Abstract
ErbB2 tyrosine kinase inhibitors (TKI) block tyrosine autophosphorylation and activation of the full-length transmembrane ErbB2 receptor (p185ErbB2). In addition to p185ErbB2 truncated forms of ErbB2 exist in breast cancer cell lines and clinical tumors. The contribution of these truncated forms, specifically those expressed in tumor cell nuclei, to the development of therapeutic resistance to ErbB2 TKIs has not been previously demonstrated. Here we show that expression of a 95 kDa tyrosine phosphorylated form of ErbB2, herein referred to as p95L (lapatinib-induced p95) was increased in ErbB2+ breast cancer cells treated with potent ErbB2 TKIs (lapatinib, GW2974). Expressed in tumor cell nuclei, tyrosine phosphorylation of p95L was resistant to inhibition by ErbB2 TKIs. Furthermore, the expression of p95L was increased in ErbB2+ breast cancer models of acquired therapeutic resistance to lapatinib that mimic the clinical setting. Pretreatment with proteasome inhibitors blocked p95L induction in response to ErbB2 TKIs, implicating the role of the proteasome in the regulation of p95L expression. In addition, tyrosine phosphorylated c-terminal fragments of ErbB2, generated by alternate initiation of translation and similar in molecular weight to p95L, were expressed in tumor cell nuclei, where they too were resistant to inhibition by ErbB2 TKIs. When expressed in the nuclei of lapatinib sensitive ErbB2+ breast cancer cells, truncated ErbB2 rendered cells resistant to lapatinib-induced apoptosis. Elucidating the function of nuclear truncated forms of ErbB2, and developing therapeutic strategies to block their expression and/or activation, may enhance the clinical efficacy of ErbB2 TKIs.
Keywords: Truncated, nuclear, ErbB2, resistance, tyrosine kinase inhibitors
Introduction
ErbB2, a 185 kDa transmembrane receptor tyrosine kinase (p185ErbB2), is deregulated in 25% of all breast cancers, where it predicts for a poor clinical outcome.(1) ErbB2 activation requires autophosphorylation of tyrosine (Y) residues within the cytoplasmic domain of the receptor e.g. Y1248.(2) These phosphotyrosine residues serve as docking sites for adaptor proteins that link ErbB2 to downstream mitogen activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K) signaling networks that promote the growth and survival of breast cancer cells.(2-6) In addition to p185ErbB2, truncated forms of ErbB2 lacking all or most of the N-terminus extracellular domain (ECD) exist in ErbB2+ breast cancer cell lines and clinical tumors.(7-10) The most extensively studied truncated forms retain the transmembrane region and are expressed at the cell surface. Historically referred to as “p95”, truncated forms of ErbB2 expressed at the cell surface form heterodimers with other ErbB receptors,(11) and interact with the p85 subunit of PI3K,(12) thereby activating downstream signal transduction cascades in a manner similar to p185ErbB2. The generation of p95 has been shown to be dependent upon metalloproteinase activity.(7) P95 positive breast cancers exhibit an aggressive clinical phenotype characterized by an increased incidence of lymph node involvement at the time of initial diagnosis,(13, 14) and are more resistant to trastuzumab since they lack the ECD.(15, 16)
Lapatinib is a highly selective small molecule inhibitor of the ErbB2 and EGFR tyrosine kinases. Inhibition of ErbB2 tyrosine autophosphorylation by lapatinib leads to the inactivation of downstream cell growth and survival signals.(17-19) Although a significant advancement in the treatment of breast cancer, the clinical efficacy of lapatinib has been limited by the development of acquired therapeutic resistance.(20, 21) To address this problem, we generated clinically relevant models of acquired resistance to lapatinib using human ErbB2+ breast cancer cell lines.(22, 23)
We now show that treatment with ErbB2 TKIs increased the expression of a tyrosine phosphorylated, truncated form of ErbB2 that was expressed in the nuclei of ErbB2+ breast cancer cells, which will herein be referred to as p95L (lapatinib-induced p95). In contrast to truncated forms of ErbB2 expressed at the cell surface, the phosphorylation of p95L, and similar truncated forms that were also expressed in tumor cell nuclei, was resistant to ErbB2 TKI. The data supporting the activation and nuclear localization of p95L in response to ErbB2 TKI, and the role of nuclear, truncated forms of ErbB2 in the development of therapeutic resistance to ErbB2 TKIs, will be discussed.
Materials & Methods
Cell culture and reagents
BT474, SKBR3, Au565, MCF7, and T47D breast cancer cell lines were obtained from the American Type Culture Collection (Manassas, VA). Lapatinib resistant breast cancer cells were generated as previously described.(22) All cells were cultured as previously described.(11, 17, 22) No independent authentication of these cells was done by the authors. Anti-phosphotyrosine (p-tyr) antibody, GW2974, and calpain inhibitor 1 were purchased from Sigma-Aldrich (St. Louis, MO). Anti-c-ErbB2 (Ab -11) monoclonal antibody was from Neo Markers (Union City, CA). Anti-ErbB2 (AA1243-1255) and anti-phospho-ErbB2 (Y1248) antibodies were from Upstate Biotechnology (Lake Placid, NY). MG132, gamma-secretase inhibitor, and lactacystin were from Calbiochem (San Diego, CA). BB94 (Batimastat) was from Kimia Corp (Santa Clara, CA). Protein G agarose was purchased from Boehringer Mannheim (Germany). IRDye800 conjugated affinity purified anti-rabbit IgG and anti-mouse IgG were from Rockland (Gilbertsville, PA). Alexa Fluor680 goat anti-rabbit IgG was obtained from Molecular Probes (Eugene, OR). Lapatinib (GW572016), N-{3-Chloro-4-[(3-fluorobenzyl)oxy]phenyl}-6-[5-({[2(methylsulfonyl)ethyl]amino}methyl)-2-furyl]-4-quinazolinamine, was purchased from LC Laboratories (Woburn, CA). Lapatinib for cell culture work was dissolved in DMSO (0.01%).
