Abstract
Although increasing evidence suggests a relationship between bacterial dysbiosis and colorectal cancer (CRC), few studies have identified specific microbes as etiologic factors. Recent studies have implicated overabundance of Fusobacterium species in association with colorectal adenomas and cancer. Two articles published in the current issue of Cell Host and Microbe provide insights into the mechanisms for this Fusobacterium-CRC relationship.
Several recent studies suggest a link between bacterial dysbiosis in the gut and colorectal adenomas and cancer [1–3]. However, the precise mechanisms by which bacterial dysbiosis may be related to colorectal cancer (CRC) are not known. Moreover, only a limited number of studies have linked specific bacteria to colorectal adenomas (CRA) and cancer (CRC). In particular, increased abundance of Fusobacterium sp. has been observed in colorectal adenomas and cancer [4–6]. However, the mechanism underlying this association has yet to be defined. It is also not clear whether Fusobacterium colonization is a consequence or a cause of CRC. In particular, it is uncertain whether Fusobacterium is only a commensal bacteria associated with the modified CRC microenvironment or an opportunistic pathogen, responsible for that modification and, in turn, promotes CRA and CRC.
In current issue of Cell Host and Microbe, two studies by Kostic et al.[7] and Rubinstein et al.[8] provide mechanistic insights into the relationship between Fusobacterium nucleatum and CRC. Through a series of elegant in vitro, in vivo animal experiments and human studies, the authors investigated the mechanisms by which F. nucleatum in the gut could be associated with CRC.
Kostic et al. [7]confirmed findings from previous studies that indicated that Fusobacterium is enriched in colorectal adenomas [6] and therefore may be involved in early tumorigenesis. They examined adenomas versus surrounding non-adenoma tissue and found that F. nucleatum was significantly more abundant in adenomas compared to surrounding normal tissue. Evaluation of stool samples from adenoma and CRC patients also showed increased enrichment of Fusobacterium sp. relative to healthy controls. Taken together, higher abundance of Fusobacterium in CRC tissue and feces of CRC patients may be a common feature of CRC.
In animal experiments using the APC Min mouse model of intestinal tumorigenesis, Kostic et al. [7] showed that F. nucleatum increased tumor burden in these mice as well as increased infiltration of myeloid immune cells and proinflammatory markers. They introduced human isolates of F. nucleatum, Streptococcus sp. (positive controls) or tryptic soy broth (TSB) into APC Min, IL-10−/− and Rag2−/−/T-bet−/− mice and observed that F. nucleatum treated mice had a higher number of colonic tumors than control mice. However, F. nucleatum did not induce colitis or promote tumorigenesis in the two models of colitis (IL-10−/− and Rag2−/−/T-bet−/−). This would suggest that F. nucleatum may not require an existing inflammatory environment to promote tumorigenesis but rather its presence induces inflammation and promotes CRC. As with all monoassociation experiments, these results must be taken with caution since the introduction of isolated strains does not represent what occurs in the complex microenvironment containing a whole microbiome consortium.
In order to understand how F. nucleatum induces inflammation and promotes CRC, Kostic et al. [7] evaluated whether F. nucleatum affected intratumoral immune cells in tumor tissue. They characterized infiltrating immune cells from intestinal tumors of APC Min animals and controls. They observed increased infiltrating cells of the myeloid lineage in tumors from F. nucleatum treated APC Min mice compared to controls. In particular, CD11b+ cells including macrophages, dendritic cells and granulocytes were significantly increased. Together, these findings suggest that F.nucleatum promotes inflammation and tumorigenesis by modulating the tumor immune microenvironment via expansion of myeloid derived immune cells. The findings from the mouse experiments were confirmed in human colon samples in which they observed a strong correlation between F. nucleatum abundance and expression of proinflammatory markers such as COX-2, IL-8, IL-6, IL-1β and TNF-α. These observations are compatible with previous reports [9, 10] and show a specific human expression profile associated with this strain of Fusobacterium, which was not shared by other bacterial species normally present in high numbers in the colon. Interestingly, the findings from Kostic et al.[7] suggest that F. nucleatum induces an NFκβ-driven pro-inflammatory response to promote CRC.
