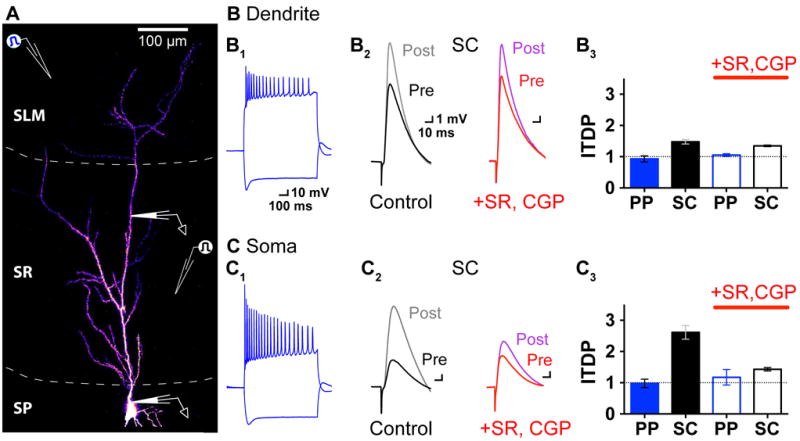
(A) Experimental scheme for somatic and dendritic whole cell recordings.
(B) Dendritic voltage responses. (B1) Intrinsic firing (top) and depolarizing sag (bottom) elicited by 700 ms depolarizing and hyperpolarizing current pulses (200 pA), respectively; (B2) SC-evoked PSPs before (Pre) and after (Post) ITDP induction with inhibition intact or blocked (+SR, CGP) throughout experiment. (B3) Mean ITDP (± SEM) for the PP-evoked (blue) and SC-evoked (black) dendritic PSPs, with inhibition intact (filled bars) or blocked (open bars, +SR, CGP).
(C) Somatic PSPs corresponding to conditions for dendritic recording shown in B. See also Figure S2
The small size of dendritic ITDP is surprising as the induction of ITDP requires summation of distal and proximal PSPs, which should be greatest in the PN dendrite. Might the difference between somatic and dendritic ITDP arise from a differential suppression of inhibition at the two compartments? In support of this idea, we found that dendritic ITDP was not altered when GABARs were blocked continuously throughout the experiment (p=0.812, dendritic SC ITDP, Control vs. +SR, CGP; Figures 3B2 and 3B3). This contrasts with the large decrease in somatic ITDP during GABAR blockade (Figures 3C2 and 3C3). These results suggest that dendritic ITDP results almost exclusively from eLTP, which is similar in size to the eLTP of the somatic PSP. Although it may seem surprising that the increased somatic PSP during ITDP does not passively propagate to cause a larger increase in the dendritic PSP (Figure 3B3-C3), our computational model confirms that a selective loss of somatic inhibition does not significantly boost the local dendritic PSP evoked by SC inputs (Figure S2).
iLTD results from a decreased inhibitory drive from CCK INs
To identify the specific class of perisomatic-targeting interneurons involved in ITDP, we used optogenetics to focus on the two major IN classes known to target the CA1 PN soma and perisomatic dendrites: the PV and CCK basket cells (Freund and Katona, 2007). We used a recombinant adeno-associated virus (rAAV) to express channelrhodopsin-2 fused to EYFP (ChR2-EYFP) (Boyden et al., 2005) selectively in cells that expressed Cre recombinase. Injection of this virus (rAAV-DIO-EF1α-ChR2-EYFP (Zhang et al., 2010)) in the CA1 region of mouse lines expressing Cre, either in CCK neurons (CCK-ires-Cre driver mice; Taniguchi et al., 2011) or PV neurons (PV-ires-Cre driver mice; Hippenmeyer et al., 2005), resulted in selective expression of ChR2-EYFP (ChR2+) in the two classes of neurons (Figures 4 A-C). Because CCK or its preprohormone is expressed at low levels in a small fraction of hippocampal PNs (Taniguchi et al., 2011) we used stereotactic injections of virus localized to CA1 to prevent photoactivation of excitatory projections to CA1. Pulses of 470 nm light generated large excitatory whole cell photocurrents in infected PV or CCK INs (Figures 4D2 and 4D3). In cell-attached recordings from ChR2+ INs, a brief train of light pulses at 10 Hz reliably elicited a train of time-locked extracellular currents that reflect reliable spiking.
Figure 4. CA1 PN IPSPs elicited by optical activation of CCK versus PV interneurons.

(A) Experimental scheme for recording IPSCs in CA1 PNs by photostimulation (470 nm light spot around soma) of ChR2 expressed in CCK (green) or PV (magenta) Cre INs.
(B-C) ChR2-EYFP (green) expression in the hippocampus of CCK-Cre (B) and PV Cre (C) mouse lines shown in a 20X confocal tiled image of the whole hippocampus (top) and higher magnification views of CA1 (bottom) overlaid with DAPI staining (blue). Uninfected CA1 PN filled during intracellular recording with neurobiotin (streptavidin-Alexa 555, white). Note the CA1-specific ChR2 expression in the CCK Cre slice.
(D) Extracellular (left trace of pair) and intracellular (right trace of pair) photocurrents from a ChR2-negative CA1 PN (D1), a ChR2-positive CCK IN (D2) and a ChR2-positive PV IN (D3) in response to a 10Hz train of 10 light pulses (left, under cell attached mode, blue bars 0.5 ms light pulse) and a low intensity 500-ms light pulse (right, under whole cell VC at -70mV). Note the large light-evoked extracellular and intracellular currents in the ChR2-expressing interneurons (D2 and D3).
(E-F) IPSCs elicited by photostimulation of ChR2-expressing CCK INs (E) recorded from uninfected CA1 PNs (VC +10 mV) using (E1 and F1) a 1 ms light pulse at 50% of maximal intensity focused over proximal SP (150 μm, black) or the CA1 soma (50 μm, gray); (E2 and F2) a range of photostimulation intensities (color-coded) using a somatic targeting light spot. Black dotted lines show onset latencies for IPSCs (at lowest and highest illumination intensity) from the start of light stimulus (blue bar and dotted line).
(G) Frequency histograms of the maximal amplitude of IPSCs elicited in CA1 PNs by photostimulation of ChR2-expressing PV (magenta) and CCK (green) INs.
(H) IPSC amplitude vs. light intensity input-output curves with 50μm beam spot (left) and IPSC onset latency (right). See also Figure S3.