Isolation of nuclear extracts, SDS-PAGE, and Western blot analysis
Details of cell fractionation, immunoprecipitation, SDS-PAGE, and Western blot analysis were previously described.(22) Membranes were probed with specific antibodies recognizing target proteins, and visualized using the Odyssey Infrared Imaging System (LI-COR, Inc., Lincoln, NE). Membranes were incubated with fluorescent-labeled secondary antibody at a 1:10000 dilution with 3% BSA in PBS for 60 min protected from light. After washing in PBS + 0.1% tween-20, the membranes were scanned using an Odyssey imaging system.
Human tumor xengrafts, animal treatment, and human tumor biopsies
NOD.CB17-Prkdcscid/J (NOD/SCID) mice were purchased from Jackson Labs (Bar Harbor, ME) and bred in the Duke Comprehensive Cancer Center Isolation Facility. BT474 and rBT474 cells were suspended in Hank's Balanced Salt Solution and mixed with Matrigel (BD Biosciences, San Jose, CA) at 1:1 ratio to make final concentrations of 1×104 cells/50μl. Fifty μl of tumor cell suspension was inoculated into bilateral mammary fat pads of female NOD/SCID mice (5∼6 weeks old, 4 mice/group). Animals were treated with lapatinib (75 mg/kg/day) by oral gavage until they were sacrificed. Tumor dimensions were measured serially, and tumor volumes calculated using the following formula: long axis × (short axis)2 × 0.52. The mice were euthanized with CO2 inhalation and tumor xenografts excised 59 days after implantation of tumor cells. All animal studies were conducted in compliance with Duke animal care regulations. Human biopsies were collected from breast cancer skin metastasis after informed written consent was obtained as part of an IRB approved tissue collection protocol. Tumor specimens were flash frozen in liquid nitrogen, and stored at −80 °C. Tissue extracts were prepared for Western blot analysis by homogenization in RIPA buffer at 4°C.
Expression of truncated forms of ErbB2 in human breast cancer cell lines
C-terminal fragments (c-611; c-676 and c-678) were generated based on ErbB2 open reading frames from LTR-2/ErbB2(8) and subcloned into the pcDNA 3.1 (+). C-611, c-676 and c-678 were subcloned into the pcDNA3.1 vector (Invitrogen) with forward primers: 5′-ACAAGCTT ACCATGCCCATCTGGAAG-3′, 5′-ACAAGCTTACCATGAAGCGACGGCAGCA-3 and 5′-ACAAGCTT ACCATGCGGAGACTGCTG-3′, and reverse primer: 5′-AACTCGAG TCACACTGGCACGTCCAG-3′. MCF-7 and T47D breast cancer cells were transfected with empty vector alone (controls) or the same vector containing p185ErbB2 or the various CTF's using the Lipofectamine™ 2000 Reagent from Invitrogen (Carlsbad, CA) according to the manufacturer's protocol. Stably transfected cells were selected using G418 (400 μg/ml) and the expression levels of CTF's were confirmed by Western blot analysis.
Immunofluorescence microscopy
Cells were cultured in 6 well plates with or without the indicated treatments. After washing with PBS, cells were fixed with 4% paraformaldehyde for 30 min, permeabilized with 0.2% Triton X-100 for 20 min, and blocked with 2% BSA in PBS at room temperature followed by washing with PBS and incubated with anti-ErbB2 or anti-phosphotyrosine specific antibodies overnight at 4°C. After extensive washings, the cells were incubated with FITC-conjugated swine anti-rabbit or rabbit anti-mouse antibodies followed by counterstaining with 1.5 μg/ml DAPI from Vector Labs (Burlingame, CA). An Olympus L Fluoview FV1000 was used for all photographs.
Proliferation and apoptosis assay
The proliferation assay was carried out in a 96 well plate format in a final volume of 100 ul/well cell culture medium with the cell proliferation reagent WST-1 from Roche Diagnostics (Mannheim, Germany). Details of the WST-1 profileration and annexin V/nexin 7-AAD apoptosis assays were previously published.(17, 22)
Statistical analysis
Data were expressed as means with standard error bars included. Student's t-test was used to determine statistical significance between 2 groups. P<0.05 was considered a statistically significant difference.
Results
ErbB2 TKIs increase the expression of phospho-p95L in tumor cell nuclei
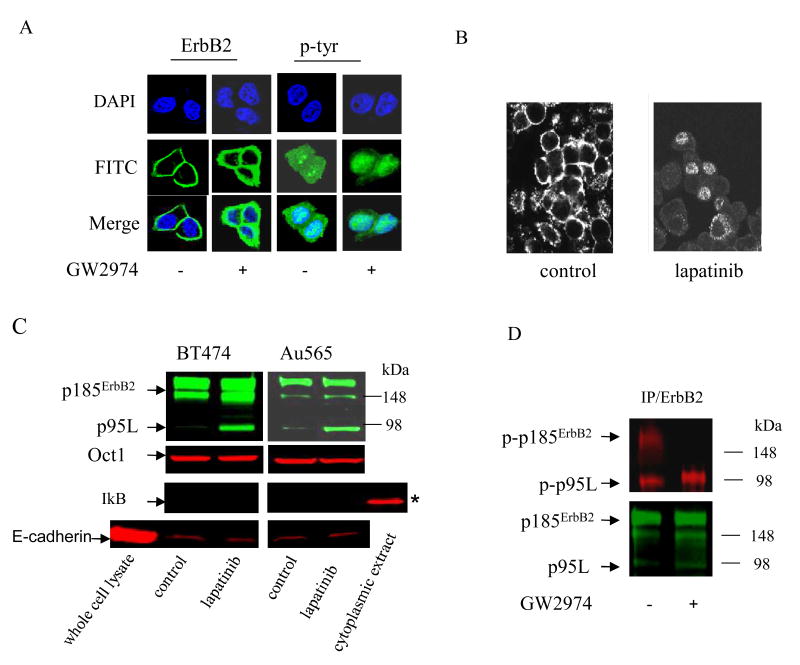
The effects of ErbB2 TKI on ErbB2 tyrosine phosphorylation were determined in BT474 cells, a human ErbB2+ breast cancer cell line, using immunofluorescence microscopy (IF). Total ErbB2 protein and phosphotyrosine expression were determined using an ErbB2 specific antibody and a phosphotyrosine (p-tyr) antibody, respectively. ErbB2 and p-tyr signals were visualized using a secondary FITC-conjugated antibody (green). Total ErbB2 expression was unchanged in response to GW2974, an ErbB2 TKI (Figure 1A). The p-tyr signal primarily localized to the cell surface and cytoplasm in vehicle treated controls (-). Relatively little p-tyr signal was seen in the nuclei (blue/DAPI) of control cells (Merge). Whereas cell surface and cytoplasmic p-tyr were markedly reduced in response to GW2974, nuclear p-tyr persisted (Figure 1A, Merge). We treated another ErbB2+ breast cancer cell line, Au565, with lapatinib and examined phospho-ErbB2 (p-ErbB2) expression using an ErbB2 phosphotyrosine specific antibody and a FITC-conjugated secondary antibody (green). Similar to BT474 cells, p-ErbB2 at the cell surface, but not in the nuclei of some ErbB2 cells, was markedly reduced by lapatinib, (Figure 1B).
