Abstract
Regulation of skeletal muscle capillarization involves distinct signaling pathways and growth factors including nitric oxide and vascular endothelial growth factor. Our understanding of this complex regulation continues to expand with the identification of new angiogenic growth factors. Future work needs to increase the use of advanced molecular techniques to expand our knowledge of the regulation of basal and exercise-induced capillarization.
Keywords: capillaries, exercise, mitochondria, muscle fiber size, VEGF, nitric oxide, 5′-AMP activated protein kinase
Introduction
Skeletal muscle accounts for 30–40% of body mass and is both heterogeneous and plastic. In adult humans, skeletal muscle contains a combination of different muscle fibers types: I, IIa, and IId/x. These different fiber types demonstrate different phenotypes with type I fibers having the greatest and IId/x fibers having the least mitochondrial volume and capillarization (1). Capillaries are important in the delivery of hormones and substrates such as O2, insulin, and glucose to resting and active muscle.
In skeletal muscle, exercise training increases muscle oxidative capacity (mitochondrial biogenesis) and increases the formation of new capillaries (angiogenesis) (1). Muscle unloading via hindlimb suspension reduces skeletal muscle capillarization (2) demonstrating that otherwise untrained muscle represents a condition of activity related to normal weight bearing activity. Increases in muscle capillarization with exercise training precede the exercise-induced fiber type transition from IIb+IId/x fibers to IIa (3) suggesting that angiogenesis is necessary to support the more oxidative muscle phenotype of exercise training. These observations provoke the question, which intracellular signals and angiogenic growth factors are responsible for the regulation of skeletal muscle capillarization.
In attempting to identify the intracellular signals involved in regulating skeletal muscle capillarization, it is critical to understand which factors are indeed regulated variables. In muscle, two intersecting characteristics of muscle fibers, namely oxidative capacity (mitochondrial volume) and fiber size (fiber volume) must be satisfied to provide the fiber with adequate ATP generation to meet metabolic demand and adequate strength to meet the challenges of everyday living. The interaction of these key variables was elegantly indentified in human muscle by Brodal et al. (1). Several relevant points are observed that are true both for untrained and endurance trained muscle. First, greater capillarization is present around fibers with greater mitochondrial content. Second, fiber type specific differences in the muscle capillary bed exist, likely owing to factors that regulate muscle fiber type and associated differences in mitochondrial content. Third, there are more capillaries surrounding larger than smaller fibers of a given fiber type. Fourth, endurance exercise training increases mitochondrial content and capillarization. Based on our work in clinical populations (aged and obese) (4, 5) and the seminal work of Brodal et al. (1) in untrained and trained individuals, we hypothesize that capillarization is regulated primarily by metabolic pathways in skeletal muscle, but that the size of the muscle fiber also influences the number of capillaries surrounding the fiber.
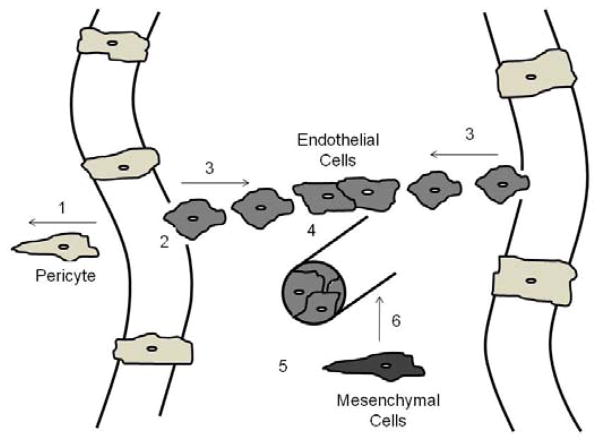
The majority of work on skeletal muscle capillaries has focused on angiogenesis. While our understanding of the steps involved in physiological muscle-specific angiogenesis remains limited, in general angiogenesis is known to depend upon the coordination of several independent processes as outlined in Figure 1.
Figure 1.
Steps and factors involved in angiogenesis. Step 1. Vessel destabilization through pericyte removal; angiopoietin (Ang) 2/Tie receptor for Ang1 and Ang2 (Tie2). Step 2. Vessel hyperpermeability and proteolysis of the basement membrane; vascular endothelial growth factor (VEGF), VE cadherin, matrix metalloproteinases (MMPs), transforming growth factor-β (TGF-β). Step 3. Endothelial cell (EC) proliferation and migration; VEGF, fibroblast growth factor (FGF), epidermal growth factor (EGF), integrins. Step 4. Cell-to-cell contact; VE cadherin, ephrins. Step 5. Tube formation; FGF, platelet-derived growth factor (PDGF), TNF-α, tumor necrosis factor-α 1 (TNF-α). Step 6. Vessel stabilization through mesenchymal cell recruitment and differentiation into pericytes; Ang1/Tie2, PDGF, VE cadherin, TGF-β. Original drawing based on ideas from Papetti and Herman (32).