To examine the inhibitory influence of the CCK and PV INs, we recorded light-evoked IPSCs under voltage clamp conditions (Vm +10 mV) from uninfected CA1 PNs (Figure 4D1). Activation of either ChR2+ PV or CCK INs with a single brief (1-2 ms) light pulse focused on the CA1 PN soma layer (Figure S3A) generated large, rapid IPSCs in the PNs (Figures 4E and 4F). Importantly, the light-evoked IPSCs in the CCK-Cre mice showed little or no change upon application of GluR antagonists, confirming that the IPSCs were caused by direct activation of the CCK INs, rather than disynaptic excitation of INs by ChR2-expressing CCK PNs (Figure S3B).
Photoactivation of ChR2+ CCK INs (Figure 4E1) evoked IPSCs in the CA1 PN that were 140% larger than those with photoactivation of ChR2+ PV INs (Figure 4F,G; CCK-Cre mice: 1.584 ± 0.1 nA, n=25; PV-Cre mice: 0.661 ± 0.05 nA, n=23; p<0.0001, CCK vs. PV, unpaired t-test), a difference maintained across a range of light intensities (p<0.0001, ANOVA with Tukey’s multiple comparisons test, n=8; Figures 4E, F and H).
The IPSCs mediated by PV INs had more rapid kinetics, with a shorter rise time and half-width, compared to CCK INs. Focal delivery of light over the PN soma at low light intensities (2-3%) elicited small (50-80 pA) miniature IPSC-like events with a 50% failure rate. Consistent with previous paired recordings data (Glickfeld and Scanziani, 2006), the response latency of light-evoked low-amplitude IPSCs was greater for CCK INs (7.58 ± 0.37 ms, n=8) compared to the PV INs (3.68 ± 0.13 ms, n=8; p<0.0001, CCK vs PV, unpaired t-test; Figures 4E, 4F and H).
Next we assessed whether the induction of ITDP modulates the light-evoked IPSCs. With ChR2 expressed in the CCK INs, the ITDP pairing protocol produced a reliable ~50% decrease in the light-evoked IPSC in CA1 PNs. The IPSC evoked by a 25% maximal light intensity pulse decreased from 1.24 ± 0.19 nA before ITDP to 0.67 ± 0.21 nA after ITDP (mean ± SEM; p<0.05, paired t-test, n=5; Figure 5A–C). In contrast, when ChR2 was expressed in PV INs, the IPSC evoked by identical photostimulation was unchanged with ITDP (0.69 ± 0.25 nA before versus 0.66 ± 0.25 nA after ITDP, p=0.996, paired t-test, n=5; Figures 5A–C). These effects were observed across the entire light intensity input-output relation (Figure 5B; CCK-Cre: p<0.05; PV-Cre: p=0.995; Two way ANOVA with Sidak multiple comparison correction). Thus, ITDP causes a significant iLTD of the CCK IN-mediated inhibitory response in CA1 PNs with little effect on inhibition mediated by PV INs (Figure 5C, p<0.0005, unpaired t-test, CCK vs. PV INs).
Figure 5. ITDP depresses the IPSC in CA1 PNs in response to photostimulation of CCK but not PV INs.

(A) Light-evoked IPSCs in CA1 PNs from ChR2+ CCK (A1) and PV (A2) INs obtained 5 mins before (blue) and 40 mins after (red) induction of ITDP using 10% (left) and 3% (right) perisomatic light intensities.
(B) IPSC vs. light intensity input-output relations (± SEM) before (Pre) and after (Post) induction of ITDP with ChR2+ CCK (B1) or PV (B2) INs. Individual IPSCs evoked at each light intensity were first normalized to that evoked at 25% light power for each cell and then averaged across all cells for a given light intensity.
(C) Magnitude of iLTD (± SEM) of light-evoked IPSCs from ChR2+ CCK (green, closed circles) and PV (magenta, open squares) INs as a function of photostimulation light intensity.
CCK INs contribute significantly to feed-forward inhibition of CA1 PNs
Our finding that ITDP may involve a selective decrease in CCK IN-mediated inhibition implies that the CCK INs must be major contributors to SC-evoked FFI under basal conditions given the near complete loss of FFI during ITDP. This is somewhat surprising as previous studies using paired recordings between single INs and CA1 PNs indicate that CCK INs are less suited than PV INs for mediating rapid FFI (Daw et al., 2009; Glickfeld and Scanziani, 2006; Hefft and Jonas, 2005). Because the ChR2-evoked inhibitory response may differ from that evoked synaptically during FFI, we used pharmacogenetic silencing of CCK INs to determine their contribution to FFI driven by electrical stimulation of the SC inputs. In this pharmacogenetic approach, a Cre-dependent viral vector was used to co-express a chimeric ligand-gated Cl- channel, the glycine receptor- based pharmacologically selective actuator module (PSAMY115F, L141F-GlyR referred to as PSAM) with ChR2 (rAAV-CAG-FLEX-ChR2-2A-PSAM, Magnus et al., 2011) in the CA1 region of CCK-ires-Cre mice (Figures 6A and 6B). Rapid and selective silencing of the virally-infected CCK IN population was achieved by applying a cognate synthetic ligand (PSEM, pharmacologically selective effector module) that binds to PSAM and activates a shunting Cl- conductance in the PSAM+ neurons (Magnus et al., 2011). Photostimulation of ChR2 produced large, CCK IN-mediated IPSCs in uninfected CA1 PNs (Vm +10mV) that were fully blocked within 6-10 min of bath application of 3 μM PSEM308 (Lovett-Barron et al., 2012), indicating the efficacy of this approach (Figure 6C).
Silencing of CCK INs by PSEM produced a 70% reduction in the IPSC amplitude in CA1 PN soma in response to electrical stimulation of the SC inputs (from 0.84 ± 0.11 nA to 0.26 ± 0.05 nA, p<0.001, paired t-test, n=6; Figure 6D1). The CCK INs accounted for the majority of the IPSC evoked by SC stimulation over a range of stimulus intensities (p<0.0001, SC IPSC, two-way ANOVA with Sidak correction for multiple comparisons; Figure 6D2). Pharmacogenetic removal of CCK INs increased the SC PSP amplitude at the CA1 PN soma by ~100%, from 4.32 ± 0.35 mV to 8.74 ± 0.92 mV, using a fixed stimulus intensity (50% of spike threshold intensity; p<0.005, paired t-test, n=6; Figure 6E1). A similar increase was seen over the entire stimulus input-output relation (p<0.0001, two way ANOVA with Sidak correction for multiple comparisons, n=5; Figure 6E2). CCK IN silencing also lowered the SC spike threshold intensity from 100 μA to 70 μA. Application of GABAAR and GABABR blockers to PSEM-treated slices produced only a ~12% further increase in the SC-evoked PSP (to 9.77 ± 1.01 mV, p<0.01, n=6; Figure 6E1). Thus CCK IN silencing blocks almost all SC-evoked FFI.