Figure 1. Phosphorylation of nuclear truncated ErbB2 is resistant to ErbB2 TKI.
(A) BT474 cells were treated for 48 h with GW2974 (1 μM) or vehicle alone (-). Total ErbB2 and phosphotyrosine (p-tyr) signals (green) were visualized by IF microscopy as described in Methods. Cell nuclei were counterstained blue with DAPI. The lower row merges FITC and DAPI signals. (B) Au565 cells were treated with lapatinib (1 μM) or vehicle alone (control) for 24 h, and p-ErbB2 was assessed by IF microscopy using an ErbB2 phosphotyrosine specific primary antibody and a FITC-conjugated secondary antibody. (C) Steady-state protein levels of p185ErbB2 and p95L were determined in nuclear extracts from BT474 and Au565 cells treated for 24 h with lapatinib (500 nM) or vehicle alone (control). Steady-state protein levels of Oct 1, IκB, and E-cadherin, which represent nuclear, cytoplasmic, and cell membrane proteins, respectively, were used to confirm the purity of nuclear extracts. (D) Au565 cells were treated with GW2974 (1 μM) or vehicle alone (-) for 24 h. Steady-state levels of total and phosphorylated p185ErbB2 and p95L were analyzed by ErbB2 IP/Western blot from nuclear extracts. Total (green) and phosphorylated (red) forms of the indicated proteins are shown. Cells treated with vehicle alone (0.01% DMSO) served as controls for all of the experiments in Figure 1.
We next isolated nuclear extracts from BT474 and Au565 cells treated with vehicle alone (controls) or lapatinib. The purity of nuclear extracts was confirmed using Oct 1, IkB, and E-cadherin, which represent nuclear, cytoplasmic, and cell membrane proteins, respectively. Steady-state levels of total p95L protein increased in lapatinib-treated cells without an appreciable change in p185ErbB2 (Figure 1C). Additional molecular weight bands >98 kDa, which have been seen previously in ErbB2 blots, were observed.
In Figure 1D, total ErbB2 protein was immunoprecipitated (IP) from nuclear extracts isolated from Au565 cells treated with vehicle alone (control) or GW2974. Steady-state levels of total (green) and phosphorylated (red) p185ErbB2 and p95L were determined by Western blot. Although both p185ErbB2 and p95L were expressed in a phosphorylated state, GW2974 inhibited phosphorylation of p185ErbB2 but not p95L. Similar results were seen in other ErbB2+ breast cancer cell lines (data not shown).
Increased expression of p95L in lapatinib resistant breast cancer cell lines and tumor xenografts
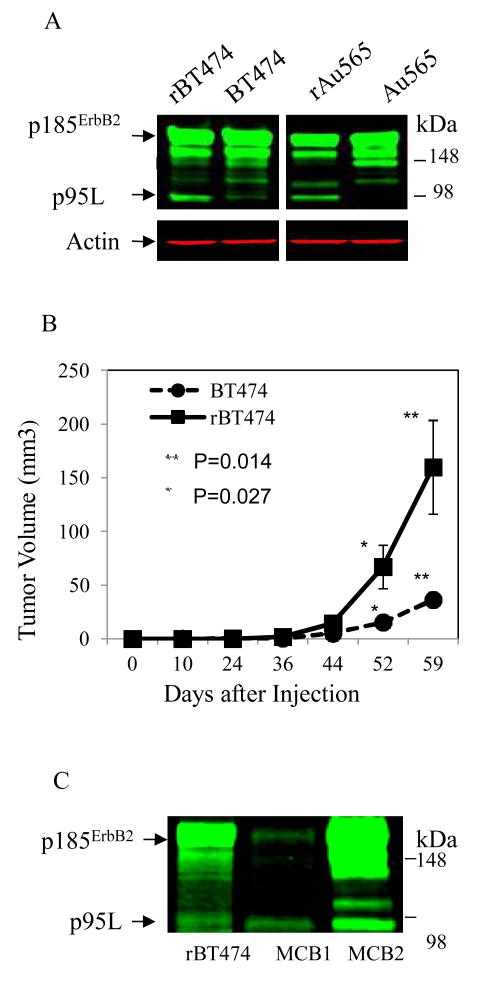
P95L protein levels were increased in models of acquired resistance to lapatinib (e.g. rBT474; rAu565)(22) compared to their lapatinib-sensitive cell counterparts (Figure 2A). In Figure 2B, the growth of tumor xenografts established from resistant cells (rBT474) was significantly increased compared with tumors derived from parental cells (BT474), in animals treated with lapatinib (p < 0.05). Steady-state p95L protein levels were increased in rBT474 compared with BT474 tumor xenografts (data not shown).