The current work will review our understanding of basal and exercise-induced regulation of these processes where possible and to identify future directions that will enhance our understanding of skeletal muscle capillary regulation. This review is not intended to provide an exhaustive review of the literature surrounding the regulation of skeletal muscle capillarization, the reader is directed to several such excellent reviews (6, 7). Skeletal muscle angiogenesis arises from existing capillaries either through sprouting or non-sprouting angiogenesis. Aerobic exercise and mechanical loading of muscle promotes sprouting angiogenesis, while vasodilation such as by the α-adrenergic blocker prazosin promotes non-sprouting muscle angiogenesis (6, 7). The current review will focus on factors associated with activity-induced sprouting angiogenesis.
Exercise-induced regulation
In reality whole body exercise represents a continuum with regard to metabolic demand though traditionally exercise is most easily classified as either aerobic or resistance exercise. Typical examples of aerobic exercise are running and cycling and for resistance exercise weight lifting. During aerobic exercise, energy demand is supplied predominantly through continuous mitochondrial ATP generation and thus adaptations are directed toward increasing oxygen supply to muscle and mitochondrial ATP generation. With resistance exercise, high mechanical loads promote adaptations to increase muscle mass and strength.
While it is well accepted that aerobic exercise promotes angiogenesis (1), less well known is that resistance exercise also increases muscle capillarization (8). A possibility behind this discrepancy may be attributed to findings that while the capillary-to-fiber ratio (C/F) is increased in response to resistance exercise the accompanying muscle hypertrophy results in no change in capillary density (CD). In contrast, aerobic exercise increases both C/F and CD. Greater muscle CD increases the diffusive potential of the capillary bed in support of greater oxidative capacity. In spite of the lack of an increase in CD, resistance exercise does promote the formation of new capillaries or angiogenesis. The disturbance in homeostasis that prompts the angiogenic response to resistance exercise remains elusive.
VEGF and VEGF receptors
By far, the best characterized angiogenic growth factor signaling pathway with regard to muscle capillarization is vascular endothelial growth factor (VEGF). VEGF is a predominantly endothelial cell-specific, heparin-binding, 35–45 kDa homodimeric, secretable, glycoprotein mitogen. VEGF is encoded by a single gene with post-transcriptional splicing resulting in several different isoforms including 121, 165, and 189. Both VEGF165 and VEGF189 have a heparin binding domain that facilitates binding to heparin sulfate in the extracellular matrix where is can subsequently be cleaved to facilitate interaction with the VEGF receptors located predominantly on endothelial cells (ECs). VEGF121 does not contain the heparin binding domain and thus is freely secreted from muscle cells. Exercise increases the release or secretion of VEGF in muscle. VEGF binds to two tyrosine kinase receptors, VEGFR-1 (Flt-1) and VEGFR-2 (KDR). The predominance of VEGF biological activity occurs through binding to VEGFR-2, which is facilitated by neuropilin 1 (Nrp1). Of the VEGF isoforms, VEGF165 is the most potent because it binds the Nrp1 receptor to enhance signaling through VEGFR-2, whereas other VEGF isoforms may not bind Nrp1. Loss of a single VEGF allele results in embryonic lethality.