Furthermore, we found that CCK INs also make a dominant contribution to the FFI in CA1 PNs evoked by PP stimulation (Figure S4). Selective silencing of PSAM+ CCK INs with PSEM application produced an 80% reduction in the amplitude of the PP-evoked somatic IPSC (p<0.0005, n=5) and a corresponding increase in the PP-evoked PSP (p<0.0001, two-way ANOVA with Sidak’s multiple comparison test, n=5).
These silencing experiments demonstrate that the CCK INs are responsible for the majority of FFI that controls synaptic responses of CA1 PNs elicited by both the SC and PP inputs. The findings that CCK IN silencing robustly increased the PSP amplitude (by ~100%) and occluded any further increase in the PSP upon subsequent GABAR blockade resemble the effects seen upon induction of ITDP (Figure 2). Such results support the view that selective silencing of CCK INs produces a large reduction in inhibition capable of accounting for the magnitude of iLTD observed during ITDP.
CCK INs are required for the expression of iLTD
To determine whether the CCK INs are indeed required for expression of iLTD during ITDP, we examined the effects of PSEM-mediated silencing on the magnitude of ITDP. PSEM ligand was applied to hippocampal slices either from CCK-ires-Cre mice injected with rAAV that expressed PSAM in a Cre-dependent manner (CCK-Cre-PSAM) or from uninjected control littermates (CCK-Cre). When the control slices were exposed to PSEM, the pairing protocol elicited a normal-sized ITDP (2.9 ± 0.26 fold) (Figures 1C and 2A). In contrast, there was a strong suppression of ITDP when the PSAM-expressing slices were exposed to PSEM (p<0.0002, unpaired t-test; CCK-Cre PSAM group, n=7; CCK-Cre group, n=6). With CCK INs silenced, the pairing protocol produced only a 1.42 ± 0.09 fold increase in the SC-evoked PSP, similar to the magnitude of ITDP during GABAR blockade (Figure 1C). Silencing of CCK INs also significantly reduced the extent of iLTD of the IPSC during ITDP. Thus, PSAM-expressing slices exposed to PSEM displayed only an 8.3 ± 1.7% decrease in the SC-evoked IPSC following induction of ITDP compared to the 60.5 ± 3.2% decrease in the IPSC seen with control slices (p<0.0001; Figure 7B). Application of GABAR antagonists 30-40 min after ITDP induction caused only a small increase (~15%) in the SC PSP in both groups (p=0.7273, one-way ANOVA; Figure 7A3), indicating a similar extent of loss of inhibition. These findings support the hypothesis that iLTD during ITDP results from a selective depression of FFI mediated by CCK INs.
Figure 7. Silencing CCK INs with PSEM reduces magnitude of ITDP and occludes iLTD.

(A) Effect of PSEM-mediated silencing of CCK INs on ITDP. Example PSPs (A1), time course (A2) and summary plot (A3) of individual and mean (± SEM) SC PSPs in CA1 PNs in the continuous presence of PSEM in slices from CCK-Cre mice in which PSAM was either expressed (CCK-Cre-PSAM) or absent (CCK-Cre). PSPs (A1 and A3) measured 5 mins before pairing (Pre, blue), 40 mins after pairing (Post, black), and 10 mins later following GABAR blockade (Post +SR, CGP, grey). (A2) Mean normalized PSP amplitude with PSEM present as a function of time before and after induction of ITDP (arrow) in slices in which PSAM was expressed (red squares) or absent (blue circles).
(B) CCK IN silencing prevents iLTD of SC-evoked IPSC during ITDP. Representative IPSCs (B1) and plot of individual and mean IPSC amplitude (B3) recorded in same cells as (A) before (blue) and after (black) induction of ITDP. (B2) shows extent of LTD of SC IPSC (iLTD %) in response to induction of ITDP in presence of PSEM in slices that expressed PSAM (red) or in which PSAM was absent (blue). (Error bars show SEM).
ITDP reduces presynaptic GABA release from CCK INs
How does ITDP reduce SC-evoked FFI from CCK INs? We examined whether the reduction in inhibition results from: 1. A decrease in excitatory synaptic transmission onto CCK INs; 2. A decrease in the postsynaptic GABA response of CA1 PNs; or 3. A decrease in presynaptic GABA release from inhibitory terminals onto CA1 PNs (see Kullmann et al., 2012 for review).
To determine whether the excitatory drive onto CCK INs was altered during ITDP, we used fluorescence-guided whole cell recordings to monitor the SC-evoked EPSPs in CCK INs expressing GFP. GFP was restricted to CCK expressing GABAergic INs using an intersectional genetic approach (Taniguchi et al., 2011; Figure S5A, see Experimental Procedures). We also recorded SC-evoked EPSPS in tdTomato labeled PV INs. We found that ITDP induction did not alter the magnitude of the EPSP evoked by SC stimulation in either CCK or PV INs (Figure 8A), ruling out either general or specific changes in synaptic excitation.
Figure 8. iLTD during ITDP involves decrease in GABA release from CCK INs.

(A) Experimental schematic (A1), example PSPs (A2) and ITDP timecourse (A3) showing ITDP does not alter SC-evoked PSPs recorded from CCK (green) or PV (magenta) INs. See also Figure S5.
(B) IPSCs (uIPSCs) in CA1 PNs evoked by photorelease of GABA from caged Rubi-GABA (B1, uncaging spot in cyan) are not altered by ITDP. (B2) uIPSCs at two time scales before and after ITDP induction. (B3) Individual and mean uIPSCs before (blue) and 40 mins after (black) induction of ITDP.
(C) Paired-pulse ratio (PPR) of IPSCs in CA1 PNs evoked by paired electrical stimulation of the SC inputs (C1) or photostimulation of ChR2+ CCK (C2) or PV (C3) INs before (Pre, blue) and after (Post, black) induction of ITDP. Top, IPSCs; middle, IPSCs normalized by peak amplitude of IPSC1; bottom, individual and mean PPR (IPSC2/IPSC1).
Next we tested whether the postsynaptic GABA response was altered in CA1 PNs using the photoactivatable caged compound RuBi-GABA (Rial Verde et al., 2008). The peak amplitude and rise time of uncaging IPSCs in CA1 PNs evoked by a single 470 nm light pulse on the perisomatic space (using 5 μM RuBi-GABA) was unchanged during ITDP (Figure 8B). Thus, ITDP does not alter the postsynaptic GABA response.
