Abstract
Alzheimer’s disease (AD) and traumatic brain injury (TBI) are both significant clinical problems characterized by debilitating symptoms with limited available treatments. Interestingly, both neurological diseases are characterized by neurovascular damage. This impaired brain vasculature correlates with the onset of dementia, a symptom associated with hippocampal degeneration seen in both diseases. We posit that vascular damage is a major pathological link between TBI and AD, in that TBI victims are predisposed to AD symptoms due to altered brain vasculature; vice versa, the progression of AD pathology may be accelerated by TBI especially when the brain insult worsens hippocampal degeneration. Our hypothesis is supported by recent data reporting expedited AD pathology in presymptomatic transgenic AD mice subjected to TBI. If our hypothesis is correct, treatments targeted at repairing the vasculature may prove effective at treating both diseases and preventing the evolution of AD symptoms in TBI victims.
Keywords: Traumatic Brain Injury, Alzheimer’s disease, Neurovascular Damage
Alzheimer’s disease and vascular damage
Each year, Alzheimer’s Disease (AD) affects over five million people in the United States. In most cases, symptoms emerge after the age of sixty, and progress on a spectrum of three stages. Memory loss and poor judgment are among the most prominent early signs and symptoms. AD is a neurodegenerative disorder [1] primarily tied to the hippocampus region of the brain with patients exhibiting an inability to communicate and a lack of control of bowel and bladder. As the disease progresses, brain tissue in the surrounding hippocampal areas undergoes similar neurodegeneration, characterized by aberrant tau and amyloid protein deposits [1]. AD is a debilitating, irreversible brain condition that eventually leads to death.
AD’s major pathological feature is the degeneration and loss of cholinergic neurons and synapses [2], which compromises the brain. This neuronal death is prominent in areas such as the basal forebrain, amygdala, hippocampus, and cortical area. Over time, memory and cognitive function decline in patients thus causing dementia and eventually death [3–5]. Currently the only positive treatment is acetylcholinesterase inhibitors that merely relieve pain but not cure the disease. Exposing cell death mechanisms brought by AD may reveal novel curative strategies. The amyloid cascade theory [6] states that extracellular plaques containing β-amyloid (Aβ) peptides pathologically cause AD. These Aβ plaques mature into β-plated sheets and fibrilise into neuritic plates, which consequently results in microglial and astrocytic activation, oxidative injury, tau aggregation and phosphorylation, culminating in neuronal loss and synaptic dysfunction resulting in dementia [6]. Studies show that an increase in Aβ peptides triggers memory deficits [7]. These peptides are derived from the bigger amyloid precursor protein (APP).
Vascular damage is another important component of AD pathology. Degradation of the neurovascular unit (NVU) is characteristic of many neurological diseases [8]. In addition, AD vascular risk factors such as hypoglycemia, hypertension, etc. cause BBB dysfunction and damage the NVU during the process of aging [9,10]. The dysregulation of NVU leads to degeneration of nerve endings and retrograde death of cholinergic neurons [8]. This can also hinder the BBB functions, weakening the BBB’s ability to clear Aβ. The Aβ then accumulates within the brain, causing chronic inflammation and further damage to the NVU [8].
Traumatic brain injury and vascular damage
BBB leakage and vascular breakdown, including NVU impairment, have been recognized in acute brain injuries. Yearly, an estimated 235,000 Americans are hospitalized for non-fatal traumatic brain injury (TBI), 1.1 million are treated in emergency, and 50,000 die as a result of the injury [11]. Additionally, it is estimated that 43.1% of patients discharged from hospitals with acute TBI suffer from TBI-related long-term debilitation [12]. TBI is brain damage typically caused by a violent impact, blow, or jolt that results in the brain striking the inside of the skull [13]. A head puncture can also lead to TBI if the object reaches brain tissue [13]. Improvised explosive devices (IED) are often responsible for war-related TBI [14,15] because when a frontal blast wave encounters the head, the shockwave is transferred through skull, cerebrospinal fluid (CSF), and tissue. The shockwave creates negative pressure at the countercoup and has the potential to cause cavitation [16]. In addition to the current TBI target population being war fighters and veterans, TBI is also common in our daily lives, such as vehicular accidents, elderly falls, and baby accidents.