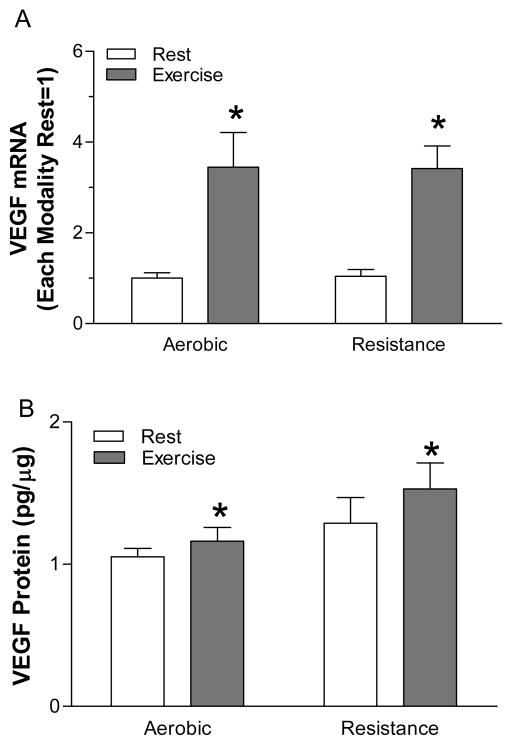
VEGF mRNA and protein are elevated by acute aerobic and resistance exercise (Figure 2). Typically, acute exercise increases VEGF mRNA 3- to 4-fold and VEGF protein 25–50% in humans. Acute exercise increases the mRNA for VEGF121, VEGF165, and VEGF189 (9). After an exercise training program, VEGF protein is increased 2-fold in humans. Aerobic and resistance exercise increases VEGFR-2 and Nrp-1 mRNA (9–11), while only aerobic exercise increases VEGFR-1 mRNA (10, 12). Increases in both VEGF and the VEGF receptors are consistent with a coordinated VEGF signaling pathway response to exercise. Data from Wagner et al. suggest that the lifelong deletion of muscle-specific VEGF in late fetal development inhibits aerobic exercise-induced angiogenesis (13). Inhibition of VEGF using a VEGF Trap prevents muscle overload-induced angiogenesis (7). While the magnitude and the time course of the VEGF responses to acute exercise are similar between aerobic and resistance exercise, these significantly different stimuli are sure to activate different intracellular signaling pathways and very likely regulate VEGF through different intracellular mechanisms.
Figure 2.
At 4 h after the completion of aerobic (45 min of cycling at 50% of maximum) or resistance exercise (3 × 10 repetitions per set) VEGF mRNA and protein are increased. * - significantly different than rest for each modality. Original drawing based on data previously published in (10, 11).
Several intracellular signals have been proposed to regulate VEGF and thus capillarization in response to exercise. The regulation of VEGF expression occurs predominantly via changes in mRNA (transcription and stabilization) and less through post-transcriptional modification. Hypoxia is a well-known regulator of skeletal muscle VEGF in vitro and in vivo. Exposure of human skeletal muscle cells to hypoxia increases VEGF expression (14). However, the VEGF mRNA response to acute exercise is similar in normoxia (muscle PO2 = 7.2 mm Hg) and acute hypoxia (muscle PO2 = 3.8 mm Hg) in untrained humans (15). Does this mean that hypoxia is not involved in exercise-induced VEGF regulation? It is quite possible that low PO2 is necessary for the increase in VEGF with exercise, but that lowering intracellular PO2 beyond some point during exercise does not produce further increases in VEGF. Consistent with this rationale, there is a plateau in the VEGF response to increasing exercise intensity between 55 and 65% of maximum when intracellular PO2 is believed to reach a nadir.
Nitric oxide (NO) is involved in muscle VEGF regulation. Nitric oxide is generated by nitric oxide synthase (NOS) and is produced in muscle by neuronal NOS (nNOS) and in endothelial cells by endothelial NOS (eNOS). nNOS production of NO regulates in part muscle mitochondrial respiration, while eNOS production of NO is involved in blood flow regulation. NOS inhibition attenuates the VEGF and VEGFR-1 mRNA increases to acute treadmill exercise (16, 17) and electrical-stimulation-induced increases in VEGF mRNA, VEGFR-1 mRNA, VEGFR-2 mRNA, and angiogenesis (18). However, mice lacking either eNOS or nNOS do not demonstrate deficiencies in muscle overload-induced angiogenesis (7). It should be highlighted that while experimental models such as electrical stimulation and muscle overload induced by synergistic muscle ablation are useful in investigating the regulation of angiogenesis, these models may not fully mimic the angiogenic process that occurs with traditional aerobic and resistance exercise.