These results imply that iLTD during ITDP is most likely mediated by a decrease in GABA release from CCK INs. To test this idea, we measured the paired-pulse ratio (PPR) of IPSCs evoked in CA1 PNs by two closely spaced stimuli (50 ms inter-pulse interval) as an increase in PPR is thought to reflect a decrease in the probability of transmitter release (Dobrunz and Stevens, 1997). We found that ITDP was indeed associated with an increase in the PPR, either when IPSCs were evoked by electrical stimulation of the SC pathway (73.13 ± 7.6% increase, p<0.0001, n=13) or by photostimulation of ChR2+ CCK INs (63.59 ± 14.6% increase, p<0.01, paired t-test, n=5; Figure 8C). In contrast the PPR for IPSCs evoked by photostimulation of ChR2+ PV INs was unaltered by ITDP (p=0.8741, paired t-test, n=4). This supports the view that iLTD during ITDP results from a selective decrease in GABA release from perisomatic-targeting CCK INs.
A local retrograde messenger signaling pathway mediates iLTD
One well-characterized mechanism that decreases GABA release from CCK INs is through the action of endocannabinoids (eCBs), retrograde messengers that act on G-protein-coupled CB1 receptors (CB1Rs) abundantly localized to CCK presynaptic terminals (Castillo et al., 2012). These molecules have been implicated in a form of iLTD induced by high frequency SC stimulation (Chevaleyre and Castillo, 2003). A recent study found that the induction of ITDP in CA1 PNs also requires eCB release and activation of CB1Rs (Xu et al., 2012). However this latter study used a protocol that was suited neither for examining FFI nor the iLTD component of ITDP (see Discussion). Given our findings that iLTD accounts for the major synaptic change during ITDP, we investigated the role of eCBs in this process. Similar to the results of Xu et al., we found that blockade of CB1Rs with AM251 (2 μM) inhibited the induction of ITDP (Figure 9A). However, we also found that the block of ITDP was incomplete, with a residual 1.36 ± 0.31 fold (p<0.005, n=8) potentiation of the PSP, which matches the residual ITDP observed in the presence of GABAR blockers (or following PSEM-mediated silencing of CCK INs). This suggests that the activation of CB1Rs by eCBs may be selectively required for the iLTD, but not eLTP, component of ITDP.
Figure 9. iLTD is mediated by endocannabinoids and localized to inhibitory synapses on CA1 PNs activated by the ITDP induction protocol.

(A) Time course of ITDP in CA1 PNs in drug-free slices (control, blue) and slices continuously exposed to the CB1 receptor antagonist AM251 (black). Asterisk (*) refers to continuous presence of AM251 in the bath.
(B-C) Inhibition is intact following induction of ITDP in presence of AM251. B1, PSPs before (Control, blue) and after GABAR block (+SR, CGP, red) in slices without induction of ITDP; AM251 present throughout. B2, PSPs before (Pre, blue) and 30 min after (Post, black) induction of ITDP, and after subsequent GABAR block (Post +SR, CGP, red).
(C) PSP input-output plots showing effect of GABAR block in slices 30 min after induction of ITDP (C2) or with pairing protocol omitted (C1) in AM251.
(D-F) Suppression of inhibition is local and selectively targets active CA1 pyramidal neurons. Experimental scheme (D1), CCD based IR (top) and epifluorescence (Alexa 594, bottom) images (D2). Sample traces (E1) and ITDP time course plots (E2) from dual recordings from neighboring PNs where one cell was voltage clamped at -85 mV during pairing protocol (VC, black, bottom trace) and the other held under current clamp and allowed to depolarize normally (IC, blue, top trace). Summary plot (F) of increase in SC PSP amplitude with ITDP and following GABAR blockade after ITDP induction for the same conditions and cells as in D-E. See also Figure S6.
To test this idea, we examined the extent of inhibition remaining after ITDP was induced in the continuous presence of AM251 (2 μM). We first applied GABAR antagonists to slices exposed to AM251 (no ITDP pairing). GABAR blockade produced a large increase in the SC-evoked PSP in CA1 PNs (110.8 ± 14.6%, p<0.001, n=6; Figures 9B1 and 9C1) similar to the increase seen in the absence of AM251 (Figures 2C and S1E), indicating that CB1R blockade did not alter basal FFI under the conditions of our experiments (cf. Losonczy et al., 2004). Next, we applied GABAR antagonists 30-40 min after the induction of ITDP in slices continuously exposed to AM251 to assess the residual IPSP. The CB1 antagonist effectively blocked the suppression of inhibition that normally accompanies ITDP (Figures 9B2 and 9C2). After induction of ITDP with CB1Rs blocked, the GABAR antagonists produced a large increase in the SC PSP (112.4 ± 24.2%, p<0.003, n=5), similar to that seen in slices where ITDP was not induced (p=0.194, unpaired t-test). These results indicate that the eCB pathway is necessary for the iLTD component of ITDP.
Previous studies report that hippocampal ITDP is sensitive to antagonists of Group I mGluRs (mGluR1 and mGluR5) (Dudman et al., 2007; Xu et al., 2012) and the mGluR1 subtype mediates eCB release during 100Hz iLTD (Chevaleyre and Castillo, 2003). We extended the characterization of the mGluR subtypes required for ITDP and found that selective blockade of mGluR1a using LY367385 (100 μM) eliminated the iLTD component of ITDP but left intact a residual potentiation most likely resulting from eLTP (Figure S6).
ITDP represents a local cell-specific activity-dependent learning rule
As eCBs are diffusible lipid molecules, we asked whether iLTD during ITDP represents a global depression of inhibition by CCK INs or is limited to those CCK IN terminals that contact CA1 PNs activated during the pairing protocol. We addressed this by obtaining whole cell recordings from two neighboring CA1 PNs, with one cell voltage clamped at -85 mV to prevent its depolarization during the pairing protocol and the other cell current-clamped to allow for depolarization (Figures 9D and 9E).
ITDP was almost fully blocked in the voltage-clamped cell (1.17 ± 0.12 fold potentiation, p=0.1849, paired t-test, n=14), whereas it was expressed normally in the adjacent current-clamped cell (2.67 ± 0.4 fold potentiation, p<0.0001, paired t-test, n=11) (Figure 9E and F). Thus, postsynaptic depolarization of the CA1 PN is a prerequisite for induction of ITDP. Moreover, ITDP can be differentially expressed in a cell-autonomous, activity-dependent manner (p<0.0001 for voltage-clamped vs. current-clamped cells, unpaired t-test). Importantly, the voltage-clamped cells displayed a normal amount of inhibition 30-40 min after the induction of ITDP, based on the 114.3 ± 17.5% increase in the SC-evoked PSP upon application of GABAR antagonists (p<0.003, paired t-test; Figure 9F), similar to results with unpaired slices (Figure 2C). In contrast, inhibition was largely eliminated in cells held under current clamp conditions, which displayed only a 12.2 ± 3.3% increase in the PSP with GABAR blockers after pairing (p<0.01, paired t-test, n=5). These results indicate that both the eLTP and iLTD components of ITDP are local events restricted to postsynaptic CA1 PNs that are actively depolarized during pairing.