Figure 2. Increased expression of p95L in ErbB2+ breast cancer models of acquired lapatinib resistance and clinical tumor samples.
(A) Steady-state protein levels of p185ErbB2 and p95L in rBT474 and rAu565 cells and their lapatinib-sensitive parental cell counterparts (BT474, Au565) were determined by Western blot. Actin steady-state protein levels served as a control for equal loading of protein. The results are representative of three independent experiments. (B) Tumor xenografts from rBT474 and BT474 cells were established bilaterally in mammary fat pads of NOD/SCID female mice treated with lapatinib 75 mg/kg/day by oral gavage for 59 days. Four mice were in each group, with 2 tumors in each mouse. Mean tumor volume (mm3) for rBT474 and BT474 are indicated (with error bars). Differences in mean tumor volumes between the two groups were statistically significant (p<0.05). (C) Total ErbB2 steady-state protein levels were analyzed in two metastatic sites of ErbB2+ breast cancer (MCB1, MCB2) that had progressed in women taking lapatinib. Cell lysate from rBT474 is shown with p95L indicated.
To determine whether a truncated form(s) of ErbB2 similar to p95L could be detected in clinical tumors, we analyzed steady-state ErbB2 protein levels in biopsies from metastatic breast cancer sites that had developed while patients were on lapatinib therapy. A truncated form of ErbB2, similar in molecular weight to p95L in rBT474 cells, was seen in both clinical samples (Figure 2C, MCB1 and 2).
Comparison of p95L with c-terminal fragments of ErbB2 generated by alternate initiation of translation
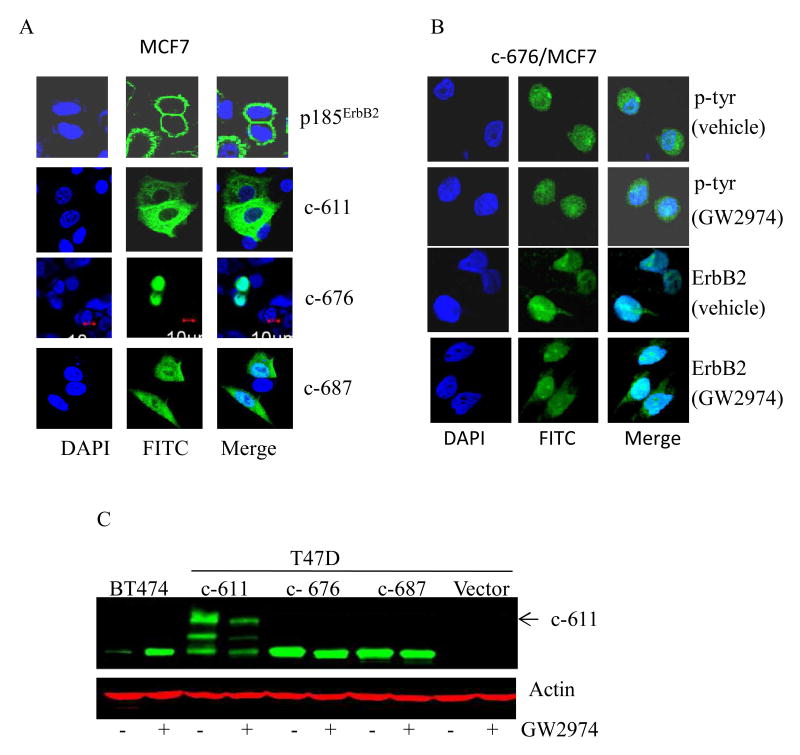
C-terminal fragments (CTFs) of ErbB2 generated by alternate initiation of translation (e.g. c-611; c-676; c-687) have been reported.(8) C-611 lacks most of the ECD, while c-676 and c-687 lack the ECD and transmembrane regions. We synthesized CTF's by alternate initiation of translation from methionines 611 (c-611) and 676 (c-676), and used the pcDNA3.1 vector to express them in non-ErbB2 overexpressing MCF7 and T47D breast cancer cells. In Figure 3A, the subcellular localization of p185ErbB2, c-611, c-676, and c-687 expressed in MCF7 transfected cells was determined by IF microscopy using an ErbB2 specific primary and FITC-conjugated secondary antibody (green). While c-611 localized to the cell membrane and cytoplasm, c-676 was seen primarily in tumor cell nuclei (blue). In Figure 3B, the effects of GW2974 on the phosphorylation of c-676 expressed in MCF7 transfected cells were examined by IF microscopy using a phosphotyrosine (p-tyr) antibody and FITC-conjugated secondary antibody (green). Phosphorylation of nuclear c-676 was not inhibited by GW2974. The effect of GW2974 on steady-state phosphoprotein levels of the indicated CTFs was next determined by Western blot using an ErbB2 phosphotyrosine specific antibody in whole cell extracts from T47D cells transfected with c-611, c-676, c-687, or vector alone (Figure 3C). GW2974 inhibited tyrosine phosphorylation of c-611, but not c-676 or c-687. Expression of p95L in BT474 cells treated with GW2974 was included as a reference. Similar results were observed in MCF7 transfected cells (data not shown).
Figure 3. Similarity of p95L induced by ErbB2 TKI and ErbB2 c-terminal fragments (CTFs) generated by alternate translation initiation.