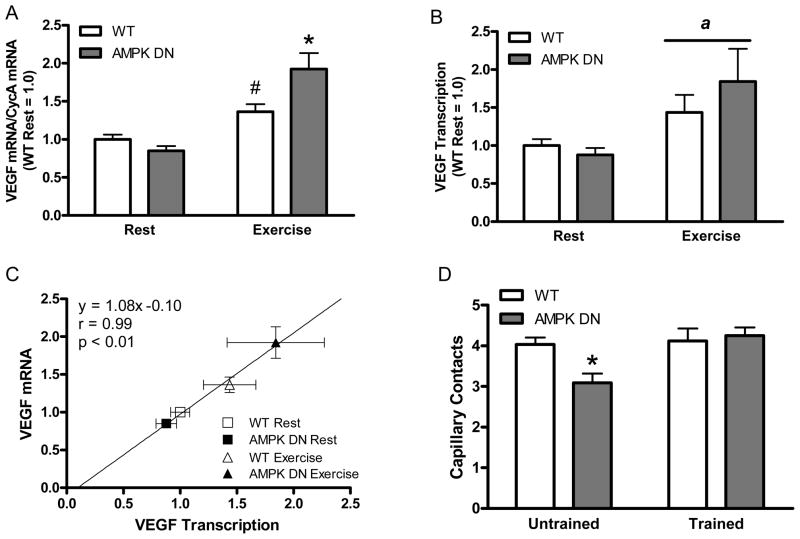
Recently we investigated if 5′-AMP-activated protein kinase (AMPK) was involved in VEGF expression and angiogenesis by using a mouse model in which a dominant negative (DN) form of AMPK is expressed rendering endogenous muscle AMPK inactive (19). AMPK is a heterotrimeric enzyme that acts as a metabolic fuel gauge to monitor cellular energy charge and AMPK activity is increased in response to hypoxia and exercise. We had hypothesized that AMPK could be the signaling pathway linking muscle metabolism and capillarization and thus a likely regulator of exercise-induced angiogenesis. AMPK is important for VEGF expression and angiogenesis induced by chronic hindlimb ischemia. In contrast to our hypothesis, we discovered that AMPK is not necessary for increases in VEGF expression or VEGF transcription in response to acute exercise (Figure 3). AMPK is also not necessary for exercise-induced angiogenesis; though basal capillarization is reduced in AMPK DN mice compared to WT mice possibly through reduced VEGF.
Figure 3.
AMPK is not necessary for the increase in VEGF mRNA in response to acute treadmill exercise. Exercise-induced increases in VEGF mRNA are due to increases in VEGF transcription (B and C). AMPK is important for basal muscle capillarization, but not exercise-induced angiogenesis (D). * - significantly different than all other groups. # - significantly different than WT Rest. a – main effect of exercise. Original drawing based on data previously published in (19).
In addition to AMPK, exercise activates several signaling pathways that may be the link between metabolism and capillarization including calcium (Ca2+) regulated pathways. Large, but transient increases in intracellular Ca2+ occur during exercise as a result of neural activation and muscle cell depolarization. Ca2+ is intimately involved in actin-myosin crossbridge formation and is responsible in part for exercise-induced improvements in insulin sensitivity that occur in part through increases in glucose transporter 4 (GLUT4). Increasing intracellular Ca2+ leads to the activation of several downstream signaling proteins including calmodulin-dependent kinase (CaMK) and calcineurin. Preliminary data from our laboratory suggest that calcium may be involved in muscle VEGF regulation. Increasing intracellular Ca2+ with caffeine appears to increase VEGF mRNA in primary human myotubes in vitro. It must be stressed that these are preliminary findings and much more work needs to be done before these studies are finalized. Consistent with a role for Ca2+ in muscle angiogenesis, recent findings from Gaudel et al. demonstrate that pharmacological activation of peroxisome proliferator-activated receptor-β (PPARβ), which is also known as PPARδ and is involved in muscle development and metabolism, increases VEGF and promotes muscle angiogenesis through a calcineurin dependent pathway (20).
Perhaps the persistent observation of a strong relationship between muscle mitochondrial volume and the size of the capillary bed suggests that the factors that regulate mitochondrial biogenesis may also regulate angiogenesis. Aerobic exercise promotes mitochondrial biogenesis and increases the expression of the transcriptional coactivator PGC-1α (peroxisome-proliferator-activated receptor-γ coactivator-1α) (21) a regulator of mitochondrial biogenesis. Muscle specific overexpression of PGC-1α increases muscle VEGF and capillarization (22). An attractive hypothesis is that exercise-induced regulation of mitochondria biogenesis and angiogenesis is regulated by PGC-1α though this hypothesis remains to be tested.
Several transcription factors can bind to the promoter region of the VEGF gene and regulate VEGF transcription. Of these primary transcription factors, only hypoxia-inducible factor 1 (HIF-1) and signal transducer and activator of transcription 3 (STAT3) are known to be responsive to exercise in muscle (23, 24). HIF-1 is a well known regulator of VEGF transcription in response to hypoxia. In humans, exercise increases the nuclear localization of HIF-1 and promotes the binding of HIF-1 to the hypoxia response element of the erythropoietin (EPO) gene in muscle (23). However, there is no statistical difference in the VEGF mRNA response to acute exercise between wild type (WT) mice and mice with skeletal muscle deletion (KO) of the regulated subunit of HIF-1, HIF-1α (25). HIF-1α KO mice demonstrate increased skeletal muscle capillarization in the untrained condition to a magnitude similar to that resulting from aerobic exercise training suggesting that the loss of HIF-1α does not negatively impact endurance training (26). Interestingly, exercise training does not produce any further increase in muscle capillarization in the HIF-1 KO mice. The greater capillarization in the HIF-1α KO has been speculated to occur via increases in AMPK (26). Given the endurance training like adaptations in muscle with lifelong deletion of HIF-1α, our understanding of the involvement of HIF-1 in the regulation of VEGF and muscle capillarization likely requires the development of muscle specific, conditional HIF-1α deletion in adult animals.