What voltage-dependent processes are required for induction of ITDP? We found that activation of NMDARs and a rise in postsynaptic Ca2+ in the CA1 PN are required for both eLTP and iLTD. Thus ITDP and iLTD were fully blocked by application of an NMDAR antagonist (100 μM D-APV) or when the whole-cell pipette solution contained the Ca2+ chelator 20 mM BAPTA (Figure S6). These findings are consistent with previous results that PP-SC synaptic pairing at the -20 ms interval results in a non-linear NMDAR-dependent increase in the Ca2+ transient in CA1 PN dendritic spines that receive SC input (Dudman et al., 2007).
Discussion
This study demonstrates how dynamic regulation of feed-forward inhibition (FFI) exerted by a local inhibitory microcircuit contributes to the enhancement of cortico-hippocampal information flow through implementation of a temporally precise synaptic learning rule, input-timing-dependent plasticity (ITDP). We find that the expression of this heterosynaptic plasticity results from complementary long-term changes in excitatory and inhibitory synaptic transmission activated by the Schaffer collateral (SC) inputs from hippocampal CA3 PNs onto the CA1 region. Thus, induction of ITDP enhances the depolarization of CA1 PNs by their SC inputs through both a long-term potentiation of excitatory synaptic transmission (eLTP) and a long-term depression of FFI (iLTD). Through this combination of enhanced excitation and diminished inhibition, ITDP may act as a gate to promote propagation of contextually relevant information through the hippocampal circuit.
A second key finding of our study is that the iLTD component of ITDP selectively targets FFI mediated by the soma-targeting CCK-positive class of INs. Moreover, we find that the CCK INs play a predominant role in FFI activated by both the cortical (PP) and hippocampal (SC) inputs to CA1 PNs under basal conditions. This latter result is surprising given the widespread view that the synaptic and biophysical properties of CCK INs make them less well suited to be efficiently driven by synaptic inputs and generate rapid inhibition compared to the PV INs (Glickfeld and Scanziani, 2006), which we find make a modest contribution to FFI.
Comparison with previous studies on ITDP
Our results complement and extend the findings of previous studies on hippocampal ITDP (Dudman et al., 2007; Xu et al., 2012). Similar to previous results, induction of both ITDP and iLTD are sharply tuned to the -20 ms pairing interval (Dudman et al., 2007), require activation of NMDA and mGluR1a receptors and Ca2+-dependent signaling (Dudman et al., 2007; Xu et al., 2012); and involve eCB retrograde messengers (Xu et al., 2012, but see below).
However, in contrast to previous conclusions that hippocampal ITDP is mediated solely by long-term changes in excitation (Dudman et al., 2007; Xu et al., 2012), the core novel finding of our study is that the major mechanism contributing to the enhanced synaptic depolarization during ITDP results from the long-term depression of synaptic inhibition (iLTD). Although Xu et al., (2012) did find that ITDP was suppressed by GABAR antagonists and required eCB signaling, the targets of eCB action were not identified and that study concluded that ITDP does not alter synaptic inhibition. This latter conclusion was based on the authors’ finding that the ITDP pairing protocol had no effect on IPSCs evoked by direct stimulation of GABAergic axons (see Figure 2D of Xu et al., 2012). However, this result is confounded by the fact that direct inhibition was measured in the continuous presence of AMPAR and NMDAR antagonists, which will prevent the postsynaptic depolarization and NMDAR-mediated Ca2+ influx necessary to induce both eLTP (Dudman et al., 2007) and iLTD (see Figures 9 and S6). Unlike the results of Xu et al., (2012) and this study, an earlier study from our laboratory reported that the magnitude of ITDP was not altered by the continuous blockade of GABARs (Dudman et al. 2007). Although we cannot fully explain the discrepancy between our present results and this previous study, the standard errors in the earlier data with GABAR antagonists were quite large owing to a small number of experiments and large experimental variability, which may have obscured the change in the magnitude of ITDP.
CCK INs are key modulators of CA1 PN activity
A number of studies indicate that CCK INs mediate relatively slow, long lasting inhibition, compared to the more rapid inhibition mediated by PV INs (Daw et al., 2009; Glickfeld and Scanziani, 2006; Hefft and Jonas, 2005). The slow CCK IN-mediated IPSP results, in part, from an asynchronous component of GABA release and the slower postsynaptic current mediated by α2 subunit-containing GABAARs (Freund and Katona, 2007). This has led to the idea that the CCK INs are best suited for regulating sustained activation of principle neurons, rather than for regulating fast depolarization elicited by excitatory synaptic input. However we found that optogenetic activation of the CCK IN population produces a prominent fast IPSC that is even larger than that elicited by PV IN stimulation. Moreover, pharmacogenetic silencing demonstrates that the CCK INs are responsible for the major component of fast FFI elicited by low frequency electrical stimulation of the SC or PP inputs. Such results are consistent with in vitro (Hefft and Jonas, 2005) and in vivo (Klausberger et al 2005) data that the CCK INs can fire synchronously with precision and fidelity during low frequency patterns of activity.
Our finding that CCK INs effectively control the input-output gain of CA1 PNs during cortico-hippocampal activity is of interest given the in vivo firing pattern of these neurons during gamma and theta oscillations, in which CCK IN firing immediately precedes CA1 PN firing (Klausberger and Somogyi 2008). By mediating rapid FFI, the timing of CCK IN activity makes them poised to powerfully regulate PN firing. Moreover, our results reveal that, through iLTD, ITDP specifically targets this dominant role of CCK INs in FFI elicited by SC activation. Given their expression of CB1, 5-HT3 and ACh receptors, the CCK IN basket cells provide a rich substrate for a variety of modulatory mechanisms.
Consistent with previous findings that eCBs act on presynaptic CB1 receptors (Katona et al., 1999) to mediate short-term (Wilson and Nicoll, 2001) and long-term (Chevaleyre and Castillo, 2003) depression of GABA release from CCK IN terminals, we find that the ITDP pairing protocol recruits this signaling pathway to orchestrate the iLTD of CCK-mediated inhibition. However, unlike previously characterized forms of activity-dependent eCB release, which require strong depolarization of the postsynaptic cell or strong tetanic stimulation of presynaptic glutamatergic inputs, the recruitment of eCBs during ITDP involves relatively weak but precisely timed paired cortical and hippocampal synaptic activity. Like cerebellar short-term associative plasticity (Brenowitz and Regehr, 2005) and cortical spike-timing-dependent plasticity (Bender et al., 2006), eCB release during ITDP requires coincident activation of mGluRs and a rise in postsynaptic Ca2+ (Castillo et al., 2012).
