Abstract
Cognitive dysfunction is a common symptom of Parkinson’s disease that causes significant morbidity and mortality. The severity of these symptoms ranges from minor executive symptoms to frank dementia involving multiple domains. In the present review, we will concentrate on the aspects of cognitive impairment associated with prefrontal dopaminergic dysfunction seen in non-demented patients with PD. These symptoms include executive dysfunction and disorders of thought such as hallucinations and psychosis. Such symptoms may go on to predict dementia related to Parkinson’s disease, which involves amnestic dysfunction and is typically seen later in the disease. Cognitive symptoms are associated with dysfunction in cholinergic circuits in addition to the abnormalities in the prefrontal dopaminergic system. These circuits can be carefully studied and evaluated in Parkinson’s disease, and could be leveraged to treat difficult clinical problems related to cognitive symptoms of PD.
Introduction
Parkinson’s disease (PD) is a neurodegenerative condition in which midbrain dopamine neurons inexorably die (Hughes et al., 1992) resulting in impaired motor function (Fahn et al., 2004). Recently, non-motor symptoms have been recognized as a prominent part of PD (Chaudhuri and Schapira, 2009). These symptoms can precede diagnosis of motor symptoms by several decades (Claassen et al., 2010). One non-motor symptom that causes particular morbidity and mortality in PD is cognitive dysfunction (Santangelo et al., 2007; Forsaa et al., 2010a). In the present review, we discuss cognitive dysfunction in PD and relate it to processes that localize to the prefrontal cortex. We show how mesocortical dopaminergic projections to the prefrontal cortex can be involved in the genesis of these symptoms, and how these projections interact with ascending cholinergic projections.
Identifying the mechanism of cognitive dysfunction in PD is crucial for treating cognitive symptoms of PD, as currently we have few effective treatments for this difficult clinical problem. Levodopa inconsistently improves cognitive symptoms of PD (Müller et al., 2001; Cools, 2006; Pascual-Sedano et al., 2008), depending on disease state and the integrity of striatal dopamine signaling (Cools et al., 2001).. Some data suggest that cholinesterase inhibitors can be somewhat beneficial for executive symptoms of PD (Reading et al., 2001; Poewe et al., 2006; Schmitt et al., 2010) in addition to their known benefits for dementia ((Emre et al., 2004)). Further elucidation of how these circuits influence cognition in PD may help rationally target therapies for this difficult clinical problem.
Spectrum of cognitive dysfunction in PD
Although mild visuospatial and amnestic impairments can also be seen, typically non-demented PD patients can manifest executive dysfunction with no amnestic component at incident diagnosis of PD (Foltynie et al., 2004a; Aarsland et al., 2009). These include impaired working memory, planning, attention (Aarsland et al., 2011), decreased speed of processing (Uc et al., 2005), impulse control disorders (Pontone et al., 2006) associated with dopamine agonists (Weintraub D, 2006), and disordered thought (Factor et al., 2003). These are distinct from fluctuating disturbances of memory, calculation, visuospatial function, and behavioral control associated diffusely with Lewy Body Dementia (Lennox, 1992; McKeith et al., 1996) and PD-related dementia (Aarsland et al., 2003, 2009). Crucially, PD is heterogeneous with respect to cognitive impairments, complicating interpretation of clinical data thus far (Wurtman, 2012). In addition, epidemiological studies draw on a diversity of methods, and there is no uniform definition of mild cognitive impairment in PD (Goldman and Litvan, 2011).
Cognitive dysfunction is common in PD (Muslimovic et al., 2005). In early Parkinson’s disease, up to 36% of Parkinson’s patients had some evidence of cognitive impairment at disease onset (Foltynie et al., 2004a). In a population-based study in Norway of untreated PD (Aarsland et al., 2009), 19% of patients had mild cognitive impairment, as defined by poor performance on neuropsychological testing. Interestingly, two-thirds of patients in this category had intact performance on tests that measured amnestic performance. The CamPaIGN study followed 126 PD patients for 5 years from incident diagnosis, and revealed a population with dementia that was unrelated to executive dysfunction as measured by the Tower of London task (Aarsland et al., 2010). In a Swedish study, 88 patients with newly diagnosed Parkinson’s disease performed consistently worse in neuropsychological testing, and 30% of patients had executive deficits (Elgh et al., 2009). This deficit may be independent of overall motor disability (Cooper et al., 1991; Kieburtz et al., 1994); however, some studies suggest some patterns linking movement speed and axial signs to cognitive dysfunction (e.g. Aarsland et al., 2003; Domellöf et al., 2011).
Cognitive symptoms can be influenced by disease progression. This pattern of both non-amnestic and amnestic patterns of cognitive dysfunction has commonly been observed in multi-center population studies of PD (Aarsland et al., 2009; Dalrymple-Alford et al., 2011; Monastero et al., 2012). A meta-analyses revealed that on average across a diversity of methodologies, 27% of patients with PD had non-amnestic cognitive dysfunction (Litvan et al., 2011). Several epidemiological studies have revealed that patients with PD develop dementia at 4-6 times the rate of normal aging (Aarsland et al., 2001, 2005; Hobson and Meara, 2004). In a longitudinal study of idiopathic PD, cognitive symptoms continued to develop over the course of the disease at rates much higher than normal aging (Williams-Gray et al., 2007) and are associated with a shorter time to dementia (Aarsland and Kurz, 2010). Within 5 years of diagnosis, up to 17% of patients developed dementia (Aarsland et al., 2010). In advanced PD, cognitive symptoms can dominate later stages of the disease (Ferreri et al., 2006).
At all stages, cognitive dysfunction can cause significant morbidity and mortality (Forsaa et al., 2010a). These symptoms at first diagnosis lead to nearly double the mortality rate in PD (Levy et al., 2002) and predict nursing home placement (Aarsland et al., 2000). This is associated with significant costs at all stages of disease (Kaltenboeck et al., 2012). Even in earlier stages of disease, cognitive dysfunction can interfere with key activities such as driving (Stolwyk et al., 2005; Uc et al., 2006, 2007, 2011; Crizzle et al., 2012) and predict loss of independence (Uc et al., 2011). Future observational data on the natural history of cognitive symptoms and dementia in PD will help further define this issue (Balzer-Geldsetzer et al., 2011)
Executive dysfunction
Executive functions are a group of psychological processes that regulate and control other cognitive processing. These include functions such as working memory, planning, reasoning, timing, and inhibitory control (Baddeley, 1998). Executive processes can be measured through standard neuropsychological testing such clock-drawing, Wisconsin-Card-Sorting, Stroop interference tasks, or trailmaking (Litvan et al., 1991), and are distinct from non-executive functions such as memory, language and motor control. Screening instruments that assay executive function may be useful in PD patients (Dalrymple-Alford et al., 2010, 2011).
Patients with very early PD can have impaired executive functions (Caballol et al., 2007). An early study reported that executive dysfunction in PD was not correlated with motor function (Van Spaendonck et al., 1996). As mentioned above, the Norwegian ParkWest study noted mild cognitive impairment among 196 non-demented patients with PD (Aarsland et al., 2009) compared to age-matched controls. A multicenter study from the same group of 1,346 actively managed patients with PD found that 26% of PD patients had MCI and 10% had executive dysfunction (Aarsland et al., 2010). Small studies have reported that dopaminergic therapy does not reliably improve executive dysfunction in high-functioning (Pascual-Sedano et al., 2008) or moderate PD (Morrison et al., 2004), and can potentially have detrimental effects (Cools et al., 2001; Cools and D’Esposito, 2011).