HIF-1 is not the only hypoxia-induced transcriptional regulator of VEGF. Hypoxia-induced PGC-1α transcriptional regulation of VEGF is dependent upon the coactivation of the orphan nuclear receptor ERR-α (estrogen-related receptor-α) and not HIF-1 (22). This is important because exercise is known to increase muscle PGC1- α (21), however there are no known reports of exercise increasing ERR-α.
It is apparent that several different intracellular signaling pathways likely contribute to the regulation of VEGF in response to aerobic activity. While aerobic exercise promotes increases in metabolic pathways involved in oxidative metabolism, in general resistance exercise does not. As mentioned above, acute resistance exercise increases VEGF mRNA and protein in human skeletal muscle. One possible signaling pathway for overload-induced VEGF and capillary regulation is Akt. Overexpression of Akt induces myotube hypertrophy and increases VEGF in C2C12 cells and increases muscle fiber size, VEGF, and capillary density in vivo (14). Increased VEGF transcription by Akt occurs through a transcription factor that binds between 0.35 kbp and 0.20 kbp upstream of the transcription initiation site in the VEGF promoter. This section of the VEGF promoter includes binding sites for the transcription factor specificity protein (Sp) 1/3. In response to resistance exercise, increases in VEGF mRNA temporally coincide with the activation of STAT3, a proposed regulator of muscle hypertrophy (24) and known transcriptional regulator of VEGF. Much more work remains to understand how resistance exercise promotes angiogenesis.
Activity-induced Regulation of Vessel Stabilization and Proteolysis of the Basement Membrane
Destabilization of capillaries is necessary for sprouting. The angiopoietin system consists of angiopoietin 1 and 2 (Ang1 and Ang2) and their common receptor Tie2. The angiopoietins are not prototypical angiogenic growth factors, but rather permissively allow for the proper interaction between endothelial cells and supporting cells. Capillary stability is promoted when the concentration of Ang1 is greater than Ang2, while capillary instability is promoted when Ang2 is greater than Ang1 thus Ang2 acts as a naturally occurring antagonist to Ang1. In humans, aerobic exercise training increases the expression of Ang2 mRNA/Ang1 mRNA indicative of a pro-angiogenic state without increases in Tie2 mRNA (12), while acute resistance exercise increases Tie2 mRNA expression without altering Ang1, Ang2, or Ang2/Ang1 mRNA (10). Ang2 protein is increased in response synergistic ablation-induced muscle overload (7). Taken together and given the importance of the angiopoietin pathway in angiogenesis, it appears that exercise does activate the angiopoietin pathway though considerable work is warranted to understand the regulation the this pathway.
Proteolysis of the capillary basement is necessary in angiogenesis to allow for among other functions endothelial cell migration. Matrix metalloproteinases (MMPs) constitute the major class of proteases responsible for degradation of basement membrane proteins. A single bout of aerobic exercise increases MMP-9 in humans (27). In response to muscle electrical stimulation, MMP-2 and membrane type 1 (MT1)-MMP are increased (28). Inhibition of MMPs inhibits electrical stimulation-induced angiogenesis (28).
Endothelial Progenitor Cells (EPCs)
Traditionally, angiogenesis was considered to result exclusively from the proliferation, migration, and remodeling of preexisting, fully differentiated endothelial cells (ECs) residing in the parent vessels. However, recent evidence suggests that neovascularization may not rely exclusively upon cells residing within the vessel wall but also appears to be modulated by circulating cells derived from bone marrow. A specific subset of these cells, referred to as endothelial progenitor cells (EPCs), can enhance blood flow recovery and angiogenesis in response to hindlimb ischemia and can differentiate into ECs in situ. Exercise training increases circulating EPCs. Ablation of resident stem cell activity by X-ray irradiation in muscle does not prevent exercise-induced angiogenesis (29). Thus, either resident skeletal muscle ECs are resistant to X-ray irradiation or the ECs are derived from extra-muscular progenitors. Given that similar dosages of X-ray irradiation block EC in vitro, the work from Li et al. are consistent with circulating EPCs being involved in exercise-induced angiogenesis.