TBI has a wide range of symptoms that vary with the severity of injury. Mild TBI is typically characterized by headache, confusion, lightheadedness, dizziness, blurred vision, ringing in the ears, bad taste in the mouth, fatigue, altered sleep patterns, and behavioral or mood changes [13]. In more severe TBI cases, symptoms may be more exaggerated. For example, more severe TBI is marked by repeated vomiting or nausea, inability to awaken from sleep, seizures, pupil dilation, slurred speech, coordination loss, and confusion [13]. Many patients suffer from TBI’s debilitating symptoms for the rest of their lives.
Although widely considered as an acute brain disease, TBI has been shown to be associated with chronic secondary cell death mediated by many cell death mechanisms including excitotoxicity, free radical formation, disrupted metabolism, and brain swelling [17]. Following the initial trauma, TBI continues to impair the brain by secondary neurodegeneration [17]. Therefore, it is imperative that strategies are developed to combat secondary brain tissue damage and thus limit the impact of TBI.
A major exacerbating neurodegenerative factor accompanying TBI involves a robust inflammatory reaction after the initial injury occurs [18]. What remains highly controversial is that blood-borne white cells serve as a casual factor in secondary brain injury [18]. Polymorphonuclear leukocytes and lymphocytes penetrate brain parenchyma within an hour of the primary brain insult [19]. Once the leukocytes have infiltrated the brain parenchyma, they can increase the vascular permeability in addition to releasing free radicals, activating proteases, altering the cerebral blood flow, and/or producing deleterious pro-inflammatory chemokines and cytokines [20–22].
Hypothesis: Vascular Damage is a Common Pathological Denominator in Alzheimer’s Disease and Traumatic Brain Injury
With the breach in vascular integrity, BBB breakdown follows TBI. Compelling evidence also shows that BBB leakage can arise secondarily to the abnormal brain activity (i.e., as seen with AD pathology) [21]. Therefore, a compromised BBB after TBI can last for years after an acute event [23]. This BBB breakdown is directly associated with an increase in the number of endothelial caveolae [24], resulting in transcytosis of plasma proteins [25,26] and a decrease in tight junction proteins [27,28]. Additionally, the BBB leakage is regarded as the arbitrator of neuroinflammatory responses, which are known to be a key pathological consequence of myriad brain pathologies including stroke, TBI, and AD [29–31].
Although the role of the BBB in the disease pathogenesis is still a developing interest of study, TBI can strongly increase the risk of developing AD [32, 33]. TBI has been found to increase Aβ secretion in the brain [34] and, as discussed previously, the accumulation of Aβ in the brain can trigger AD [35].