Impaired impulse control is another executive deficit seen in PD patients. It is manifested as pathological gambling, hypersexuality, compulsive shopping, and compulsive eating. These were recently detected in up to 13% of structured surveys of Parkinson’s patients (Voon et al., 2006b), and prospectively related to exposure to dopamine agonists (Voon et al., 2006a) that may be dose-dependent (Lee et al., 2010). In a large cross-sectional multicenter study, impulse control disorders were found in 13.6% of patients with PD (Weintraub et al., 2010). In this study, dopaminergic agonists were associated with a 2.5 times greater risk of developing complications. Patients who were more likely to develop impulse control disorders were younger, depressed, anxious, impulsive, and obsessive (Weintraub et al., 2010; Voon et al., 2011). The primary treatment for this disorder is withdrawal of dopaminergic agonists (Ávila et al., 2011) if practical.
Thought disorders in PD
Disorders of thought are commonly seen in psychiatric disease such as schizophrenia or bipolar disorder, and are hypothesized to involve dopaminergic circuitry (Braver et al., 1999). In PD, which also involves dysfunction in dopamine systems, disorders of thought have been recognized for decades (Knopp, 1970). A community survey of 245 patients in Norway reported that 10% of patients had symptoms of disordered thought, the risk of which correlated with age and disease severity (Aarsland et al., 1999). Following this cohort over time suggested that a majority of Parkinson’s patients experienced thought disorders at some time (Forsaa et al., 2010b) associated with high doses of levodopa and REM sleep disorder. Such symptoms could be a surrogate of disease severity, and could be associated with poor outcomes such as nursing home placement, death, and dementia (Factor et al., 2003). Atypical antipsychotics such as clozapine (Wolk and Douglas, 1992) can be effective at controlling hallucinations (Parkinson’s Study Group, 1999), and cholinesterase inhibitors have modest benefit (Williams-Gray et al., 2006). More recently, serotonin 5-HT2A agonists have demonstrated anti-psychotic efficacy in both animal models and human subjects (Acadia Pharmaceuticals, 2012).
Dementia
One hallmark of dementia is that it involves amnestic dysfunction. Some PD patients with cognitive symptoms have no memory loss (Williams-Gray et al., 2006 p.-; Aarsland et al., 2009, 2010). Although executive dysfunction and thought disorders can be seen in other types of dementias (Swanberg et al., 2004; Aarsland et al., 2010), in Parkinson’s disease these features often predict the early development of dementia (Aarsland and Kurz, 2010; Forsaa et al., 2010b) with associated increases in morbidity and mortality (Forsaa et al., 2010a). Dementia can be highly prevalent across the entire course of Parkinson’s disease, affecting up to 80% of patients (Aarsland et al., 2001, 2003; Aarsland and Kurz, 2010), and may be associated with early hallucinations and akinetic-dominant variants of PD (Aarsland et al., 2003; Emre et al., 2007).
Idiopathic PD involves extensive accumulation of Lewy-bodies, eosinophilic intracellular inclusions within the midbrain. However, they can be observed in normal aging and other dementias (Gibb and Lees, 1988). One possibility is that Lewy-Body pathology spreads from peripheral nuclei to the neocortex (Braak et al., 2003). This idea has been recently bolstered by description of cell-to-cell transmission of pathological a-synuclein (Dunning et al., 2012; Luk et al., 2012b, 2012a). According to this hypothesis, cognitive symptoms of PD would be caused by spread of Lewy-bodies to the cognitive areas of the neocortex, such as medial prefrontal cortex (Huang et al., 2007).
Indeed, quantitative counts of synuclein pathology seemed to loosely associate with MMSE, although in this data, there were high-pathological stage PD patients with low impairments, and low pathological stage PD patients with high impairment (Braak et al., 2006). Multivariate regression of the brains of 148 patients with PD revealed that Braak’s specific pathological staging was not predictive of dementia (Gibb and Lees, 1988; Burton et al., 2004; Compta et al., 2011; Irwin et al., 2012). However, distribution of Lewy-body burden did roughly correlate with clinical diagnosis of dementia (Irwin et al., 2012). These data, while not supporting the explicit rostro-caudal progression of synuclein (Jellinger, 2009), largely support the Braak hypothesis. However, the specific patterns in PD, PD-related dementia, and Lewy-body dementia are yet to be defined (Lippa et al., 2007).
Other pathological processes may contribute to cognitive symptoms in PD (Kempster et al., 2010). For instance, tau and amyloid are common in PD-dementia (Kempster et al., 2010; Compta et al., 2011; Kotzbauer et al., 2012; Irwin et al., 2012) and while these are correlated with PD-dementia (Compta et al., 2011), they shed little insight on cognitive dysfunction present earlier in the disease. Furthermore, the association with Alzheimer’s risk factors, AB42 (Montine et al., 2010), ApoE4 (Irwin et al., 2012), the tau-related gene MAPT (Aarsland et al., 2010) or white matter changes (Kim et al., 2012) suggest that other processes may contribute to cognitive dysfunction in PD.
One possibility is that AD-pathology involving tau and amyloid deposition contributes to dementia, and separate synuclein-related processes involving cholinergic and dopaminergic systems contribute to executive dysfunction in PD (Fig 1). Future work may be able to perform molecular imaging of synuclein with molecular imaging of AD pathology (Burack et al., 2010) and explicitly test this idea and further define the role of synuclein pathology and cognitive symptoms of PD.
Figure 1.
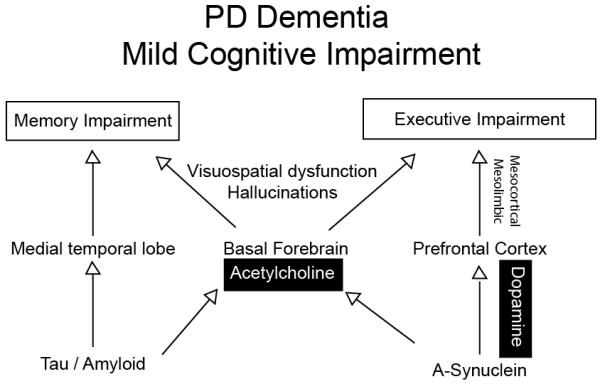
Whereas alpha-synuclein in mesocortical dopamine projections likely contributes cellular dysfunction leading executive impairment, tau and alpha-synuclein may impair basal forebrain and acetylcholine signaling contributing to both executive and memory impairment.
Prefrontal dopamine signaling and executive dysfunction in PD
Executive dysfunction and thought disorders localize to the prefrontal cortex (Goldman-Rakic, 1998; Baddeley, 1998; Fuster, 2008). For instance, brain imaging of executive processes such as working memory (Narayanan et al., 2005), planning (Miller and Cohen, 2001), conflict monitoring (Botvinick et al., 2004), inhibitory control (Dias et al., 2006), and timing (Coull et al., 2011) reliably show activation of prefrontal regions, including dorsolateral prefrontal cortex (BA 9/46) and medial prefrontal regions (BA 25/32). Diseases that involve impaired executive function such as schizophrenia involve deactivation in these regions (Weinberger et al., 1986). Prefrontal dysfunction has long been linked to disordered thought (Dolan et al., 1993), and localization of processes that are affected by PD might facilitate insight into possible mechanisms, as any pathophysiological explanation for the cognitive symptoms of PD must account for prefrontal dysfunction.