Basal Regulation of the Capillary Bed
Very little is known regarding basal regulation of VEGF or muscle capillarization. Using Cre-Lox P technology, the deletion of VEGF from skeletal muscle decreases muscle capillary-to-fiber ratio by 67% and capillary density by 69% (13). Equally important is that VEGF deletion also results in muscle fiber apoptosis suggesting that VEGF is critical for the maintenance of muscle mass. Whether this is a direct effect of VEGF on the muscle fiber or an indirect effect due to capillary rarefaction remains to be determined.
Basal expression of VEGF is sensitive to reductions in muscular activity. Hindlimb suspension for 7 d decreases muscle VEGF mRNA expression by 90% (2) (Figure 4). When muscle is reloaded for 7 d following suspension, VEGF mRNA is increased by 90% compared to controls. Thus, VEGF regulation is dependent upon variations in basal activity levels which is likely due to signaling pathways activated by weight bearing activity or by neural activity associated with weight bearing activity.
Figure 4.
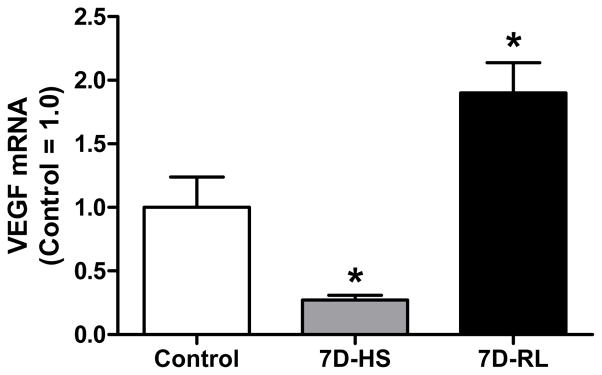
Compared to control, 7 d of hindlimb suspension (7D-HS) decreases VEGF mRNA 70% and 7 d of reloading (7D-RL) after of suspension increases VEGF mRNA 90%. Basal weight bearing activity tremendously impacts VEGF regulation. * - significantly different than all other groups. Original drawing based on data previously published in the supplemental material of Dapp et al. 2004 (2).
Given the relationship between muscle fiber type, mitochondrial capacity, and capillarization, the factors that regulate muscle fiber type may also regulate basal muscle capillarization. A major contributing factor to the establishment of muscle fiber type is neural control. Motor neurons contact muscle fibers and transmit electrical signals that modulate calcium concentrations, which in turn play a critical role in determining, maintaining, and transforming muscle fiber types. Calcineurin is a calcium dependent protein phosphatase that is regulates in part muscle fiber type. Treating rodents with cyclosporine A (CsA), an inhibitor of calcineurin activity, results in an increase in glycolytic enzymes, a decrease in type I myosin heavy chain (MHC) expression, an increase in type IIB MHC expression, and a 10% decrease in soleus muscle C/F (30). Consistent with this, preliminary findings in our laboratory in primary human myotubes suggest that inhibition of calcineurin by CsA reduces basal VEGF mRNA, while KN93 which binds to CaMK does not.
Future Directions
In spite of our knowledge of the importance of VEGF, angiopoietins, and MMP in skeletal muscle, recent work demonstrated that Akt increases the expression of Follistatin-like 1 which is secreted from muscle cells and induces angiogenesis (31). This new discovery reminds us that much still remains to be discovered regarding regulation of the muscle capillary bed. To further our understanding of basal and exercise-induced regulation capillarization, an increase in the use of cell culture based strategies is needed. Increased use of muscle cell cultures will permit for investigating the intracellular signaling pathways and transcription factors involved in growth factor regulation and the use of electrical stimulation of these cell cultures would be a useful model of activity-induced regulation. Consistent with this, initial experiments in our laboratory suggest that electrical stimulation of primary human myotubes increases VEGF mRNA. Findings from cell culture can then be expanded into in vivo conditions that provide the necessary integrative approach to understand activity-induced changes in muscle capillarization. In addition to increased use of cell culture, greater use of transgenic animals is needed to translate findings from in vitro systems and apply them to in vivo exercise models.
Clinical Implications
Several conditions including type 2 diabetes (T2DM) and advanced age are associated with reduced angiogenic potential in response to limb ischemia. Interestingly, the angiogenic response to exercise training is maintained in both T2DM and aged individuals. As with differences between aerobic and resistance exercise, the intracellular signals regulating the angiogenic responses to ischemia and exercise in muscle may be quite different. For example, AMPK is important for ischemia-induced angiogenesis, but not exercise-induced angiogenesis. Understanding how exercise promotes angiogenesis may provide novel insight into new approaches to treating ischemic disease.