(A) The subcellular localization of the indicated CTF (c-611; c-676; c687) and p185ErbB2 expressed in MCF7 cells was determined by IF microscopy using an ErbB2 specific antibody and a FITC-conjugated secondary antibody (green). Nuclei were counterstained blue with DAPI and the overlap of DAPI and FITC is shown (Merge). (B) MCF7 cells expressing c-676 were treated for 24 h with GW2974 (8 μM) or vehicle alone (DMSO). The subcellular localization of p-tyr and c-676 signals was determined by IF microscopy using phosphotyrosine (p-tyr) and ErbB2 specific antibodies, respectively, and visualized with a FITC-conjugated secondary antibody (green). The overlap between DAPI and FITC is shown (Merge). (C) Phospho-ErbB2 steady-state protein levels (green) were determined by Western blot in T47D cells transfected with the indicated CTF's following treatment with GW2974 (8 μM) or vehicle (DMSO) alone for 24 h. Actin (red) steady-state protein levels served as a control for equal loading of protein. The arrow indicates the mature form of c-611. Results shown in Figure 3 are representative of three independent experiments.
Proteasome inhibitors block p95L induction by ErbB2 TKI
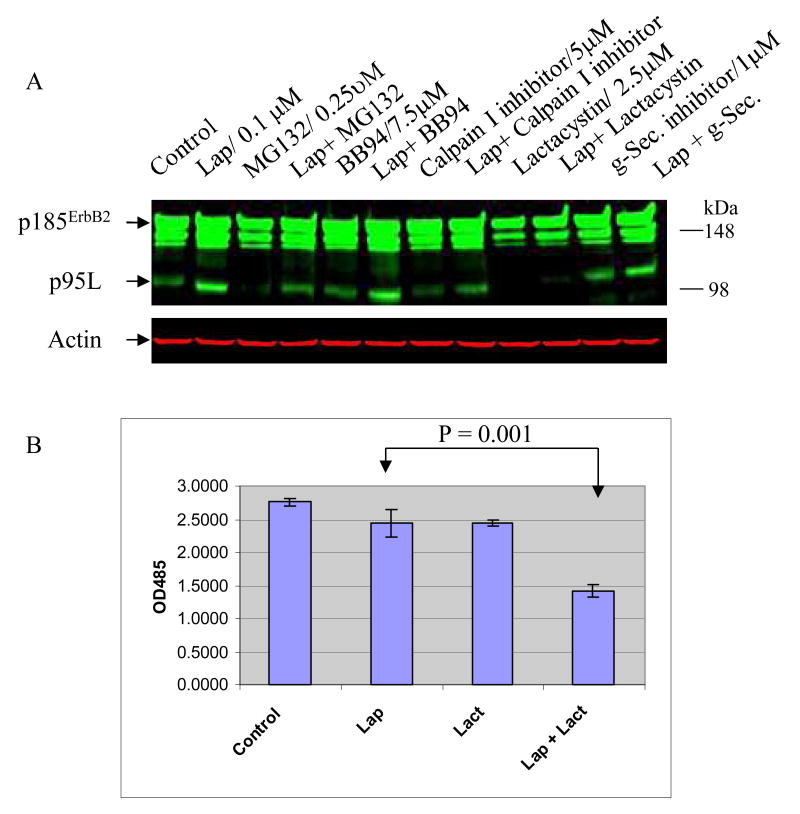
We examined the effects of protease inhibitors on p95L expression in lapatinib-treated Au565 cells. Cells were treated as indicated in Figure 4A. Briefly, cells were treated with (i) lapatinib alone, (ii) the indicated protease inhibitors alone, or (24) a combination of lapatinib plus protease inhibitor. Included among the protease inhibitors were BB-94, a metalloproteinase inhibitor that blocked phorbol ester-induced p95 expression,(7) and a γ secretase inhibitor that reduced ErbB4 truncation.(25) BB-94 and the γ secretase inhibitor had little effect on the induction of p95L by lapatinib (Figure 4A). However, inhibitors of the 20S proteasomal subunit (lactacystin, MG132, calpain I inhibitor) blocked the induction of p95L in lapatinib-treated Au565 cells (Figure 4A). Cells treated with vehicle alone served as controls.
Figure 4. Increased p95L following treatment with ErbB2 TKI is proteasome-dependent.
(A) Au565 cells were treated lapatinib in the presence or absence of the indicated protease inhibitors. The concentrations of lapatinib and individual protease inhibitors used alone, are shown. The same concentrations were used in combination treatments. After 72 h, cells were harvested and p185ErbB2 and p95L steady-state protein levels were assessed (green). Actin steady-state protein levels served controls for equal loading of protein. (B) Effects of lapatinib (0.1 μM) alone, lactacystin (2.5 μM) alone, and lapatinib (0.1 μM) + lactacystin (2.5 μM) on Au565 cell proliferation after 24 h. Cells treated with 0.01% DMSO served as controls. Studies were conducted in triplicates with error bars included. Differences were statistically significant (p = 0.001). Results were confirmed in three independent experiments.
Treatment with lactacystin alone, at the same concentration that blocked induction of p95L, had relatively little antitumor activity in Au565 cells (Figure 4B). However, there was enhanced antitumor activity when lactacystin was combined with a sub-lethal concentration of lapatinib (0.1 μM) that was otherwise sufficient to induce p95L.
Expression of truncated ErbB2 reduces the antitumor activity of lapatinib
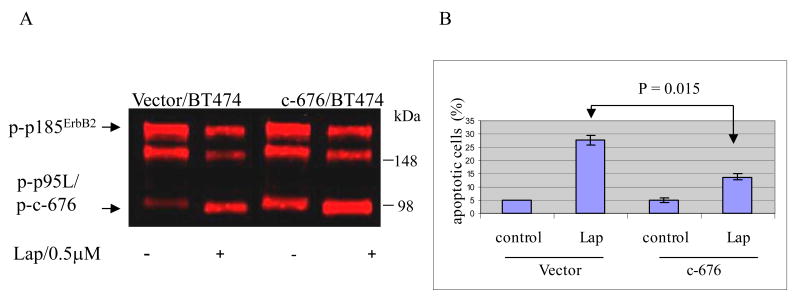
To determine the impact of nuclear, truncated forms of ErbB2 on the antitumor activity of lapatinib, we expressed c-676 in BT474 cells (Figure 5A). We chose c-676 because of its similarities to p95L e.g. molecular weight, nuclear localization, resistant to ErbB2 TKI. Using an ErbB2 phosphotyrosine specific antibody in Western blot analysis, we found that lapatinib increased steady-state p95L phosphoprotein levels in cells transfected with vector alone (Figure 5A). In contrast, the phosphorylation of c-676 and p95L was unaffected by lapatinib. Cells transfected with vector alone served as controls.