Neuropsychological evidence reveals that Parkinson’s patients are impaired on tests that are sensitive to prefrontal function (Zgaljardic et al., 2006), such as the Wisconsin-Card-Sorting Task (Gotham et al., 1988), attentional set-shifting (Cools et al., 2001), and spatial working memory (Lange et al., 1992). Metabolic imaging evidence has implicated a medial frontal network in which deactivation in medial frontal networks is correlated with poor performance on cognitive tests (Huang et al., 2007). Brain imaging studies have consistently demonstrated hypoactivation of prefrontal areas in Parkinson’s patients. For instance, during a random number generation task, controls activated medial frontal networks more robustly than Parkinson’s patients (Dirnberger et al., 2005). The same group observed similar deactivation during a motor timing task; in this task, medial frontal activation could be modulated by levodopa (Jahanshahi et al., 2010). A similar pattern was found in a time perception task (Harrington et al., 2011). During performance of the Tower of London planning tasks, L-dopa restored prefrontal blood flow as measured by PET (Cools et al., 2002). Finally, in PD decreased performance on attentional set-shifting is correlated with impaired metabolic activity in prefrontal cortex (Sawada et al., 2012). This line of work consistently describes less prefrontal activity in PD that is correlated with impaired executive performance.
Prefrontal dopamine
Patients with PD have impaired prefrontal dopamine signaling (Dubois and Pillon, 1995, 1997). Prefrontal regions receive dopamine not from the nigrostriatal projections; rather, they receive dopamine from ventral tegmental and medial nigral regions of the midbrain (Williams and Goldman-Rakic, 1998). These nuclei do degenerate in some patients with PD (Javoy-Agid and Agid, 1980; Javoy-Agid et al., 1981; Dymecki et al., 1996). To date, there is no evidence explicitly linking VTA loss with cognitive dysfunction in PD (Jellinger, 1999), although it is clear that mesocortical dopaminergic projections can influence cognitive function (Narayanan et al., 2012).
Clinically, there is no correlation with classical nigrostriatal functions, such as bradykinesia or tremor (Cooper et al., 1991; Kieburtz et al., 1994). Attempts to link cognitive dysfunction with motor features noted an association with akinetic forms of PD (Aarsland et al., 2003; Uc et al., 2009) and with axial/visuomotor dysfunction (Domellöf et al., 2011) insinuate that other dopaminergic systems, such as mesocortical projections originating from the ventral tegmental area, may be involved. Future in-vivo molecular imaging studies of patients with early PD and cognitive dysfunction may address this question.
Dopamine signaling in prefrontal cortex influences executive function (Arnsten and Li, 2005; Cools and D’Esposito, 2011). For instance, seminal work by Goldman-Rakic and colleagues demonstrated that blocking D1 receptors in prefrontal cortex degraded single neuron activity encoding working memory (Williams and Goldman-Rakic, 1995; Wang et al., 2004; Goldman-Rakic et al., 2004). In rodents, selectively blocking prefrontal D1 receptors impairs cognitive functions such as interval timing (Narayanan et al., 2012) and memory (Seamans et al., 1998; Floresco and Phillips, 2001). Furthermore, dopamine release measured by microdialysis is correlated with working memory performance (Phillips et al., 2004).
In humans, dopamine can be imaged using molecular imaging techniques, such as PET or SPECT. However, these studies are complicated because prefrontal signal is difficult to isolate (Vrieze et al., 2011). Metabolic imaging (Huang et al., 2007) and presynaptic dopamine imaging (Ekman et al., 2012) have suggested an association with decreased anterior cingulate and caudate activation in patients with cognitive impairment in PD. In prefrontal regions, D1-type dopamine receptors are more prominent than D2-type receptors (Gaspar et al., 1995; Seong and Carter, 2012). Despite these challenges, PET imaging shows that D1 signaling is modulated in frontal regions during working memory tasks (Okubo et al., 1997; Abi-Dargham et al., 2002). PET scanning revealed marked decrease in lateral and medial prefrontal dopamine and in D2 receptors in PD, albeit later in the disease (Rakshi et al., 1999; Kaasinen et al., 2000). Curiously, in early PD dopamine uptake has been reported to be increased in prefrontal regions (Rakshi et al., 1999; Kaasinen et al., 2001; Cools et al., 2001), presumably related to frontal compensation. Increased dopamine could also produce cognitive dysfunction observed at first diagnosis (Aarsland et al., 2009) as cognitive processes require optimal dopamine (Cools and D’Esposito, 2011).
One other possibility is that dysfunctional striatal networks in PD involve both motor and cognitive pathways (Graybiel et al., 1994). For instance, decreased metabolic signal in frontal circuits is correlated with altered signal in the pallidum (Dirnberger et al., 2005), and that connectivity analysis revealed that pallido-frontal analysis could be modulated by levodopa (Jahanshahi et al., 2010). Another study described altered dopamine release in the caudate and not the medial frontal cortex (Sawamoto et al., 2008), and this altered dopamine release could explain dysfunctional prefrontal networks (Polito et al., 2012).
Finally, genetic polymorphisms in frontostriatal circuits may interact with cognitive dysfunction. For instance, a polymorphism in catechol-o-methyl transferase was associated with poor planning in PD patients as measured by the Tower of London task (Foltynie et al., 2004b). Mutations in this gene also influenced attention (Williams-Gray et al., 2008), and potently modulated fronto-striatal circuitry (Wu et al., 2012).
Cholinergic dysfunction
A final mechanistic contributor to cognitive symptoms of PD is the degeneration of non-dopaminergic ascending projection nuclei. Further analysis of the DATATOP study (Anon, 1989) found that cognitive symptoms of PD were correlated with subcortical features such as bulbar and gastrointestinal symptoms, suggesting that autonomic, non-dopaminergic projections may be involved (Uc et al., 2009).
Indeed, PD involves degeneration of multiple ascending projection systems (Fig 2), including norepinephrine, serotonin, and dopamine (Scatton et al., 1983; Jellinger, 2011), and prominently, acetylcholine. Cholinergic neurons in the basal forebrain are decimated in PD (Arendt et al., 1983) and develop Lewy-bodies (Candy 1983). Early pathological work linked cognitive symptoms in PD with decreased choline acetyltransferase (Fonnum, 1966), an enzyme necessary for presynaptic synthesis of acetylcholine, and neuronal counts in the basal nucleus of Meynert (Perry et al., 1985).
Figure 2.
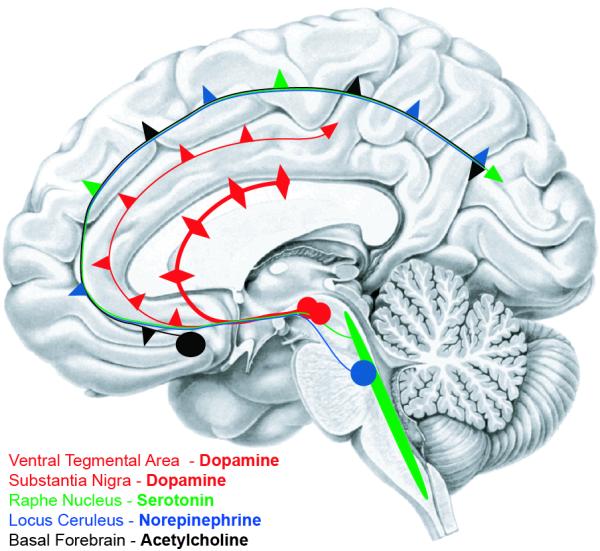
Ascending projection systems that degenerate in Parkinson’s disease.
More recent molecular imaging techniques have identified cholinergic deficits in Parkinson’s patients. For instance, PET and SPECT of nicotinic receptors have revealed widespread loss in Parkinson’s patients with cognitive impairments (Fujita et al., 2006; Meyer et al., 2009). In fact, cortical cholinergic function is more compromised in Parkinson’s disease with dementia compared to Parkinson’s disease without dementia, which in turn shows worse cortical cholinergic function compared to Alzheimer’s disease (Bohnen et al., 2003). PET studies have also found decreased cortical and subcortical acetylcholinesterase in PD (Gilman et al., 2010). Acetylcholinesterase activity correlates with impaired executive function but not motor symptoms (Bohnen et al., 2006). Furthermore, acetylcholine has a potent modulatory role in thought disorders (Bosboom et al., 2003).