Implications of iLTD for sparse coding and formation of neuronal assemblies
Synapse specificity during activity-dependent plasticity is considered a crucial feature of memory storage and the construction of neuronal assemblies that encode a given context (Buzsaki, 2010). However, the promiscuity of inhibition, in which a single IN contacts hundreds of local PNs (Isaacson and Scanziani, 2011), poses a problem for achieving synapse-specific interneuron plasticity (Kullmann et al., 2012). Our finding that iLTD is expressed only at those inhibitory synapses that contact postsynaptic CA1 PNs activated during the pairing protocol (Figure 9) provides a mechanism for enabling ITDP and iLTD to enhance the excitation of specific co-activated ensembles of PNs. This may contribute to the emergence of high-contrast, sparsely-coded cell assemblies (Klausberger and Somogyi, 2008).
What is the function of ITDP in information processing?
Both theoretical and experimental studies support the view that activity-dependent synaptic plasticity represents an important cellular and molecular mechanism for memory storage and that distinct forms of plasticity provide distinct synaptic learning rules for different forms of memory (Abbott and Regehr, 2004; Mayford et al., 2012). In contrast to classical Hebbian forms of associative homosynaptic plasticity, such as spike-timing-dependent plasticity, in which synapses are rewarded by potentiation if the presynaptic neuron participates in the firing of the postsynaptic neuron (Feldman, 2012), heterosynaptic learning rules such as ITDP may be used for salience or error detection during contextual learning. For example, in cerebellar LTD, a heterosynaptic learning rule also linked to eCB signaling, an error signal carried by climbing fibers results in the LTD of sensory information carried by coactive parallel fibers onto Purkinje neurons (Ito, 2001; Safo and Regehr, 2008). A form of ITDP, recently described in lateral nucleus principal neurons of the amygdala following paired activation of cortical and thalamic inputs, is recruited during contextual fear learning (Cho et al., 2012). The convergence of precisely timed, behaviorally relevant inputs from distinct brain regions is likely to reflect a common feature of circuit architecture in many brain areas, including neocortex, where there is an abundance of CCK INs. Thus the long-term suppression of CCK IN-mediated inhibition following paired input activation may prove of general importance for regulating cortical plasticity and activity.
Although the precise function of hippocampal ITDP is not known, it is interesting that the pairing interval (20 ms) for ITDP coincides temporally with both the circuit timing delay (Yeckel and Berger, 1990) and gamma oscillation period (Buzsaki and Wang, 2012) in the cortico-hippocampal circuit. The requirement for precise temporal tuning of paired PP and SC input activity might enable CA1 PNs to assess the salience of information propagated through the hippocampal circuit based on the immediate sensory context conveyed directly by the cortex. A timing-dependent learning rule such as ITDP may be particularly useful in mnemonic processing for reading out temporal correlations to create salient windows for information storage.
Experimental Procedures
Animals
All experiments were conducted in accordance with the National Institutes of Health guidelines and with the approval of the Columbia University Institutional Animal Care and Use Committee. PV-ires-Cre (Hippenmeyer et al.) and Ai14-tdTomato (Madisen et al., 2010) mouse lines were obtained from the Jackson laboratory (JAX). The CCK-ires-Cre driver (Taniguchi et al., 2011) mice were crossed with the Dlx5/6-Flpe driver mice (generous gift from Gordon Fishell, New York University, (Miyoshi et al., 2010)) and a Cre- and Flp-dependent EGFP reporter strain, RCE-Dual (generous gift from Gordon Fishell (Sousa et al., 2009)) or R26NZG (JAX, (Yamamoto et al., 2009)) to generate the CCK IN specific EGFP labeled line as described in Taniguchi et al 2011 (see Supplementary Procedures for details).
Viruses and Surgery
Commercially generated (UNC Vector and Penn Vector) rAAVs encoding ChR2-EYFP (generous gift from Karl Deisseroth, Stanford University) and ChR2-2A-PSAM (generous gift from Scott Sternson, Janelia Farm Research Campus) was injected into the hippocampal CA1 region under stereotactic control. Mice were allowed to recover for 2-4 weeks to allow for viral expression before electrophysiology and imaging were performed. See Supplemental Information for details.
Solutions
Standard artificial cerebrospinal fluid (ACSF) consisted of (in mM): NaCl (125), NaHCO3 (25), KCl (2.5), NaH2PO4 (1.25), MgCl2 (1), CaCl2 (2), glucose (22.5), Na-pyruvate (3), ascorbate (1). Sucrose-enriched modified dissection ACSF contained (in mM): NaCl (10), NaH2PO4 (1.2), KCl (2.5), NaHCO3 (25), glucose (25), CaCl2 (0.5), MgCl2 (7), sucrose (190), pyruvate (2). The ACSF had a pH of 7.3, osmolarity of 305-320 mOsm and was saturated with 95% O2 and 5% CO2. The intracellular solution contained (in mM): KMeSO4 (135) (for current clamp recordings) or CsMeSO4 (135) (for voltage clamp recordings), KCl (5), NaCl (2), EGTA (0.2), HEPES (10), phosphocreatineNa2 (10), MgATP (5), Na2GTP (0.4), Alexa Fluor 594 (0.1) and Biocytin (0.2%).
In a subset of experiments, the following drugs (Tocris) were used at the following concentrations via bath application (unless otherwise noted): SR95531 (2 μM), CGP55845 (1 μM), AM251 (2 μM), NBQX (10 μM), D-APV (100 μM), LY 367385 (100 μM). RuBiGABA was obtained from Tocris or Ascent and PSEM308 was a generous gift from Scott Sternson, and used at a concentration of 5 μM and 3 μM, respectively.
Slice Preparation and Electrophysiology
A vibrating microtome (Vibratome 1000 or Leica VT1200S) was used to obtain 400 μm thick horizontal or transverse sections of brains from mice that were transcardially perfused with ice-cold dissection ACSF. Slices were allowed to recover for at least 30 mins at 34 °C and then stored at room temperature in a 50% dissection: 50% standard ACSF solution.
Infrared- or fluorescence-guided whole cell patch clamp recordings were performed at 34°C in standard ACSF. Fire-polished borosilicate glass pipettes (Sutter) were used with tip resistances of 3.5-4.5 MΩ for somatic and 8-10 MΩ for dendritic recordings. A Multiclamp 700B Amplifier and pClamp 9 software (Axon Inst) were used for data acquisition. The average series resistance for whole cell voltage clamp recordings was kept between 9-15 MΩ; 75-80% of the resistance was compensated. Current clamp recordings were obtained with access resistances of 10-20 MΩ for the soma and 10-40 MΩ for the dendrites, compensated in bridge mode.