Figure 5. Expressing of c-676 reduces BT474 cells sensitivity to lapatinib.
(A) BT474 cells transiently transfected with c-676 were treated for 48 h with 0.5 μM lapatinib or vehicle (DMSO) alone (-). Steady-state phosphoprotein levels of p185ErbB2 and p95L/c-676 were determined using an ErbB2 phosphotyrosine specific antibody. Cells transfected with vector alone served as controls. (B) Apoptosis was assessed by annexin V staining and flow cytometry in c-676 expressing BT474 cells treated as described above. Cells transfected with vector alone served as controls. Experiments were conducted in triplicates with error bars included. Results were statistically significant (p=0.015). The data is representative of three independent experiments.
Importantly, BT474 cells, which are normally highly sensitive to the antitumor effects of lapatinib, became significantly more resistant to lapatinib after c-676 was expressed in the nuclei of BT474 cells (p= 0.015) (Figure 5B). Cells transfected with vector alone served as controls.
Discussion
The development of acquired therapeutic resistance represents a significant barrier limiting the clinical efficacy of lapatinib. Acquired resistance to lapatinib does not appear to be related to loss of target sensitivity as tyrosine phosphorylation of p185ErbB2 remains inhibited in ErbB2+ breast cancer cells that have developed resistance to lapatinib.(22, 26) We now show that p95L is expressed in the nuclei of ErbB2+ breast cancer cells, in a tyrosine phosphorylated, presumably activated state. In contrast to truncated forms of ErbB2 expressed at the cell surface, the phosphorylation of truncated ErbB2 in the nucleus was resistant to ErbB2 TKIs. Importantly, expression of a truncated form of ErbB2 (c-676) in the nuclei of ErbB2+ breast cancer cells rendered cells resistant to the antitumor effects of lapatinib (Figure 5).
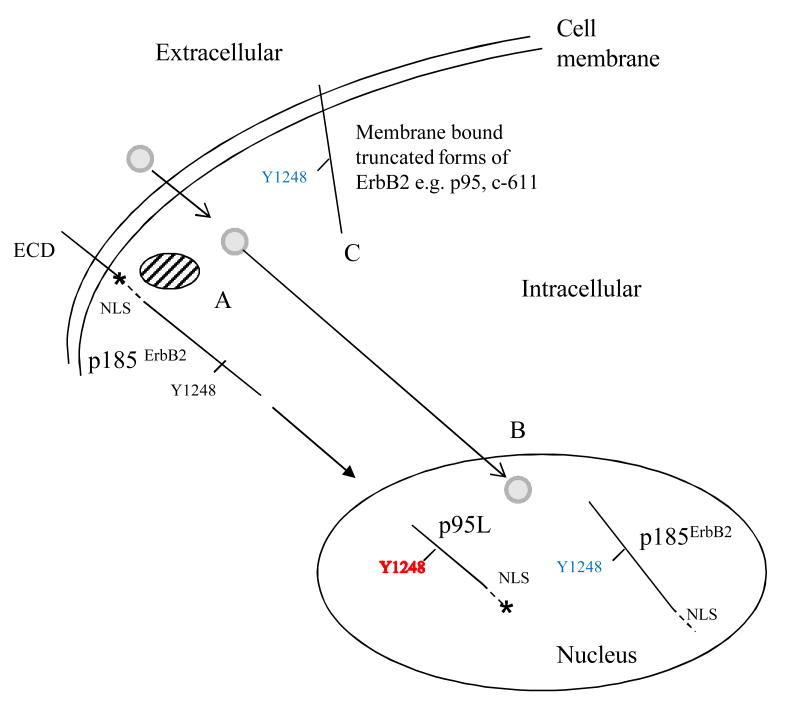
Distinct forms of ErbB2 differing in their (i) subcellular localization, (ii) regulation of expression, and (iii) sensitivity to ErbB2 targeted therapies, exist in breast cancer cells. Expressed at the cell surface, p95 and c-611 mediate resistance to trastuzumab, but not ErbB2 TKIs.(11, 15) In contrast, truncated forms of ErbB2 expressed in tumor cell nuclei in a tyrosine phosphorylated state, are resistant to inhibition by ErbB2 TKIs. Whereas some nuclear truncated forms of ErbB2 are generated through alternate initiation of translation,(8) p95L appears to be mediated by the activation of the proteasome, as proteasome inhibitors block its induction (Figure 4A). As summarized in Figure 6, cleavage at a putative proteasome recognition site located within the intracellular domain (see *) would generate a truncated form of ErbB2 with a predicted molecular weight similar to p95L. This truncated form contains tyrosine autophosphorylation sites (e.g. Y1248) and the nuclear localization signal (NLS). In the nucleus, tyrosine phosphorylation (Y1248) of p95L is resistant to lapatinib (B). In contrast, phosphorylation of p185ErbB2, which is also expressed in the nucleus, is inhibited by lapatinib. The role of the proteasome in the induction of p95L is consistent with recent findings from our laboratory showing evidence of proteasome activation in lapatinib-treated ErbB2+ breast cancer cells.(27) It is tempting to speculate that deregulation of intracellular calcium, which occurs in lapatinib-treated ErbB2+ cells,(28) leads to the accumulation of unfolded proteins, which in turn activates the proteasome.
Figure 6. Summary of our working model.