The strongest clinical link between cholinergic projections and PD comes from the EXPRESS study, which described several beneficial effects of treatment with rivastigmine, a cholinesterase inhibitor which presumably increases the amount of acetylcholine present at the synaptic cleft. This study, a randomized, placebo-controlled study of 410 PD patients, reported a moderate improvement in a cognitive battery for PD patients with mild-moderate dementia (Emre et al., 2004; Poewe et al., 2006). Similar efforts with donepezil have been promising (Aarsland et al., 2002; Rowan et al., 2007) but have been limited by side effects (Fabbrini et al., 2002; Müller et al., 2006). This literature suggests that cholinergic signaling can be effectively modulated by oral drugs to improve executive function, psychosis, and dementia in Parkinson’s patients, and future drugs might modulate this system while minimizing dose-limiting side effects.
An interesting interface exists between cholinergic projection systems and dopamine systems involved in cognition. Parkinson’s patients given small doses of anticholinergics such as scopolamine had impaired working memory (Dubois et al., 1987). A follow-up study reported decreases specifically on executive tests with both trihexiphenadyl and scopolamine (Bédard et al., 1999). We propose a model whereby cholinergic and dopaminergic projections to prefrontal cortex (Fig 1 and 2) interact in the prefrontal cortex and lead to executive dysfunction. This model makes testable predictions that cholinergic and dopaminergic signaling interact on prefrontal neurons, and that modulation of these receptors should profoundly influence neuronal networks correlated with executive function (Williams and Goldman-Rakic, 1995; Asaad et al., 1998; Narayanan and Laubach, 2006, 2009) and can be systematically investigated in animal models of PD and in PD patients. We are optimistic that these avenues may lead to new treatment paradigms for this difficult clinical problem.
Summary
In the present review, we have discussed cognitive dysfunction of PD involving executive dysfunction and disordered thought. There are no effective treatments for these symptoms, which may predict later amnestic disturbance, and lead to considerable morbidity and mortality. We evaluate a mechanistic possibility for executive dysfunction in PD revolving around prefrontal dopamine systems, which could interact with cholinergic circuitry to produce executive dysfunction (Fig 1). We are hopeful that further evaluation of the pathophysiology of cognitive dysfunction in Parkinson’s disease will explore these ideas, as they may lead to new treatments that take advantage of either of these systems.
References
- Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sørensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Brønnick K, Fladby T. Mild cognitive impairment in Parkinson’s disease. Curr Neurol Neurosci Rep. 2011;11:371–378. doi: 10.1007/s11910-011-0203-1. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Brønnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Kurz MW. The Epidemiology of Dementia Associated with Parkinson’s Disease. Brain Pathology. 2010;20:633–639. doi: 10.1111/j.1750-3639.2009.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Laake K, Larsen JP, Janvin C. Donepezil for cognitive impairment in Parkinson’s disease: a randomised controlled study. J. Neurol. Neurosurg. Psychiatr. 2002;72:708–712. doi: 10.1136/jnnp.72.6.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch. Neurol. 1999;56:595–601. doi: 10.1001/archneur.56.5.595. [DOI] [PubMed] [Google Scholar]
- Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov. Disord. 2005;20:1255–1263. doi: 10.1002/mds.20527. [DOI] [PubMed] [Google Scholar]
- Abi-Dargham A, Mawlawi O, Lombardo I, Gil R, Martinez D, Huang Y, Hwang D-R, Keilp J, Kochan L, Van Heertum R, Gorman JM, Laruelle M. Prefrontal dopamine D1 receptors and working memory in schizophrenia. J. Neurosci. 2002;22:3708–3719. doi: 10.1523/JNEUROSCI.22-09-03708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acadia Pharmaceuticals (first) ACADIA Announces Pimavanserin Meets Primary and Key Secondary Endpoints in Pivotal Phase III Parkinson’s Disease Psychosis Trial. 2012 http://news.acadia-pharm.com/phoenix.zhtml?c=125180&p=irol-newsArticle&ID=1761922&highlight=
- Anon. Parkinson Study Group DATATOP: a multicenter controlled clinical trial in early Parkinson’s disease. Arch. Neurol. 1989;46:1052–1060. doi: 10.1001/archneur.1989.00520460028009. [DOI] [PubMed] [Google Scholar]
- Anon. The Parkinson Study Group Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. N. Engl. J. Med. 1999;340:757–763. doi: 10.1056/NEJM199903113401003. [DOI] [PubMed] [Google Scholar]
- Arendt T, Bigl V, Arendt A, Tennstedt A. Loss of neurons in the nucleus basalis of Meynert in Alzheimer’s disease, paralysis agitans and Korsakoff’s Disease. Acta Neuropathol. 1983;61:101–108. doi: 10.1007/BF00697388. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Li B-M. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol. Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Asaad WF, Rainer G, Miller EK. Neural activity in the primate prefrontal cortex during associative learning. Neuron. 1998;21:1399–407. doi: 10.1016/s0896-6273(00)80658-3. [DOI] [PubMed] [Google Scholar]
- Ávila A, Cardona X, Martín-Baranera M, Bello J, Sastre F. Impulsive and compulsive behaviors in Parkinson’s disease: a one-year follow-up study. J. Neurol. Sci. 2011;310:197–201. doi: 10.1016/j.jns.2011.05.044. [DOI] [PubMed] [Google Scholar]
- Baddeley A. The central executive: a concept and some misconceptions. J Int Neuropsychol Soc. 1998;4:523–526. doi: 10.1017/s135561779800513x. [DOI] [PubMed] [Google Scholar]
- Balzer-Geldsetzer M, et al. Parkinson’s disease and dementia: a longitudinal study (DEMPARK) Neuroepidemiology. 2011;37:168–176. doi: 10.1159/000331490. [DOI] [PubMed] [Google Scholar]
- Bédard MA, Pillon B, Dubois B, Duchesne N, Masson H, Agid Y. Acute and long-term administration of anticholinergics in Parkinson’s disease: specific effects on the subcortico-frontal syndrome. Brain Cogn. 1999;40:289–313. doi: 10.1006/brcg.1999.1083. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Hendrickson R, Ivanco LS, Lopresti BJ, Constantine GM, Mathis CA, Davis JG, Moore RY, Dekosky ST. Cognitive correlates of cortical cholinergic denervation in Parkinson’s disease and parkinsonian dementia. J. Neurol. 2006;253:242–247. doi: 10.1007/s00415-005-0971-0. [DOI] [PubMed] [Google Scholar]
- Bohnen NI, Kaufer DI, Ivanco LS, Lopresti B, Koeppe RA, Davis JG, Mathis CA, Moore RY, DeKosky ST. Cortical cholinergic function is more severely affected in parkinsonian dementia than in Alzheimer disease: an in vivo positron emission tomographic study. Arch. Neurol. 2003;60:1745–1748. doi: 10.1001/archneur.60.12.1745. [DOI] [PubMed] [Google Scholar]