ITDP was induced by paired PP and SC electrical stimulation at a -20 ms interval (PP before SC) at 1 Hz for 90 s using focal glass pipette stimulating electrodes coupled to constant current stimulators (WPI). Stimulus strengths were adjusted so that PP and SC PSPs were less than 50% of their maximal amplitude (typically <0.5 mV for PP and <5mV for SC). Individual and paired stimuli were always subthreshold for evoking somatic or dendritic spikes. Basal transmission was monitored every 15 s with PP and SC stimuli spaced 2 s apart. A 470 nm LED (CoolLED) or a solid-state single photon laser (OEM lasers) was routed through the 60X objective and 2 pinholes to optically stimulate ChR2 or uncage RuBiGABA. See Supplemental Procedures for details.
Immunohistochemistry and Imaging
Animals were perfused with 1X Phosphate Buffered Saline (PBS) followed by 4% paraformaldehyde (PFA) in 1X PBS. 50 μm sections were cut with a vibratome following an overnight post-fixation (4% PFA) of the brain. Slices were permeabilized, stained with antibodies, mounted on slides and imaged on an inverted laser scanning confocal microscope (Zeiss LSM 700) or multiphoton microscope (Ultima, Prairie Technologies). See Supplemental Information for all details.
Data Analysis
Axograph X and Image J were used for electrophysiology data analysis and image processing, respectively. Kaleidagraph (Synergy) and Prism (Graphpad) were used for plotting data and statistical analysis. Time course plots were generated using a box-car average of every 4 responses (1 min). For calculating the fold change in ITDP, PSP amplitudes were normalized to the mean PSP amplitude during the first 5 mins of baseline recording prior to ITDP induction for each individual experiment and then averaged to generate the mean. For comparing the effect of ITDP induction on response amplitudes, the data was derived from time points corresponding to 5 mins before (pre) and 30-40 mins after (post) induction. All statistical errors are standard errors of the population mean or boxcar mean (SEM); all p values (significance level set at p<0.05) for t-tests are two tailed and all ANOVAs were corrected for multiple comparisons using post-hoc tests as indicated. Figures were generated with Adobe Illustrator.
Computational Modeling
Neurolucida (MicroBrightField) based reconstructions of biocytin-filled CA1 PNs were used to generate a compartmental model in the NEURON simulation environment (Hines and Carnevale 1997) matching the neuron’s digitized anatomy and its measured τslow (recorded in synaptic and HCN blockers). See Supplemental Information for further details.
Supplementary Material
Acknowledgments
We thank K. Deisseroth, G. Fishell and S. Sternson for generously providing reagents; V. Chevaleyre, J. Dudman, M. Larkum, M. Lovett-Barron, R. Piskorowski, H. Takahashi, P. Trifilieff and T. Younts for technical advice; K. Franks, F. Hitti, V. Johnstone, J. Kupferman, A. Losonczy, Z. Rosen, M. Russo and B. Santoro for helpful comments on previous versions of the manuscript. This work was supported by a NARSAD Young Investigator grant (JB), a Columbia University Undergraduate WEP grant (SKC), a 5U01 MH078844-05 grant (ZJH) and the Howard Hughes Medical Institute (SAS). JB and SAS designed the study; JB performed the experiments and analyzed the data; KVS performed the computational modeling; SKC and JB performed the immunohistochemistry and imaging; HT and ZJH generated the CCK-Cre driver and intersectional CCK IN specific transgenic mice; JB and SAS wrote the paper with help from the other authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: a review of anatomical data. Neuroscience. 1989;31:571–591. doi: 10.1016/0306-4522(89)90424-7. [DOI] [PubMed] [Google Scholar]
- Bender VA, Bender KJ, Brasier DJ, Feldman DE. Two coincidence detectors for spike timing-dependent plasticity in somatosensory cortex. The Journal of neuroscience. 2006;26:4166–4177. doi: 10.1523/JNEUROSCI.0176-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nature neuroscience. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Brenowitz SD, Regehr WG. Associative short-term synaptic plasticity mediated by endocannabinoids. Neuron. 2005;45:419–431. doi: 10.1016/j.neuron.2004.12.045. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Feed-forward inhibition in the hippocampal formation. Progress in neurobiology. 1984;22:131–153. doi: 10.1016/0301-0082(84)90023-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron. 2010;68:362–385. doi: 10.1016/j.neuron.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsaki G, Wang XJ. Mechanisms of gamma oscillations. Annual review of neuroscience. 2012;35:203–225. doi: 10.1146/annurev-neuro-062111-150444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Younts TJ, Chavez AE, Hashimotodani Y. Endocannabinoid signaling and synaptic function. Neuron. 2012;76:70–81. doi: 10.1016/j.neuron.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Cho JH, Bayazitov IT, Meloni EG, Myers KM, Carlezon WA, Jr, Zakharenko SS, Bolshakov VY. Coactivation of thalamic and cortical pathways induces input timing-dependent plasticity in amygdala. Nature neuroscience. 2012;15:113–122. doi: 10.1038/nn.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Oscillatory coupling of hippocampal pyramidal cells and interneurons in the behaving Rat. The Journal of neuroscience. 1999;19:274–287. doi: 10.1523/JNEUROSCI.19-01-00274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daw MI, Tricoire L, Erdelyi F, Szabo G, McBain CJ. Asynchronous transmitter release from cholecystokinin-containing inhibitory interneurons is widespread and target-cell independent. The Journal of neuroscience. 2009;29:11112–11122. doi: 10.1523/JNEUROSCI.5760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrunz LE, Stevens CF. Heterogeneity of release probability, facilitation, and depletion at central synapses. Neuron. 1997;18:995–1008. doi: 10.1016/s0896-6273(00)80338-4. [DOI] [PubMed] [Google Scholar]
- Dudman JT, Tsay D, Siegelbaum SA. A role for synaptic inputs at distal dendrites: instructive signals for hippocampal long-term plasticity. Neuron. 2007;56:866–879. doi: 10.1016/j.neuron.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman DE. The spike-timing dependence of plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Brown EN, Wilson MA. A comparison of the firing properties of putative excitatory and inhibitory neurons from CA1 and the entorhinal cortex. Journal of neurophysiology. 2001;86:2029–2040. doi: 10.1152/jn.2001.86.4.2029. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I. Perisomatic inhibition. Neuron. 2007;56:33–42. doi: 10.1016/j.neuron.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Glickfeld LL, Scanziani M. Distinct timing in the activity of cannabinoid-sensitive and cannabinoid-insensitive basket cells. Nature neuroscience. 2006;9:807–815. doi: 10.1038/nn1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Staff NP, Spruston N. Dendritic spikes as a mechanism for cooperative long-term potentiation. Nature. 2002;418:326–331. doi: 10.1038/nature00854. [DOI] [PubMed] [Google Scholar]