(A) The proteasome (hatched circle) is activated in ErbB2+ breast cancer cells treated with lapatinib (solid circle). As a consequence, p185ErbB2 is cleaved at a putative proteasome recognition site (*), generating a truncated molecule (p95L) that retains the nuclear localization signal (NLS) and tyrosine autophosphorylation sites (e.g. Y1248). (B) P95L localizes to tumor cell nuclei where Y1248 phosphorylation is resistant to lapatinib (Y1248 +). In contrast, the tyrosine phosphorylation of nuclear p185ErbB2 is inhibited by lapatinib (Y1248 -). (C) Truncated forms of ErbB2 expressed at the cell surface e.g. p95, c-611 are inhibited by lapatinib (Y1248).
It is not clear why the phosphorylation of truncated ErbB2 in the nucleus is resistant to ErbB2 TKI. One potential explanation is that the structural conformation of p95L and c-676 differs from p185ErbB2, preventing ErbB2 TKIs to gain access to their active sites. Studies to elucidate the structural conformation of nuclear, truncated forms of ErbB2 should help answer this question.
Although the function of nuclear, truncated forms of ErbB2 is unknown, insight from studies of p185ErbB2 may be informative. When expressed at the cell surface, p185ErbB2 promotes tumor growth and survival by activating downstream cell signaling cascades.(2-6) In contrast, nuclear p185ErbB2 directly regulates gene transcription.(29-31) Nuclear p185ErbB2 has been shown to activate thymidylate synthase gene transcription, which is blocked by lapatinib.(29) This is consistent with our observation that phosphorylation of nuclear p185ErbB2 is inhibited by lapatinib, presumably abrogating its transcriptional activity. It is tempting to speculate that phosphorylated forms of truncated ErbB2 expressed in tumor cell nuclei are also involved in regulating gene transcription, especially in light of the proteasome-dependent regulation of p95L. Although generally associated with complete proteolysis of proteins, proteasomal processing has been shown to generate biologically active proteins, particularly those involved in regulating gene transcription.(32, 33) Studies to elucidate the function(s) of truncated nuclear forms of ErbB2 are warranted.
Patients take lapatinib on a chronic, daily basis. Our model would have predicted that this schedule would lead to the accumulation of p95L in breast cancer cells, thereby contributing to the development of acquired resistance. Results in models of acquired lapatinib resistance confirm this prediction (Figure 2A). In addition, lower molecular weight forms of ErbB2, similar to p95L, were expressed in clinical biopsies from ErbB2+ breast cancers that had progressed on lapatinib therapy (Figure 2C). Although intriguing, these findings will require confirmation in larger studies.
Although the exact function of nuclear, truncated forms of ErbB2 remains unknown, we have provided evidence supporting their role in the development of therapeutic resistance to lapatinib and GW2974 (Figure 5B). Strategies to enhance the clinical efficacy of ErbB2 TKIs may now include therapies that prevent induction of p95L and/or inactivate other truncated forms of ErbB2 that are expressed in tumor cell nuclei.
Acknowledgments
This work was supported by Department of Defense Breast Cancer Research Program (34 W81WXH-09-0065), Sisko Foundation and Balderacchi Gift (to N.L.S).
Abbreviations
- TKI
tyrosine kinase inhibitors
- p95L
lapatinib-induced p95
Footnotes
No author has a conflict of interest
References
- 1.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987 Jan 9;235(4785):177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Dankort D, Jeyabalan N, Jones N, Dumont DJ, Muller WJ. Multiple ErbB-2/Neu Phosphorylation Sites Mediate Transformation through Distinct Effector Proteins. J Biol Chem. 2001 Oct 19;276(42):38921–8. doi: 10.1074/jbc.M106239200. [DOI] [PubMed] [Google Scholar]
- 3.Dougall WC, Qian X, Peterson NC, Miller MJ, Samanta A, Greene MI. The neu-oncogene: signal transduction pathways, transformation mechanisms and evolving therapies. Oncogene. 1994 Aug;9(8):2109–23. [PubMed] [Google Scholar]
- 4.Olayioye MA, Graus-Porta D, Beerli RR, Rohrer J, Gay B, Hynes NE. ErbB-1 and ErbB-2 acquire distinct signaling properties dependent upon their dimerization partner. Mol Cell Biol. 1998 Sep;18(9):5042–51. doi: 10.1128/mcb.18.9.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000 Jul 3;19(13):3159–67. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001 Feb;2(2):127–37. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 7.Codony-Servat J, Albanell J, Lopez-Talavera JC, Arribas J, Baselga J. Cleavage of the HER2 ectodomain is a pervanadate-activable process that is inhibited by the tissue inhibitor of metalloproteases-1 in breast cancer cells. Cancer Res. 1999 Mar 15;59(6):1196–201. [PubMed] [Google Scholar]
- 8.Anido J, Scaltriti M, Bech Serra JJ, et al. Biosynthesis of tumorigenic HER2 C-terminal fragments by alternative initiation of translation. EMBO J. 2006 Jul 12;25(13):3234–44. doi: 10.1038/sj.emboj.7601191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin YZ, Clinton GM. A soluble protein related to the HER-2 proto-oncogene product is released from human breast carcinoma cells. Oncogene. 1991 Apr;6(4):639–43. [PubMed] [Google Scholar]
- 10.Zabrecky JR, Lam T, McKenzie SJ, Carney W. The extracellular domain of p185/neu is released from the surface of human breast carcinoma cells, SK-BR-3. J Biol Chem. 1991 Jan 25;266(3):1716–20. [PubMed] [Google Scholar]