- Bosboom JLW, Stoffers D, Wolters EC. The role of acetylcholine and dopamine in dementia and psychosis in Parkinson’s disease. J. Neural Transm. 2003;(Suppl.):185–195. doi: 10.1007/978-3-7091-0643-3_11. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn. Sci. (Regul. Ed.) 2004;8:539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Braak H, Rüb U, Del Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson’s disease. J. Neurol. Sci. 2006;248:255–258. doi: 10.1016/j.jns.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, De Vos RAI, Jansen Steur ENH, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Cohen JD. Cognition and control in schizophrenia: a computational model of dopamine and prefrontal function. Biological Psychiatry. 1999;46:312–328. doi: 10.1016/s0006-3223(99)00116-x. [DOI] [PubMed] [Google Scholar]
- Burack MA, Hartlein J, Flores HP, Taylor-Reinwald L, Perlmutter JS, Cairns NJ. In vivo amyloid imaging in autopsy-confirmed Parkinson disease with dementia. Neurology. 2010;74:77–84. doi: 10.1212/WNL.0b013e3181c7da8e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- Caballol N, Martí MJ, Tolosa E. Cognitive dysfunction and dementia in Parkinson disease. Mov. Disord. 2007;22(Suppl 17):S358–366. doi: 10.1002/mds.21677. [DOI] [PubMed] [Google Scholar]
- Chaudhuri KR, Schapira AHV. Non-motor symptoms of Parkinson’s disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8:464–474. doi: 10.1016/S1474-4422(09)70068-7. [DOI] [PubMed] [Google Scholar]
- Claassen DO, Josephs KA, Ahlskog JE, Silber MH, Tippmann-Peikert M, Boeve BF. REM sleep behavior disorder preceding other aspects of synucleinopathies by up to half a century. Neurology. 2010;75:494–499. doi: 10.1212/WNL.0b013e3181ec7fac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compta Y, Parkkinen L, O’Sullivan SS, Vandrovcova J, Holton JL, Collins C, Lashley T, Kallis C, Williams DR, De Silva R, Lees AJ, Revesz T. Lewy- and Alzheimer-type pathologies in Parkinson’s disease dementia: which is more important? Brain. 2011;134:1493–1505. doi: 10.1093/brain/awr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R. Dopaminergic modulation of cognitive function-implications for L-DOPA treatment in Parkinson’s disease. Neurosci Biobehav Rev. 2006;30:1–23. doi: 10.1016/j.neubiorev.2005.03.024. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb. Cortex. 2001;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol. Psychiatry. 2011;69:e113–125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125:584–594. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson’s disease and its relationship to motor disability. Brain. 1991;114(Pt 5):2095–2122. doi: 10.1093/brain/114.5.2095. [DOI] [PubMed] [Google Scholar]
- Coull JT, Cheng R-K, Meck WH. Neuroanatomical and neurochemical substrates of timing. Neuropsychopharmacology. 2011;36:3–25. doi: 10.1038/npp.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crizzle AM, Classen S, Uc EY. Parkinson disease and driving: an evidence-based review. Neurology. 2012;79:2067–2074. doi: 10.1212/WNL.0b013e3182749e95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, Livingston L, MacAskill MR, Graham C, Melzer TR, Porter RJ, Watts R, Anderson TJ. Characterizing mild cognitive impairment in Parkinson’s disease. Mov. Disord. 2011;26:629–636. doi: 10.1002/mds.23592. [DOI] [PubMed] [Google Scholar]
- Dalrymple-Alford JC, MacAskill MR, Nakas CT, Livingston L, Graham C, Crucian GP, Melzer TR, Kirwan J, Keenan R, Wells S, Porter RJ, Watts R, Anderson TJ. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–1725. doi: 10.1212/WNL.0b013e3181fc29c9. [DOI] [PubMed] [Google Scholar]
- Dias EC, McGinnis T, Smiley JF, Foxe JJ, Schroeder CE, Javitt DC. Changing plans: neural correlates of executive control in monkey and human frontal cortex. Exp Brain Res. 2006;174:279–291. doi: 10.1007/s00221-006-0444-4. [DOI] [PubMed] [Google Scholar]
- Dirnberger G, Frith CD, Jahanshahi M. Executive dysfunction in Parkinson’s disease is associated with altered pallidal-frontal processing. Neuroimage. 2005;25:588–599. doi: 10.1016/j.neuroimage.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Bench CJ, Liddle PF, Friston KJ, Frith CD, Grasby PM, Frackowiak RS. Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? J. Neurol. Neurosurg. Psychiatr. 1993;56:1290–1294. doi: 10.1136/jnnp.56.12.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domellöf ME, Elgh E, Forsgren L. The relation between cognition and motor dysfunction in drug-naive newly diagnosed patients with Parkinson’s disease. Mov. Disord. 2011;26:2183–2189. doi: 10.1002/mds.23814. [DOI] [PubMed] [Google Scholar]
- Dubois B, Danzé F, Pillon B, Cusimano G, Lhermitte F, Agid Y. Cholinergic-dependent cognitive deficits in Parkinson’s disease. Ann. Neurol. 1987;22:26–30. doi: 10.1002/ana.410220108. [DOI] [PubMed] [Google Scholar]
- Dubois B, Pillon B. Do cognitive changes of Parkinson’s disease result from dopamine depletion? J. Neural Transm. 1995;(Suppl. 45):27–34. [PubMed] [Google Scholar]
- Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J. Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- Dunning CJR, Reyes JF, Steiner JA, Brundin P. Can Parkinson’s disease pathology be propagated from one neuron to another? Prog. Neurobiol. 2012;97:205–219. doi: 10.1016/j.pneurobio.2011.11.003. [DOI] [PubMed] [Google Scholar]
- Dymecki J, Lechowicz W, Bertrand E, Szpak GM. Changes in dopaminergic neurons of the mesocorticolimbic system in Parkinson’s disease. Folia Neuropathol. 1996;34:102–106. [PubMed] [Google Scholar]
- Ekman U, Eriksson J, Forsgren L, Mo SJ, Riklund K, Nyberg L. Functional brain activity and presynaptic dopamine uptake in patients with Parkinson’s disease and mild cognitive impairment: a cross-sectional study. Lancet Neurol. 2012;11:679–687. doi: 10.1016/S1474-4422(12)70138-2. [DOI] [PubMed] [Google Scholar]
- Emre M, Aarsland D, Albanese A, Byrne EJ, Deuschl G, De Deyn PP, Durif F, Kulisevsky J, Van Laar T, Lees A, Poewe W, Robillard A, Rosa MM, Wolters E, Quarg P, Tekin S, Lane R. Rivastigmine for dementia associated with Parkinson’s disease. N. Engl. J. Med. 2004;351:2509–2518. doi: 10.1056/NEJMoa041470. [DOI] [PubMed] [Google Scholar]
- Fabbrini G, Barbanti P, Aurilia C, Pauletti C, Lenzi GL, Meco G. Donepezil in the treatment of hallucinations and delusions in Parkinson’s disease. Neurol. Sci. 2002;23:41–43. doi: 10.1007/s100720200022. [DOI] [PubMed] [Google Scholar]
- Factor SA, Feustel PJ, Friedman JH, Comella CL, Goetz CG, Kurlan R, Parsa M, Pfeiffer R. Longitudinal outcome of Parkinson’s disease patients with psychosis. Neurology. 2003;60:1756–1761. doi: 10.1212/01.wnl.0000068010.82167.cf. [DOI] [PubMed] [Google Scholar]
- Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K. Levodopa and the progression of Parkinson’s disease. N. Engl. J. Med. 2004;351:2498–2508. doi: 10.1056/NEJMoa033447. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Agbokou C, Gauthier S. Recognition and management of neuropsychiatric complications in Parkinson’s disease. CMAJ. 2006;175:1545–1552. doi: 10.1503/cmaj.060542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Phillips AG. Delay-dependent modulation of memory retrieval by infusion of a dopamine D1 agonist into the rat medial prefrontal cortex. Behav. Neurosci. 2001;115:934–939. [PubMed] [Google Scholar]