The second study authored by Rubinstein and colleagues provide further evidence as to the mechanisms by which Fusobacterium sp. may contribute to CRC. In particular, their report suggests that the well-studied FadA adhesion molecule encoded by Fusobacterium sp. have a role in the adhesion to and invasion of epithelial cells to promote inflammatory and pro-oncogenic responses, and stimulate epithelial cell growth. FadA is a virulence factor that is expressed on the bacterial cell surface. In both in vitro and animal studies in APC Min mice, they observed that binding of FadA to E-cadherin mediates attachment and invasion of F. nucleatum into epithelial cells. This, in turn, leads to activation β-catenin signaling and activates proinflammatory and oncogenic signals, namely, increased expression of transcription factors, oncogenes, Wnt signaling, inflammatory genes and growth stimulation of CRC cells. Further studies in humans suggest that FadA is elevated CRC tissue relative to normal tissue and increased FadA correlated with elevated expression of inflammatory genes including NFKB, IL-6, IL-8 and IL-18. These findings are similar to the observations of Kostic et al. [7]
Both studies used the APC mutation model (cell lines and animals) to assess the relationship between Fusobacterium sp. and CRC. Their observations would suggest that the APC mutation is a leading event and that promotion of oncogenesis by Fusobacterium sp. is a consequence. For example, cell lines with APC mutations (DLD1, SW-480, and HT-29) or β-catenin mutation (HCT-116) showed enhanced β-catenin activation and stimulated growth of CRC cells in the presence of either purified FadA or F. nucleatum. This was not observed in non-cancerous HEK-293 cells suggesting that promotion of tumorigenesis by F. nucleatum is subsequent to mutational events.
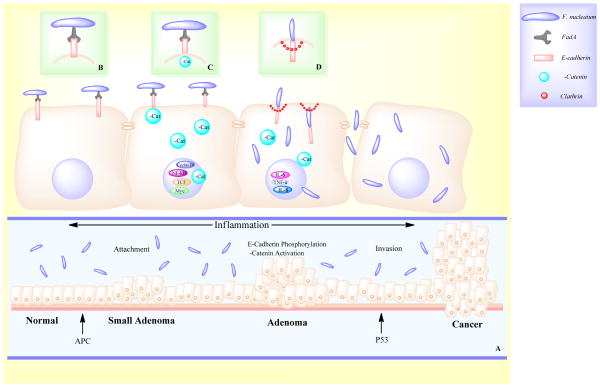
These results are intriguing and identify a potential contribution of Fusobacterium sp. to colorectal carcinogenesis. Most importantly, they provide mechanistic insights that suggest that Fusobacterium sp. actions are mediated via binding of FadA to receptors on host epithelial cells to alter barrier function, increase inflammation by modulating the tumor microenvironment, and activation of pro-oncogenic signals to promote CRC (Fig. 1). These findings have potential to impact CRC prevention, diagnosis and treatment, though additional studies are needed to evaluate the diagnostic potential of FadA.
Figure 1.
Potential mechanisms by which Fusobacterium promotes colorectal carcinogenesis. In the presence of APC mutation, F. nucleatum promotes oncogenesis in the colon via adhesin FadA through attachment, phosphorylation and invasion (A). FadA binds to E-cadherin on the surface of the cell, inhibiting its tumor-suppressor activity and activating β-catenin signaling (B). β-catenin phosphorylation is decreased, resulting in increased β-catenin in the cytoplasm and translocation into the nucleus where it modulates increased expression of LEF, TCF, NF-κβ and other oncogenes (C). F. nucleatum invades the cell via internalization of E-cadherin by clathrin (D) and stimulates expression of inflammatory genes.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sanapareddy N, et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 2012;6(10):1858–68. doi: 10.1038/ismej.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shen XJ, et al. Molecular characterization of mucosal adherent bacteria and associations with colorectal adenomas. Gut Microbes. 2010;1(3):138–47. doi: 10.4161/gmic.1.3.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sobhani I, et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS One. 2011;6(1):e16393. doi: 10.1371/journal.pone.0016393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Castellarin M, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 2012;22(2):299–306. doi: 10.1101/gr.126516.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kostic AD, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22(2):292–8. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCoy AN, et al. Fusobacterium is associated with colorectal adenomas. PLoS One. 2013;8(1):e53653. doi: 10.1371/journal.pone.0053653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostic AD, et al. Fusobacterium nucleatum Potentiates Intestinal Tumorigenesis and Modulates the Tumor-Immune Microenvironment. Cell Host Microbe. 2013;14(2):207–15. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinstein MR, et al. Fusobacterium nucleatum Promotes Colorectal Carcinogenesis by Modulating E-Cadherin/beta-Catenin Signaling via its FadA Adhesin. Cell Host Microbe. 2013;14(2):195–206. doi: 10.1016/j.chom.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dharmani P, et al. Fusobacterium nucleatum infection of colonic cells stimulates MUC2 mucin and tumor necrosis factor alpha. Infect Immun. 2011;79(7):2597–607. doi: 10.1128/IAI.05118-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grivennikov SI, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature. 2012;491(7423):254–8. doi: 10.1038/nature11465. [DOI] [PMC free article] [PubMed] [Google Scholar]