We hypothesize that impaired brain vasculature exists as a major pathological link between AD and TBI [Figure 1]. Many TBI-afflicted individuals suffer from dementia, an AD-like symptom which also suggests the role of the hippocampus in TBI disease progression [36–37]. Indeed, presymptomatic AD mice exposed to TBI displayed accelerated AD-like brain pathology including significantly increased Aβ accumulations in the cortex and hippocampus [38]. TBI is thought to induce vascular damage, resulting in activation of the cleavage of APP to Aβ, accelerating the formation of Aβ plaques. [39–41]. This TBI-like vascular damage also accompanies AD [42–46], indicating a possible pathological link between the two diseases. Furthermore, TBI initiates a pro-inflammatory signaling pathway intimately associated with alterations in vascular integrity [47,48], and these vascular disturbances are reminiscent of those seen in AD and are thought to be a result of such a inflammatory pathway [45]. Indeed, decreased cognitive performance was observed in AD mice subjected to TBI [49,50], and these accelerated AD symptoms suggest a strong link between the two diseases—TBI may be a co-morbidity factor of AD [38]. Therefore, these spatiotemporal neurodegenerative events mediated by vascular and BBB breakdowns support our hypothesis that TBI hastens the onset of AD pathology, and vice versa, the evolution of TBI secondary cell death involves an AD-like symptomatology. To this end, in order to further develop the hypothesis of AD being accompanied by vascular damage, which is exacerbated by TBI, studies should pursue temporal-based experiments (time dependent) from early AD to chronic phase to visualize the progressive plaque accumulation and disease onset coinciding with severity of vascular damage. Studies should also explore spatial-based experiments (brain location such as hippocampus) in determining the primary role of vascular damage in plaque formation and behavioral outcome. In this scenario, the recognition of vascular damage co-localized with plaque formation in the hippocampus, especially when patient suffers from TBI, with coincident cognitive impairment would most likely point to a key pathological contribution of vascular damage to both diseases. Based on these spatiotemporal studies, the target brain regions for clinical treatments of vascualr damage in TBI and may involve both the primary impacted region of cortex and the hippocampus which are brain areas vulnerable to both TBI and AD.
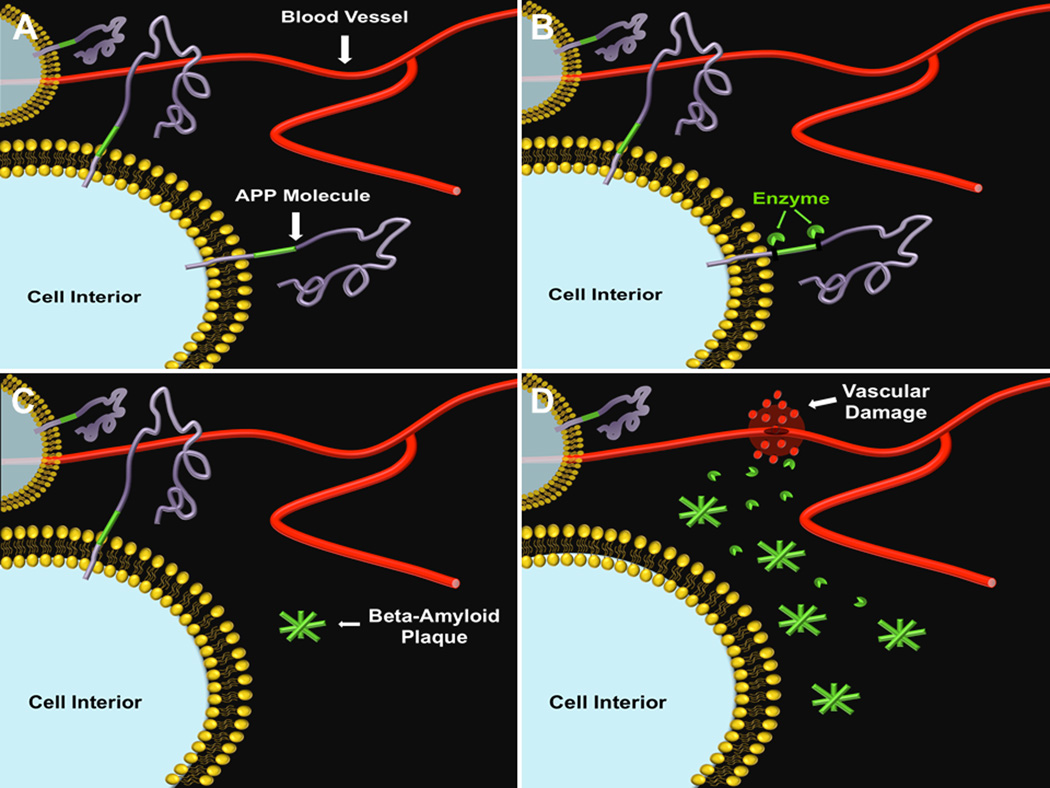
Figure 1. TBI-accelerated Aβ plaque build-up is accompanied by vascular damage.