- Foltynie T, Brayne CEG, Robbins TW, Barker RA, The CamPaIGN study The cognitive ability of an incident cohort of Parkinson’s patients in the UK. Brain. (2004)(a);127:550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Goldberg TE, Lewis SGJ, Blackwell AD, Kolachana BS, Weinberger DR, Robbins TW, Barker RA. Planning ability in Parkinson’s disease is influenced by the COMT val158met polymorphism. Mov. Disord. (2004)(b);19:885–891. doi: 10.1002/mds.20118. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Is choline acetyltransferase present in synaptic vesicles? Biochem. Pharmacol. 1966;15:1641–1643. doi: 10.1016/0006-2952(66)90216-4. [DOI] [PubMed] [Google Scholar]
- Forsaa EB, Larsen JP, Wentzel-Larsen T, Alves G. What predicts mortality in Parkinson disease?: a prospective population-based long-term study. Neurology. (2010)(a);75:1270–1276. doi: 10.1212/WNL.0b013e3181f61311. [DOI] [PubMed] [Google Scholar]
- Forsaa EB, Larsen JP, Wentzel-Larsen T, Goetz CG, Stebbins GT, Aarsland D, Alves G. A 12-year population-based study of psychosis in Parkinson disease. Arch. Neurol. (2010)(b);67:996–1001. doi: 10.1001/archneurol.2010.166. [DOI] [PubMed] [Google Scholar]
- Fujita M, Ichise M, Zoghbi SS, Liow J-S, Ghose S, Vines DC, Sangare J, Lu J-Q, Cropley VL, Iida H, Kim KM, Cohen RM, Bara-Jimenez W, Ravina B, Innis RB. Widespread decrease of nicotinic acetylcholine receptors in Parkinson’s disease. Ann. Neurol. 2006;59:174–177. doi: 10.1002/ana.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster J. 4th Edition Academic Press; New York, NY: 2008. The Prefrontal Cortex. [Google Scholar]
- Gaspar P, Bloch B, Le Moine C. D1 and D2 receptor gene expression in the rat frontal cortex: cellular localization in different classes of efferent neurons. Eur. J. Neurosci. 1995;7:1050–1063. doi: 10.1111/j.1460-9568.1995.tb01092.x. [DOI] [PubMed] [Google Scholar]
- Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatr. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Nan B, Wang C-N, Wang X, Junck L, Chervin RD, Consens F, Bhaumik A. Cerebral cortical and subcortical cholinergic deficits in parkinsonian syndromes. Neurology. 2010;74:1416–1423. doi: 10.1212/WNL.0b013e3181dc1a55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman JG, Litvan I. Mild cognitive impairment in Parkinson’s disease. Minerva Med. 2011;102:441–459. [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv. Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Castner SA, Svensson TH, Siever LJ, Williams GV. Targeting the dopamine D1 receptor in schizophrenia: insights for cognitive dysfunction. Psychopharmacology (Berl.) 2004;174:3–16. doi: 10.1007/s00213-004-1793-y. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. “Frontal” cognitive function in patients with Parkinson’s disease “on” and “off” levodopa. Brain. 1988;111(Pt 2):299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Graybiel AM, Aosaki T, Flaherty AW, Kimura M. The basal ganglia and adaptive motor control. Science. 1994;265:1826–1831. doi: 10.1126/science.8091209. [DOI] [PubMed] [Google Scholar]
- Harrington DL, Castillo GN, Greenberg PA, Song DD, Lessig S, Lee RR, Rao SM. Neurobehavioral mechanisms of temporal processing deficits in Parkinson’s disease. PLoS ONE. 2011;6:e17461. doi: 10.1371/journal.pone.0017461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson P, Meara J. Risk and incidence of dementia in a cohort of older subjects with Parkinson’s disease in the United Kingdom. Mov. Disord. 2004;19:1043–1049. doi: 10.1002/mds.20216. [DOI] [PubMed] [Google Scholar]
- Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage. 2007;34:714–723. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J. Neurol. Neurosurg. Psychiatr. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, Lee VM-Y, Leverenz JB, Montine TJ, Duda JE, Hurtig HI, Trojanowski JQ. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 2012;72:587–598. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahanshahi M, Jones CRG, Zijlmans J, Katzenschlager R, Lee L, Quinn N, Frith CD, Lees AJ. Dopaminergic modulation of striato-frontal connectivity during motor timing in Parkinson’s disease. Brain. 2010;133:727–745. doi: 10.1093/brain/awq012. [DOI] [PubMed] [Google Scholar]
- Javoy-Agid F, Agid Y. Is the mesocortical dopaminergic system involved in Parkinson disease? Neurology. 1980;30:1326–1330. doi: 10.1212/wnl.30.12.1326. [DOI] [PubMed] [Google Scholar]
- Javoy-Agid F, Ploska A, Agid Y. Microtopography of tyrosine hydroxylase, glutamic acid decarboxylase, and choline acetyltransferase in the substantia nigra and ventral tegmental area of control and Parkinsonian brains. J. Neurochem. 1981;37:1218–1227. doi: 10.1111/j.1471-4159.1981.tb04672.x. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Post mortem studies in Parkinson’s disease--is it possible to detect brain areas for specific symptoms? J. Neural Transm. 1999;(Suppl. 56):1–29. doi: 10.1007/978-3-7091-6360-3_1. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim. Biophys. Acta. 2009;1792:730–740. doi: 10.1016/j.bbadis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Synuclein deposition and non-motor symptoms in Parkinson disease. J. Neurol. Sci. 2011;310:107–111. doi: 10.1016/j.jns.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Någren K, Hietala J, Oikonen V, Vilkman H, Farde L, Halldin C, Rinne JO. Extrastriatal dopamine D2 and D3 receptors in early and advanced Parkinson’s disease. Neurology. 2000;54:1482–1487. doi: 10.1212/wnl.54.7.1482. [DOI] [PubMed] [Google Scholar]
- Kaasinen V, Nurmi E, Brück A, Eskola O, Bergman J, Solin O, Rinne JO. Increased frontal [(18)F]fluorodopa uptake in early Parkinson’s disease: sex differences in the prefrontal cortex. Brain. 2001;124:1125–1130. doi: 10.1093/brain/124.6.1125. [DOI] [PubMed] [Google Scholar]
- Kaltenboeck A, Johnson SJ, Davis MR, Birnbaum HG, Carroll CA, Tarrants ML, Siderowf AD. Direct costs and survival of medicare beneficiaries with early and advanced Parkinson’s disease. Parkinsonism Relat. Disord. 2012;18:321–326. doi: 10.1016/j.parkreldis.2011.11.015. [DOI] [PubMed] [Google Scholar]
- Kempster PA, O’Sullivan SS, Holton JL, Revesz T, Lees AJ. Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain. 2010;133:1755–1762. doi: 10.1093/brain/awq059. [DOI] [PubMed] [Google Scholar]
- Kieburtz K, McDermott M, Como P, Growdon J, Brady J, Carter J, Huber S, Kanigan B, Landow E, Rudolph A, Parkinson Study Group The effect of deprenyl and tocopherol on cognitive performance in early untreated Parkinson’s disease. Neurology. 1994;44:1756–1759. doi: 10.1212/wnl.44.9.1756. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Oh Y-S, Lee K-S, Kim Y-I, Yang D-W, Goldstein DS. Association of cognitive dysfunction with neurocirculatory abnormalities in early Parkinson disease. Neurology. 2012;79:1323–1331. doi: 10.1212/WNL.0b013e31826c1acd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopp W. Psychiatric changes in patients treated with levodopa. I. The clinical experiment. Neurology. 1970;20:23–30. [PubMed] [Google Scholar]
- Kotzbauer PT, Cairns NJ, Campbell MC, Willis AW, Racette BA, Tabbal SD, Perlmutter JS. Pathologic Accumulation of α-Synuclein and Aβ in Parkinson Disease Patients With Dementia. Arch. Neurol. 2012:1–6. doi: 10.1001/archneurol.2012.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM. L-dopa withdrawal in Parkinson’s disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology (Berl.) 1992;107:394–404. doi: 10.1007/BF02245167. [DOI] [PubMed] [Google Scholar]