Summary/Conclusions
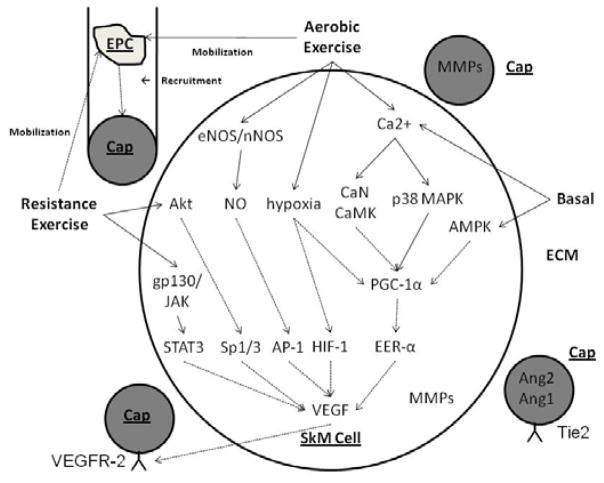
Exercise induces several pathways that are known to be important for regulating angiogenesis. VEGF is critical for basal and activity-induced regulation of skeletal muscle capillarization, while the importance of other growth factor pathways remains to be elucidated. While several signaling pathways have been identified (Figure 5), significant work remains on understanding the intracellular signaling pathways and transcription factors involved in basal and exercise-induced angiogenesis.
Figure 5.
Proposed model including intracellular signaling and growth factor regulation in skeletal muscle (SkM) cells, capillaries lined with endothelial cells (Caps), extracellular matrix (ECM), and endothelial progenitor cells (EPCs). AMPK, 5′-AMP-activated protein kinase; Ang1 and 2, angiopoietin 1 and 2; AP-1, activator protein 1; CaMK, calmodulin dependent kinase; CaN, calcineurin; eNOS, endothelial nitric oxide synthase (NOS); ERR-α, estrogen-related receptor-α; HIF-1, hypoxia-inducible factor 1; gp130/JAK, glycoprotein 130/Janus family of tyrosine kinases; MMPs, matrix metalloproteinases; nNOS, neuronal NOS; NO, nitric oxide; p38 MAPK, p38 mitogen activated protein kinase; PGC-1α, peroxisome-proliferator-activated receptor-γ coactivator-1α; STAT3, signal transducers and activator of transcription; Tie2, Tie receptor for Ang1 and Ang2; VEGF, vascular endothelial growth factor; VEGFR-2, VEGF receptor 2. Solid lines (---) are established pathways. Dashed lines (- -) are hypothesized pathways.
Acknowledgments
The author wishes to thank his collaborators without whom many of the findings presented here would not have been possible. The author regrets that owing to space limitations he has been unable to cite the volume of primary literature that has enhanced our understanding of muscle capillarization. This study was supported in part by National Institute on Aging grant AG-021891 and Mid-Atlantic American Heart Association grant 0465415U.
References
- 1.Brodal P, Ingjer F, Hermansen L. Capillary supply of skeletal muscle fibers in untrained and endurance-trained men. Am J Physiol. 1977;232:H705–12. doi: 10.1152/ajpheart.1977.232.6.H705. [DOI] [PubMed] [Google Scholar]
- 2.Dapp C, Schmutz S, Hoppeler H, Fluck M. Transcriptional reprogramming and ultrastructure during atrophy and recovery of mouse soleus muscle. Physiol Genomics. 2004;20:97–107. doi: 10.1152/physiolgenomics.00100.2004. [DOI] [PubMed] [Google Scholar]
- 3.Waters RE, Rotevatn S, Li P, Annex BH, Yan Z. Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol. 2004;287:C1342–8. doi: 10.1152/ajpcell.00247.2004. [DOI] [PubMed] [Google Scholar]
- 4.Croley AN, Zwetsloot KA, Westerkamp LM, et al. Lower capillarization, VEGF protein, and VEGF mRNA response to acute exercise in the vastus lateralis muscle of aged vs. young women. J Appl Physiol. 2005;99:1872–1879. doi: 10.1152/japplphysiol.00498.2005. [DOI] [PubMed] [Google Scholar]
- 5.Gavin TP, Stallings HW, III, Zwetsloot KA, et al. Lower capillary density but no difference in VEGF expression in obese vs. lean young skeletal muscle in humans. J Appl Physiol. 2005;98:315–321. doi: 10.1152/japplphysiol.00353.2004. [DOI] [PubMed] [Google Scholar]
- 6.Hudlicka O, Brown M, Egginton S. Angiogenesis in skeletal and cardiac muscle. Physiol Rev. 1992;72:369–417. doi: 10.1152/physrev.1992.72.2.369. [DOI] [PubMed] [Google Scholar]
- 7.Egginton S. Pflugers Arch. 2008. Invited review: activity-induced angiogenesis. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol. 1996;81:2004–12. doi: 10.1152/jappl.1996.81.5.2004. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson T, Ameln H, Fischer H, Sundberg CJ, Timmons JA, Jansson E. VEGF-A splice variants and related receptor expression in human skeletal muscle following submaximal exercise. J Appl Physiol. 2005;98:2137–2146. doi: 10.1152/japplphysiol.01402.2004. [DOI] [PubMed] [Google Scholar]