Under normal conditions, healthy blood vessels and amyloid precursor proteins can be seen in the brain (A). Either aging of the brain or TBI or even both can cause enzymatic alteration of the APP molecule (B), resulting in aberrant aggregation of Aβ plaques (C). Accumulation of Aβ plaques is accompanied by vascular damage (D), which may act as a precursor to AD, as well as an exacerbating factor to TBI.
Future Directions
While vascular damage is likely the pathological link between AD and TBI, it may also serve as an excellent therapeutic target for both diseases. A therapy which focuses on the repair of vasculature may be effective as treatment for both AD and TBI. Furthermore, a therapy that treats vascular damage resulting from TBI has the potential to prevent early onset AD by restoring normal cerebral blood flow to the penumbra. Additionally, because of the relationship between AD and TBI, close monitoring of TBI patients for AD symptoms may allow for earlier and more targeted vascular treatments for patients predisposed to AD due to TBI. Therefore, preclinical research on possible AD and TBI treatments targeting vascular damage should be conducted in order to combat both diseases. In contemplating with vascular repair, novel strategies may include stem cell therapy and electromagnetic treatment [51,52]. These findings directly advance our basic scientific knowledge about a pathological overlap between TBI and AD, and provide pivotal guidance into the translational applications of vascular treatments for TBI and AD patients.
Acknowledgements
This research was supported by the Department of Neurosurgery and Brain Repair funds. CVB is supported by National Institutes of Health, National Institute of Neurological Disorders and Stroke 1R01NS071956-01, Department of Defense W81XWH-11-1-0634, James and Esther King Foundation for Biomedical Research Program, SanBio Inc., KMPHC and NeuralStem Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
References
- 1.Shlosberg D, Benifla M, Kaufer D, Friedman A. Blood-brain barrier breakdown as a therapeutic target in traumatic brain injury. Nat Rev Neurol. 2010;6:393–403. doi: 10.1038/nrneurol.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borlongan CV. Recent preclinical evidence advancing cell therapy for Alzheimer’s disease. Exp Neurol. 2012;237:142–146. doi: 10.1016/j.expneurol.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartus R, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–411. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 4.Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- 5.Whitehouse PJ, Price DL, Clark AW, Coyle JT, DeLong MR. Alzheimer dis- ease: evidence for selective loss of cholinergic neurons in the nucleus basalis. Ann Neurol. 1981;10:122–126. doi: 10.1002/ana.410100203. [DOI] [PubMed] [Google Scholar]
- 6.Verdile G, Fuller S, Atwood CS, Laws SM, Gandy SE, Martins RN. The role of beta amyloid in Alzheimer’s disease: still a cause of everything or the only one who got caught? Pharmacol Res. 2004;50:397–409. doi: 10.1016/j.phrs.2003.12.028. [DOI] [PubMed] [Google Scholar]
- 7.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 8.Stanimirovic DB, Friedman A. Pathophysiology of the neurovascular unit: disease cause or consequence? J Cereb Blood Flow Metab. 2012;32:1207–1221. doi: 10.1038/jcbfm.2012.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zlokovic BV. Neurodegeneration and the neurovascular unit. Nat Med. 2010;16:1370–1371. doi: 10.1038/nm1210-1370. [DOI] [PubMed] [Google Scholar]