- Lee J-Y, Kim J-M, Kim JW, Cho J, Lee WY, Kim H-J, Jeon BS. Association between the dose of dopaminergic medication and the behavioral disturbances in Parkinson disease. Parkinsonism Relat. Disord. 2010;16:202–207. doi: 10.1016/j.parkreldis.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Lennox G. Lewy body dementia. Baillieres Clin Neurol. 1992;1:653–676. [PubMed] [Google Scholar]
- Levy G, Tang M-X, Louis ED, Côté LJ, Alfaro B, Mejia H, Stern Y, Marder K. The association of incident dementia with mortality in PD. Neurology. 2002;59:1708–1713. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- Lippa CF, et al. DLB and PDD boundary issues: diagnosis, treatment, molecular pathology, and biomarkers. Neurology. 2007;68:812–819. doi: 10.1212/01.wnl.0000256715.13907.d3. [DOI] [PubMed] [Google Scholar]
- Litvan I, Aarsland D, Adler CH, Goldman JG, Kulisevsky J, Mollenhauer B, Rodriguez-Oroz MC, Tröster AI, Weintraub D. MDS Task Force on mild cognitive impairment in Parkinson’s disease: critical review of PD-MCI. Mov. Disord. 2011;26:1814–1824. doi: 10.1002/mds.23823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvan I, Mohr E, Williams J, Gomez C, Chase TN. Differential memory and executive functions in demented patients with Parkinson’s and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1991;54:25–29. doi: 10.1136/jnnp.54.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM-Y. Pathological α-Synuclein Transmission Initiates Parkinson-like Neurodegeneration in Nontransgenic Mice. Science. (2012)(a);338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VMY. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. (2012)(b);209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- Meyer PM, Strecker K, Kendziorra K, Becker G, Hesse S, Woelpl D, Hensel A, Patt M, Sorger D, Wegner F, Lobsien D, Barthel H, Brust P, Gertz HJ, Sabri O, Schwarz J. Reduced alpha4beta2*-nicotinic acetylcholine receptor binding and its relationship to mild cognitive and depressive symptoms in Parkinson disease. Arch. Gen. Psychiatry. 2009;66:866–877. doi: 10.1001/archgenpsychiatry.2009.106. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Monastero R, Di Fiore P, Ventimiglia GD, Ventimiglia CC, Battaglini I, Camarda R, Camarda C. Prevalence and profile of mild cognitive impairment in Parkinson’s disease. Neurodegener Dis. 2012;10:187–190. doi: 10.1159/000335909. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Shi M, Quinn JF, Peskind ER, Craft S, Ginghina C, Chung KA, Kim H, Galasko DR, Jankovic J, Zabetian CP, Leverenz JB, Zhang J. CSF Aβ(42) and tau in Parkinson’s disease with cognitive impairment. Mov. Disord. 2010;25:2682–2685. doi: 10.1002/mds.23287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison CE, Borod JC, Brin MF, Hälbig TD, Olanow CW. Effects of levodopa on cognitive functioning in moderate-to-severe Parkinson’s disease (MSPD) J Neural Transm. 2004;111:1333–1341. doi: 10.1007/s00702-004-0145-8. [DOI] [PubMed] [Google Scholar]
- Müller T, Benz S, Börnke C. Delay of simple reaction time after levodopa intake. Clin Neurophysiol. 2001;112:2133–2137. doi: 10.1016/s1388-2457(01)00653-8. [DOI] [PubMed] [Google Scholar]
- Müller T, Welnic J, Fuchs G, Baas H, Ebersbach G, Reichmann H. The DONPAD-study--treatment of dementia in patients with Parkinson’s disease with donepezil. J. Neural Transm. 2006;(Suppl.):27–30. doi: 10.1007/978-3-211-33328-0_3. [DOI] [PubMed] [Google Scholar]
- Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- Narayanan NS, Land BB, Solder JE, Deisseroth K, Dileone RJ. Prefrontal D1 dopamine signaling is required for temporal control. Proc. Natl. Acad. Sci. U.S.A. 2012 doi: 10.1073/pnas.1211258109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Top-down control of motor cortex ensembles by dorsomedial prefrontal cortex. Neuron. 2006;52:921–931. doi: 10.1016/j.neuron.2006.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Laubach M. Delay activity in rodent frontal cortex during a simple reaction time task. J. Neurophysiol. 2009;101:2859–2871. doi: 10.1152/jn.90615.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan NS, Prabhakaran V, Bunge SA, Christoff K, Fine EM, Gabrieli JDE. The role of the prefrontal cortex in the maintenance of verbal working memory: an event-related FMRI analysis. Neuropsychology. 2005;19:223–232. doi: 10.1037/0894-4105.19.2.223. [DOI] [PubMed] [Google Scholar]
- Okubo Y, Suhara T, Suzuki K, Kobayashi K, Inoue O, Terasaki O, Someya Y, Sassa T, Sudo Y, Matsushima E, Iyo M, Tateno Y, Toru M. Decreased prefrontal dopamine D1 receptors in schizophrenia revealed by PET. Nature. 1997;385:634–636. doi: 10.1038/385634a0. [DOI] [PubMed] [Google Scholar]
- Pascual-Sedano B, Kulisevsky J, Barbanoj M, García-Sánchez C, Campolongo A, Gironell A, Pagonabarraga J, Gich I. Levodopa and executive performance in Parkinson’s disease: a randomized study. J Int Neuropsychol Soc. 2008;14:832–841. doi: 10.1017/S1355617708081010. [DOI] [PubMed] [Google Scholar]
- Perry EK, Curtis M, Dick DJ, Candy JM, Atack JR, Bloxham CA, Blessed G, Fairbairn A, Tomlinson BE, Perry RH. Cholinergic correlates of cognitive impairment in Parkinson’s disease: comparisons with Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatr. 1985;48:413–421. doi: 10.1136/jnnp.48.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AG, Ahn S, Floresco SB. Magnitude of dopamine release in medial prefrontal cortex predicts accuracy of memory on a delayed response task. J. Neurosci. 2004;24:547–553. doi: 10.1523/JNEUROSCI.4653-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poewe W, Wolters E, Emre M, Onofrj M, Hsu C, Tekin S, Lane R. Long-term benefits of rivastigmine in dementia associated with Parkinson’s disease: an active treatment extension study. Mov. Disord. 2006;21:456–461. doi: 10.1002/mds.20700. [DOI] [PubMed] [Google Scholar]
- Polito C, Berti V, Ramat S, Vanzi E, De Cristofaro MT, Pellicanò G, Mungai F, Marini P, Formiconi AR, Sorbi S, Pupi A. Interaction of caudate dopamine depletion and brain metabolic changes with cognitive dysfunction in early Parkinson’s disease. Neurobiol. Aging. 2012;33:206.e29–39. doi: 10.1016/j.neurobiolaging.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Pontone G, Williams JR, Bassett SS, Marsh L. Clinical features associated with impulse control disorders in Parkinson disease. Neurology. 2006;67:1258–1261. doi: 10.1212/01.wnl.0000238401.76928.45. [DOI] [PubMed] [Google Scholar]
- Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, Dagher A, Jenkins IH, Friston KJ, Brooks DJ. Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson’s disease A 3D [(18)F]dopa-PET study. Brain. 1999;122(Pt 9):1637–1650. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- Reading PJ, Luce AK, McKeith IG. Rivastigmine in the treatment of parkinsonian psychosis and cognitive impairment: preliminary findings from an open trial. Mov. Disord. 2001;16:1171–1174. doi: 10.1002/mds.1204. [DOI] [PubMed] [Google Scholar]