- Han EB, Heinemann SF. Distal dendritic inputs control neuronal activity by heterosynaptic potentiation of proximal inputs. The Journal of neuroscience. 2013;33:1314–1325. doi: 10.1523/JNEUROSCI.3219-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nature neuroscience. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS biology. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiological reviews. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Jarsky T, Roxin A, Kath WL, Spruston N. Conditional dendritic spike propagation following distal synaptic activation of hippocampal CA1 pyramidal neurons. Nature neuroscience. 2005;8:1667–1676. doi: 10.1038/nn1599. [DOI] [PubMed] [Google Scholar]
- Kajiwara R, Wouterlood FG, Sah A, Boekel AJ, Baks-te Bulte LT, Witter MP. Convergence of entorhinal and CA3 inputs onto pyramidal neurons and interneurons in hippocampal area CA1--an anatomical study in the rat. Hippocampus. 2008;18:266–280. doi: 10.1002/hipo.20385. [DOI] [PubMed] [Google Scholar]
- Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. The Journal of neuroscience. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Somogyi P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science. 2008;321:53–57. doi: 10.1126/science.1149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Moreau AW, Bakiri Y, Nicholson E. Plasticity of inhibition. Neuron. 2012;75:951–962. doi: 10.1016/j.neuron.2012.07.030. [DOI] [PubMed] [Google Scholar]
- Levy WB, Desmond NL, Zhang DX. Perforant path activation modulates the induction of long-term potentiation of the schaffer collateral--hippocampal CA1 response: theoretical and experimental analyses. Learn Mem. 1998;4:510–518. doi: 10.1101/lm.4.6.510. [DOI] [PubMed] [Google Scholar]
- Lovett-Barron M, Turi GF, Kaifosh P, Lee PH, Bolze F, Sun XH, Nicoud JF, Zemelman BV, Sternson SM, Losonczy A. Regulation of neuronal input transformations by tunable dendritic inhibition. Nature neuroscience. 2012;15:423–430. S421–423. doi: 10.1038/nn.3024. [DOI] [PubMed] [Google Scholar]
- Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, Gu H, Ng LL, Palmiter RD, Hawrylycz MJ, Jones AR, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nature neuroscience. 2010;13:133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnus CJ, Lee PH, Atasoy D, Su HH, Looger LL, Sternson SM. Chemical and genetic engineering of selective ion channel-ligand interactions. Science. 2011;333:1292–1296. doi: 10.1126/science.1206606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mayford M, Siegelbaum SA, Kandel ER. Synapses and memory storage. Cold Spring Harbor perspectives in biology. 2012;4 doi: 10.1101/cshperspect.a005751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi G, Hjerling-Leffler J, Karayannis T, Sousa VH, Butt SJ, Battiste J, Johnson JE, Machold RP, Fishell G. Genetic fate mapping reveals that the caudal ganglionic eminence produces a large and diverse population of superficial cortical interneurons. The Journal of neuroscience. 2010;30:1582–1594. doi: 10.1523/JNEUROSCI.4515-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouille F, Scanziani M. Enforcement of temporal fidelity in pyramidal cells by somatic feed-forward inhibition. Science. 2001;293:1159–1163. doi: 10.1126/science.1060342. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Direct cortical input modulates plasticity and spiking in CA1 pyramidal neurons. Nature. 2002;416:736–740. doi: 10.1038/416736a. [DOI] [PubMed] [Google Scholar]
- Remondes M, Schuman EM. Role for a cortical input to hippocampal area CA1 in the consolidation of a long-term memory. Nature. 2004;431:699–703. doi: 10.1038/nature02965. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Zayat L, Etchenique R, Yuste R. Photorelease of GABA with Visible Light Using an Inorganic Caging Group. Frontiers in neural circuits. 2008;2:2. doi: 10.3389/neuro.04.002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safo P, Regehr WG. Timing dependence of the induction of cerebellar LTD. Neuropharmacology. 2008;54:213–218. doi: 10.1016/j.neuropharm.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa VH, Miyoshi G, Hjerling-Leffler J, Karayannis T, Fishell G. Characterization of Nkx6-2-derived neocortical interneuron lineages. Cereb Cortex. 2009;19(Suppl 1):i1–10. doi: 10.1093/cercor/bhp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spruston N. Pyramidal neurons: dendritic structure and synaptic integration. Nature reviews Neuroscience. 2008;9:206–221. doi: 10.1038/nrn2286. [DOI] [PubMed] [Google Scholar]
- Steffenach HA, Witter M, Moser MB, Moser EI. Spatial memory in the rat requires the dorsolateral band of the entorhinal cortex. Neuron. 2005;45:301–313. doi: 10.1016/j.neuron.2004.12.044. [DOI] [PubMed] [Google Scholar]
- Suh J, Rivest AJ, Nakashiba T, Tominaga T, Tonegawa S. Entorhinal cortex layer III input to the hippocampus is crucial for temporal association memory. Science. 2011;334:1415–1420. doi: 10.1126/science.1210125. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Magee JC. Pathway interactions and synaptic plasticity in the dendritic tuft regions of CA1 pyramidal neurons. Neuron. 2009;62:102–111. doi: 10.1016/j.neuron.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigstrom H, Gustafsson B. Facilitation of hippocampal long-lasting potentiation by GABA antagonists. Acta physiologica Scandinavica. 1985;125:159–172. doi: 10.1111/j.1748-1716.1985.tb07703.x. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- Wohrl R, von Haebler D, Heinemann U. Low-frequency stimulation of the direct cortical input to area CA1 induces homosynaptic LTD and heterosynaptic LTP in the rat hippocampal-entorhinal cortex slice preparation. The European journal of neuroscience. 2007;25:251–258. doi: 10.1111/j.1460-9568.2006.05274.x. [DOI] [PubMed] [Google Scholar]
- Xu JY, Zhang J, Chen C. Long-lasting potentiation of hippocampal synaptic transmission by direct cortical input is mediated via endocannabinoids. The Journal of physiology. 2012;590:2305–2315. doi: 10.1113/jphysiol.2011.223511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Shook NA, Kanisicak O, Yamamoto S, Wosczyna MN, Camp JR, Goldhamer DJ. A multifunctional reporter mouse line for Cre- and FLP-dependent lineage analysis. Genesis. 2009;47:107–114. doi: 10.1002/dvg.20474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeckel MF, Berger TW. Feed-forward excitation of the hippocampus by afferents from the entorhinal cortex: redefinition of the role of the trisynaptic pathway. Proceedings of the National Academy of Sciences of the United States of America. 1990;87:5832–5836. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nature protocols. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