- 10.Iadecola C, Hachinski V, Rosenberg GA. Vascular cognitive impairment: introduction. Stroke. 2010;41:S127–S128. doi: 10.1161/STROKEAHA.110.595488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corrigan JD, Selassie AW, Orman JA. The epidemiology of traumatic brain injury. J Head Trauma Rehabil. 2010;25:72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 12.Selassie AW, Zaloshnja E, Langlois JA, Miller T, Jones P, Steiner C. Incidence of long-term disability following traumatic brain injury hospitalization in the United States. J Head Trauma Rehabil. 2008;23:123–131. doi: 10.1097/01.HTR.0000314531.30401.39. [DOI] [PubMed] [Google Scholar]
- 13.Hernandez-Ontiveros DG, Tajiri N, Acosta S, Giunta B, Tan J, Borlongan CV. Microglia activation as a biomarker for traumatic brain injury. Front Neurol. 2013;4:30. doi: 10.3389/fneur.2013.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bogdanova Y, Verfaellie M. Cognitive sequelae of blast-induced traumatic brain injury: recovery and rehabilitation. Neuropsychol Rev. 2012;22:4–20. doi: 10.1007/s11065-012-9192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duckworth JL, Grimes J, Ling GS. Pathophysiology of battlefield associated traumatic brain injury. Pathophysiology. 2012 doi: 10.1016/j.pathophys.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Goeller J, Wardlaw A, Treichler D, O’Bruba J, Weiss G. Investigation of cavitation as a possible damage mechanism in blast-induced traumatic brain injury. J Neurotrauma. 2012;29:1970–1981. doi: 10.1089/neu.2011.2224. [DOI] [PubMed] [Google Scholar]
- 17.Andriessen TM, Jacobs B, Vos PE. Clinical characteristics and pathophysiological mechanisms of focal and diffuse traumatic brain injury. J Cell Mol Med. 2010;14:2381–2392. doi: 10.1111/j.1582-4934.2010.01164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sahuquillo J, Poca MA, Amoros S. Current aspects of pathophysiology and cell dysfunction after severe head injury. Curr Pharm Des. 2001;7:1475–1503. doi: 10.2174/1381612013397311. [DOI] [PubMed] [Google Scholar]
- 19.Tompkins P, Tesiram Y, Lerner M, Gonzalez LP, Lightfoot SA, Raab CH, Bracket DJ. Brain injury: neuro-inflammation, cognitive deficit & MRI in a model of blast-induced TBI. J Neurotrauma. 2013 doi: 10.1089/neu.2012.2674. [DOI] [PubMed] [Google Scholar]
- 20.Morganti-Kossmann MC, Rancan M, Otto VI, Stahel PF, Kossman T. Role of cerebral inflammation after traumatic brain injury: a revised concept. Shock. 2001;16:165–177. doi: 10.1097/00024382-200116030-00001. [DOI] [PubMed] [Google Scholar]
- 21.Shojo H, Kaneko Y, Mabuchi T, Kibayashi K, Adachi N, Borlongan CV. Genetic and histological evidence implicates role of inflammation in traumatic brain injury-induced apoptosis in the rat cerebral cortex following moderate fluid percussion injury. Neuroscience. 2010;171:1273–1282. doi: 10.1016/j.neuroscience.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 22.McKeating EG, Andrews PJ, Macia L. Leukocyte adhesion molecule profiles and outcome after traumatic brain injury. Acta Neurochir Suppl. 1998;71:200–202. doi: 10.1007/978-3-7091-6475-4_57. [DOI] [PubMed] [Google Scholar]
- 23.Tompkins O, Shelef I, Kaizerman I, Eliushin A, Afawi Z, Misk A, Gidon M, Cohen A, Zumsteg D, Friedman A. Blood-brain barrier disruption in post-traumatic epilepsy. J Neurol Neurosurg Psychiatry. 2008;79:774–777. doi: 10.1136/jnnp.2007.126425. [DOI] [PubMed] [Google Scholar]
- 24.Nag S, Kapadia A, Stewart DJ. Review: molecular pathogenesis of blood-brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol. 2011;37:3–23. doi: 10.1111/j.1365-2990.2010.01138.x. [DOI] [PubMed] [Google Scholar]