- Rowan E, McKeith IG, Saxby BK, O’Brien JT, Burn D, Mosimann U, Newby J, Daniel S, Sanders J, Wesnes K. Effects of donepezil on central processing speed and attentional measures in Parkinson’s disease with dementia and dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2007;23:161–167. doi: 10.1159/000098335. [DOI] [PubMed] [Google Scholar]
- Santangelo G, Trojano L, Vitale C, Ianniciello M, Amboni M, Grossi D, Barone P. A neuropsychological longitudinal study in Parkinson’s patients with and without hallucinations. Mov. Disord. 2007;22:2418–2425. doi: 10.1002/mds.21746. [DOI] [PubMed] [Google Scholar]
- Sawada Y, Nishio Y, Suzuki K, Hirayama K, Takeda A, Hosokai Y, Ishioka T, Itoyama Y, Takahashi S, Fukuda H, Mori E. Attentional set-shifting deficit in Parkinson’s disease is associated with prefrontal dysfunction: an FDG-PET study. PLoS ONE. 2012;7:e38498. doi: 10.1371/journal.pone.0038498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawamoto N, Piccini P, Hotton G, Pavese N, Thielemans K, Brooks DJ. Cognitive deficits and striato-frontal dopamine release in Parkinson’s disease. Brain. 2008;131:1294–1302. doi: 10.1093/brain/awn054. [DOI] [PubMed] [Google Scholar]
- Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- Schmitt FA, Farlow MR, Meng X, Tekin S, Olin JT. Efficacy of rivastigmine on executive function in patients with Parkinson’s disease dementia. CNS Neurosci Ther. 2010;16:330–336. doi: 10.1111/j.1755-5949.2010.00182.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamans JK, Floresco SB, Phillips AG. D1 receptor modulation of hippocampal-prefrontal cortical circuits integrating spatial memory with executive functions in the rat. J. Neurosci. 1998;18:1613–1621. doi: 10.1523/JNEUROSCI.18-04-01613.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seong HJ, Carter AG. D1 receptor modulation of action potential firing in a subpopulation of layer 5 pyramidal neurons in the prefrontal cortex. J. Neurosci. 2012;32:10516–10521. doi: 10.1523/JNEUROSCI.1367-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Spaendonck KP, Berger HJ, Horstink MW, Buytenhuijs EL, Cools AR. Executive functions and disease characteristics in Parkinson’s disease. Neuropsychologia. 1996;34:617–626. doi: 10.1016/0028-3932(95)00159-x. [DOI] [PubMed] [Google Scholar]
- Stolwyk RJ, Triggs TJ, Charlton JL, Iansek R, Bradshaw JL. Impact of internal versus external cueing on driving performance in people with Parkinson’s disease. Mov. Disord. 2005;20:846–857. doi: 10.1002/mds.20420. [DOI] [PubMed] [Google Scholar]
- Uc EY, McDermott MP, Marder KS, Anderson SW, Litvan I, Como PG, Auinger P, Chou KL, Growdon JC. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009;73:1469–1477. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Qian S, Rodnitzky RL, Dawson JD. Visual dysfunction in Parkinson disease without dementia. Neurology. 2005;65:1907–1913. doi: 10.1212/01.wnl.0000191565.11065.11. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Driving with distraction in Parkinson disease. Neurology. 2006;67:1774–1780. doi: 10.1212/01.wnl.0000245086.32787.61. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Impaired navigation in drivers with Parkinson’s disease. Brain. 2007;130:2433–2440. doi: 10.1093/brain/awm178. [DOI] [PubMed] [Google Scholar]
- Uc EY, Rizzo M, Johnson AM, Emerson JL, Liu D, Mills ED, Anderson SW, Dawson JD. Real-life driving outcomes in Parkinson disease. Neurology. 2011;76:1894–1902. doi: 10.1212/WNL.0b013e31821d74fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, Duff-Canning S, De Souza M, Fox S, Lang AE, Miyasaki J. Prospective prevalence of pathologic gambling and medication association in Parkinson disease. Neurology. (2006)(a);66:1750–1752. doi: 10.1212/01.wnl.0000218206.20920.4d. [DOI] [PubMed] [Google Scholar]
- Voon V, Hassan K, Zurowski M, De Souza M, Thomsen T, Fox S, Lang AE, Miyasaki J. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. (2006)(b);67:1254–1257. doi: 10.1212/01.wnl.0000238503.20816.13. [DOI] [PubMed] [Google Scholar]
- Voon V, Sohr M, Lang AE, Potenza MN, Siderowf AD, Whetteckey J, Weintraub D, Wunderlich GR, Stacy M. Impulse control disorders in Parkinson disease: a multicenter case--control study. Ann. Neurol. 2011;69:986–996. doi: 10.1002/ana.22356. [DOI] [PubMed] [Google Scholar]
- Vrieze E, Ceccarini J, Pizzagalli DA, Bormans G, Vandenbulcke M, Demyttenaere K, Van Laere K, Claes S. Measuring extrastriatal dopamine release during a reward learning task. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Vijayraghavan S, Goldman-Rakic PS. Selective D2 receptor actions on the functional circuitry of working memory. Science. 2004;303:853–856. doi: 10.1126/science.1091162. [DOI] [PubMed] [Google Scholar]
- Weinberger DR, Berman KF, Zec RF. Physiologic dysfunction of dorsolateral prefrontal cortex in schizophrenia. I. Regional cerebral blood flow evidence. Arch. Gen. Psychiatry. 1986;43:114–124. doi: 10.1001/archpsyc.1986.01800020020004. [DOI] [PubMed] [Google Scholar]
- Weintraub D, Koester J, Potenza MN, Siderowf AD, Stacy M, Voon V, Whetteckey J, Wunderlich GR, Lang AE. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch. Neurol. 2010;67:589–595. doi: 10.1001/archneurol.2010.65. [DOI] [PubMed] [Google Scholar]
- Weintraub DSA. ASsociation of dopamine agonist use with impulse control disorders in parkinson disease. Arch Neurol. 2006;63:969–973. doi: 10.1001/archneur.63.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Williams SM, Goldman-Rakic PS. Widespread origin of the primate mesofrontal dopamine system. Cereb. Cortex. 1998;8:321–345. doi: 10.1093/cercor/8.4.321. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Brayne CEG, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Foltynie T, Lewis SJG, Barker RA. Cognitive deficits and psychosis in Parkinson’s disease: a review of pathophysiology and therapeutic options. CNS Drugs. 2006;20:477–505. doi: 10.2165/00023210-200620060-00004. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson’s disease is dependent on COMT val 158 met genotype. Brain. 2008;131:397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- Wolk SI, Douglas CJ. Clozapine treatment of psychosis in Parkinson’s disease: a report of five consecutive cases. J Clin Psychiatry. 1992;53:373–376. [PubMed] [Google Scholar]
- Wu K, Keeffe D, Politis M, O’Keeffe GC, Robbins TW, Bose SK, Brooks DJ, Piccini P, Barker RA. The catechol-O-methyltransferase Val(158)Met polymorphism modulates fronto-cortical dopamine turnover in early Parkinson’s disease: a PET study. Brain. 2012;135:2449–2457. doi: 10.1093/brain/aws157. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ. [Accessed September 28, 2012];Personalized medicine strategies for managing patients with Parkinsonism and cognitive deficits. Metab. Clin. Exp. 2012 doi: 10.1016/j.metabol.2012.08.025. Available at: http://www.ncbi.nlm.nih.gov/pubmed/22999712. [DOI] [PubMed]
- Zgaljardic DJ, Borod JC, Foldi NS, Mattis PJ, Gordon MF, Feigin A, Eidelberg D. An examination of executive dysfunction associated with frontostriatal circuitry in Parkinson’s disease. J Clin Exp Neuropsychol. 2006;28:1127–1144. doi: 10.1080/13803390500246910. [DOI] [PMC free article] [PubMed] [Google Scholar]