- 11.Xia W, Liu LH, Ho P, Spector NL. Truncated ErbB2 receptor (p95ErbB2) is regulated by heregulin through heterodimer formation with ErbB3 yet remains sensitive to the dual EGFR/ErbB2 kinase inhibitor GW572016. Oncogene. 2004 Jan 22;23(3):646–53. doi: 10.1038/sj.onc.1207166. [DOI] [PubMed] [Google Scholar]
- 12.Chandarlapaty S, Scaltriti M, Angelini P, et al. Inhibitors of HSP90 block p95-HER2 signaling in Trastuzumab-resistant tumors and suppress their growth. Oncogene. 2010;29(3):325–34. doi: 10.1038/onc.2009.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saez R, Molina MA, Ramsey EE, et al. p95HER-2 predicts worse outcome in patients with HER-2-positive breast cancer. Clin Cancer Res. 2006 Jan 15;12(2):424–31. doi: 10.1158/1078-0432.CCR-05-1807. [DOI] [PubMed] [Google Scholar]
- 14.Christianson TA, Doherty JK, Lin YJ, et al. NH2-terminally truncated HER-2/neu protein: relationship with shedding of the extracellular domain and with prognostic factors in breast cancer. Cancer Res. 1998 Nov 15;58(22):5123–9. [PubMed] [Google Scholar]
- 15.Scaltriti M, Rojo F, Ocana A, et al. Expression of p95HER2, a truncated form of the HER2 receptor, and response to anti-HER2 therapies in breast cancer. J Natl Cancer Inst. 2007 Apr 18;99(8):628–38. doi: 10.1093/jnci/djk134. [DOI] [PubMed] [Google Scholar]
- 16.Pedersen K, Angelini PD, Laos S, et al. A naturally occurring HER2 carboxy-terminal fragment promotes mammary tumor growth and metastasis. Mol Cell Biol. 2009 Jun;29(12):3319–31. doi: 10.1128/MCB.01803-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene. 2002 Sep 12;21(41):6255–63. doi: 10.1038/sj.onc.1205794. [DOI] [PubMed] [Google Scholar]
- 18.Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001 Dec;1(2):85–94. [PubMed] [Google Scholar]
- 19.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib (GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006 Feb 1;66(3):1630–9. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 20.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006 Dec 28;355(26):2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 21.Johnston S, Trudeau M, Kaufman B, et al. Phase II study of predictive biomarker profiles for response targeting human epidermal growth factor receptor 2 (HER-2) in advanced inflammatory breast cancer with lapatinib monotherapy. J Clin Oncol. 2008 Mar 1;26(7):1066–72. doi: 10.1200/JCO.2007.13.9949. [DOI] [PubMed] [Google Scholar]
- 22.Xia W, Bacus S, Hegde P, et al. A model of acquired autoresistance to a potent ErbB2 tyrosine kinase inhibitor and a therapeutic strategy to prevent its onset in breast cancer. Proc Natl Acad Sci U S A. 2006 May 16;103(20):7795–800. doi: 10.1073/pnas.0602468103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia W, Bacus S, Husain I, et al. Resistance to ErbB2 tyrosine kinase inhibitors in breast cancer is mediated by calcium-dependent activation of RelA. Mol Cancer Ther. 2010 Feb;9(2):292–9. doi: 10.1158/1535-7163.MCT-09-1041. [DOI] [PubMed] [Google Scholar]
- 24.Burris HA, III, Storniolo AM, Overmoyer EA, et al. A phase I, open-label study of the safety, tolerability and pharmacokinetics of lapatinib (GW572016) in combination with trastuzumab. Breast Cancer Res Treat. 2004;88 [Google Scholar]
- 25.Ni CY, Murphy MP, Golde TE, Carpenter G. gamma -Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001 Dec 7;294(5549):2179–81. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 26.Spector NL, Xia W, Burris H, 3rd, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005 Apr 10;23(11):2502–12. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 27.Xia W, Bisi J, Strum J, et al. Regulation of survivin by ErbB2 signaling: therapeutic implications for ErbB2-overexpressing breast cancers. Cancer Res. 2006 Feb 1;66(3):1640–7. doi: 10.1158/0008-5472.CAN-05-2000. [DOI] [PubMed] [Google Scholar]
- 28.Spector NL, Yarden Y, Smith B, et al. Activation of AMP-activated protein kinase by human EGF receptor 2/EGF receptor tyrosine kinase inhibitor protects cardiac cells. Proc Natl Acad Sci U S A. 2007 Jun 19;104(25):10607–12. doi: 10.1073/pnas.0701286104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim HP, Yoon YK, Kim JW, et al. Lapatinib, a dual EGFR and HER2 tyrosine kinase inhibitor, downregulates thymidylate synthase by inhibiting the nuclear translocation of EGFR and HER2. PLoS One. 2009;4(6):e5933. doi: 10.1371/journal.pone.0005933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang SC, Lien HC, Xia W, et al. Binding at and transactivation of the COX-2 promoter by nuclear tyrosine kinase receptor ErbB-2. Cancer Cell. 2004 Sep;6(3):251–61. doi: 10.1016/j.ccr.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 31.Xie Y, Hung MC. Nuclear localization of p185neu tyrosine kinase and its association with transcriptional transactivation. Biochem Biophys Res Commun. 1994 Sep 30;203(3):1589–98. doi: 10.1006/bbrc.1994.2368. [DOI] [PubMed] [Google Scholar]
- 32.Hervas-Aguilar A, Rodriguez JM, Tilburn J, Arst HN, Jr, Penalva MA. Evidence for the direct involvement of the proteasome in the proteolytic processing of the Aspergillus nidulans zinc finger transcription factor PacC. J Biol Chem. 2007 Nov 30;282(48):34735–47. doi: 10.1074/jbc.M706723200. [DOI] [PubMed] [Google Scholar]
- 33.Tian L, Holmgren RA, Matouschek A. A conserved processing mechanism regulates the activity of transcription factors Cubitus interruptus and NF-kappaB. Nat Struct Mol Biol. 2005 Dec;12(12):1045–53. doi: 10.1038/nsmb1018. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann LC, Sellers TA, Schaid DJ, et al. Efficacy of bilateral prophylactic mastectomy in BRCA1 and BRCA2 gene mutation carriers. J Natl Cancer Inst. 2001 Nov 7;93(21):1633–7. doi: 10.1093/jnci/93.21.1633. [DOI] [PubMed] [Google Scholar]