- 25.Nag S, Venugopalan R, Stewart DJ. Increased caveolin-1 expression precedes decreased expression of occludin and claudin-5 during blood–brain barrier breakdown. Acta Neuropathol. 2007;114:459–469. doi: 10.1007/s00401-007-0274-x. [DOI] [PubMed] [Google Scholar]
- 26.Nag S, Manias JL, Stewart DJ. Expression of endothelial phosphorylated caveolin-1 is increased in brain injury. Neuropathol Appl Neurobiol. 2009;35:417–426. doi: 10.1111/j.1365-2990.2008.01009.x. [DOI] [PubMed] [Google Scholar]
- 27.Zhao J, Moore AN, Redell JB, Dash PK. Enhancing expression of Nrf2-driven genes protects the blood brain barrier after brain injury. J Neurosci. 2007;27:10240–10248. doi: 10.1523/JNEUROSCI.1683-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeung D, Manias JL, Stewart DJ, Nag S. Decreased junctional adhesion molecule-A expression during blood–brain barrier breakdown. Acta Neuropathol. 2008;115:635–642. doi: 10.1007/s00401-008-0364-4. [DOI] [PubMed] [Google Scholar]
- 29.Infante-Duarte C, Waiczies S, Wuerfel J, Zipp F. New developments in understanding and treating neuroinflammation. J Mol Med. 2008;86:975–985. doi: 10.1007/s00109-007-0292-0. [DOI] [PubMed] [Google Scholar]
- 30.Oby E, Janigro D. The blood–brain barrier and epilepsy. Epilepsia. 2006;47:1761–1774. doi: 10.1111/j.1528-1167.2006.00817.x. [DOI] [PubMed] [Google Scholar]
- 31.Skaper SD. The brain as a target for inflammatory processes and neuroprotective strategies. Ann NY Acad Sci. 2007;1122:23–34. doi: 10.1196/annals.1403.002. [DOI] [PubMed] [Google Scholar]
- 32.Guo Z, Cupples LA, Kurz A, Auerbach SH, Volicer L, Chui H, Green RC, Sadovnick AD, Duara R, DeCarli C, Johnson K, Go RC, Growdon JH, Haines JL, Kukull WA, Farrer LA. Head injury and the risk of AD in the MIRAGE study. Neurology. 54:1316–1323. doi: 10.1212/wnl.54.6.1316. (200) [DOI] [PubMed] [Google Scholar]
- 33.Jellinger K, Paulus W, Wrocklage C, Litvan I. Traumatic brain injury as a risk factor for Alzheimer disease. Comparison of two retrospective autopsy cohorts with evaluation of ApoE genotype. BMC Neurol. 2001;1:3. doi: 10.1186/1471-2377-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meyer-Luehmann M, Spires-Jones TL, Prada C, Garcia-Alloza M, de Calignon A, Rozkalne A, Koenigsknecht-Talboo J, Holtzman DM, Bacskai BJ, Hyman BT. Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer’s disease. Nature. 2008;451:720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeKosky ST, Ikonomovic MD, Gandy S. Traumatic brain injury: football, warfare, and long-term effects. N Engl J Med. 2010;363:1293–1296. doi: 10.1056/NEJMp1007051. [DOI] [PubMed] [Google Scholar]
- 37.Miller G. A battle no soldier wants to fight. Science. 2011;333:517. doi: 10.1126/science.333.6042.517. [DOI] [PubMed] [Google Scholar]
- 38.Tajiri N, Glover LE, Shimizu T, Arendash GW, Borlongan CV. Traumatic brain injury expedites the presentation of Alzheimer’s disease-like histopathology in transgenic mice. International Conference on Alzheimer’s Drug Discovery. 2011;12:12. [Google Scholar]
- 39.Davies TA, Long HJ, Eisenhauer PB, Hastey R, Cribbs DH, Fine RE, Simons ER. Beta amyloid fragments derived from activated platelets deposit in cerebrovascular endothelium: usage of a novel blood brain barrier endothelial cell model system. Amyloid. 2000;7:153–165. doi: 10.3109/13506120009146830. [DOI] [PubMed] [Google Scholar]
- 40.Mlekusch R, Humpel C. Matrix metalloproteinases-2 and -3 are reduced in cerebrospinal fluid with low beta-amyloid1–42 levels. Neurosci Lett. 2009;466:135–138. doi: 10.1016/j.neulet.2009.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Palmer JC, Kehoe PG, Love S. Endothelin-converting enzyme-1 in Alzheimer's disease and vascular dementia. Neuropathol Appl Neurobiol. 2010;36:487–497. doi: 10.1111/j.1365-2990.2010.01084.x. [DOI] [PubMed] [Google Scholar]
- 42.Aliev G, Seyidova D, Lamb BT, Obrenovich ME, Siedlak SL, Vinters HV, Friedland RP, LaManna JC, Smith MA, Perry G. Mitochondria and vascular lesions as a central target for the development of Alzheimer's disease and Alzheimer disease-like pathology in transgenic mice. Neurol Res. 2003;25:665–674. doi: 10.1179/016164103101201977. [DOI] [PubMed] [Google Scholar]
- 43.Jantaratnotai N, Ryu JK, Schwab C, McGeer PL, McLarnon JG. Comparison of Vascular Perturbations in an Abeta-Injected Animal Model and in AD Brain. Int J Alzheimers Dis. doi: 10.4061/2011/918280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Borroni B, Akkawi N, Martini G, Colciaghi F, Prometti P, Rozzini L, Di Luca M, Lenzi GL, Romanelli G, Caimi L, Padovani A. Microvascular damage and platelet abnormalities in early Alzheimer's disease. J Neurol Sci. 2002;203–204:189–193. doi: 10.1016/s0022-510x(02)00289-7. [DOI] [PubMed] [Google Scholar]
- 45.Walker DG, Dalsing-Hernandez JE, Lue LF. Human postmortem brain-derived cerebrovascular smooth muscle cells express all genes of the classical complement pathway: a potential mechanism for vascular damage in cerebral amyloid angiopathy and Alzheimer's disease. Microvasc Res. 2008;75:411–419. doi: 10.1016/j.mvr.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yesil Y, Kuyumcu ME, Cankurtaran M, Uz B, Kara A, Kilic MK, Halil M, Ulger Z, Yavuz BB, Haznedaroglu IC, Ariogul S. Increased mean platelet volume (MPV) indicating the vascular risk in Alzheimer's disease (AD) Arch Gerontol Geriatr. 2011;55:257–260. doi: 10.1016/j.archger.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 47.Pettus EH, Wright DW, Stein DG, Hoffman SW. Progesterone treatment inhibits the inflammatory agents that accompany traumatic brain injury. Brain research. 2005;1049:112–119. doi: 10.1016/j.brainres.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 48.You Z, Yang J, Takahashi K, Yager PH, Kim HH, Qin T, Stahl GL, Ezekowitz RA, Carroll MC, Whalen MJ. Reduced tissue damage and improved recovery of motor function after traumatic brain injury in mice deficient in complement component C4. J Cereb Blood Flow Metab. 2007;27:1954–1964. doi: 10.1038/sj.jcbfm.9600497. [DOI] [PubMed] [Google Scholar]
- 49.Kellogg SL, Tajiri N, Shimizu T, Arendash GW, Borlongan CV. Behavioral and histological effects of traumatic brain injury on Alzheimer's disease transgenic mice. Soc Neurosci Abstr. 2011;561:20. [Google Scholar]
- 50.Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-β accumulation and independently accelerates the development of tau abnormalities. J Neurosci. 2011;31:9513–9925. doi: 10.1523/JNEUROSCI.0858-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Borlongan CV. Recent preclinical evidence advancing cell therapy for Alzheimer's disease. Exp Neurol. 2012;237:142–146. doi: 10.1016/j.expneurol.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Arendash GW, Mori T, Dorsey M, Gonzalez R, Tajiri N, Borlongan C. Electromagnetic treatment to old Alzheimer's mice reverses β-amyloid deposition, modifies cerebral blood flow, and provides selected cognitive benefit. PLoS One. 2012;7:e35751. doi: 10.1371/journal.pone.0035751. [DOI] [PMC free article] [PubMed] [Google Scholar]