- 10.Gavin TP, Drew JL, Kubik CJ, Pofahl WE, Hickner RC. Acute resistance exercise increases skeletal muscle angiogenic growth factor expression. Acta Physiol. 2007;191:139–146. doi: 10.1111/j.1748-1716.2007.01723.x. [DOI] [PubMed] [Google Scholar]
- 11.Ryan NA, Zwestloot KA, Westerkamp LM, Pofahl WE, Hickner RC, Gavin TP. Lower skeletal muscle capillarization and VEGF expression in aged versus young men. J Appl Physiol. 2006;100:178–85. doi: 10.1152/japplphysiol.00827.2005. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson T, Rundqvist H, Norrbom J, Rullman E, Jansson E, Sundberg CJ. The influence of physical training on the angiopoietin and VEGF-A systems in human skeletal muscle. J Appl Physiol. 2007;103:1012–1020. doi: 10.1152/japplphysiol.01103.2006. [DOI] [PubMed] [Google Scholar]
- 13.Wagner PD, Olfert IM, Tang K, Breen EC. Muscle-targeted deletion of VEGF and exercise capacity in mice. Respir Physiol Neurobiol. 2006;151:159–66. doi: 10.1016/j.resp.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi A, Kureishi Y, Yang J, et al. Myogenic Akt signaling regulates blood vessel recruitment during myofiber growth. Mol Cell Biol. 2002;22:4803–14. doi: 10.1128/MCB.22.13.4803-4814.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Richardson RS, Wagner H, Mudaliar SR, Henry R, Noyszewski EA, Wagner PD. Human VEGF gene expression in skeletal muscle: effect of acute normoxic and hypoxic exercise. Am J Physiol. 1999;277:H2247–52. doi: 10.1152/ajpheart.1999.277.6.H2247. [DOI] [PubMed] [Google Scholar]
- 16.Gavin TP, Spector DA, Wagner H, Breen EC, Wagner PD. Nitric oxide synthase inhibition attenuates the skeletal muscle VEGF mRNA response to exercise. J Appl Physiol. 2000;88:1192–8. doi: 10.1152/jappl.2000.88.4.1192. [DOI] [PubMed] [Google Scholar]
- 17.Gavin TP, Wagner PD. Attenuation of the exercise-induced increase in skeletal muscle Flt-1 mRNA by nitric oxide synthase inhibition. Acta Physiol Scand. 2002;175:201–9. doi: 10.1046/j.1365-201X.2002.00987.x. [DOI] [PubMed] [Google Scholar]
- 18.Milkiewicz M, Hudlicka O, Brown MD, Silgram H. Nitric oxide, VEGF and VEGFR-2: interactions in activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol. 2005;289:H336–H343. doi: 10.1152/ajpheart.01105.2004. [DOI] [PubMed] [Google Scholar]
- 19.Zwetsloot KA, Westerkamp LM, Holmes BF, Gavin TP. AMPK regulates basal skeletal muscle capillarization and VEGF expression, but is not necessary for the angiogenic response to exercise. J Physiol. 2008;586:6021–35. doi: 10.1113/jphysiol.2008.159871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaudel C, Schwartz C, Giordano C, Abumrad NA, Grimaldi PA. Pharmacological activation of PPARbeta promotes rapid and calcineurin-dependent fiber remodeling and angiogenesis in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2008;295:E297–304. doi: 10.1152/ajpendo.00581.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mathai AS, Bonen A, Benton CR, Robinson DL, Graham TE. Rapid exercise-induced changes in PGC-1α mRNA and protein in human skeletal muscle. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.00847.2007. e Pub ahead. [DOI] [PubMed] [Google Scholar]
- 22.Arany Z, Foo SY, Ma Y, et al. HIF-independent regulation of VEGF and angiogenesis by the transcriptional coactivator PGC-1α. Nature. 2008;451:1008–12. doi: 10.1038/nature06613. [DOI] [PubMed] [Google Scholar]
- 23.Ameln H, Gustafsson T, Sundberg CJ, et al. Physiological activation of hypoxia inducible factor-1 in human skeletal muscle. FASEB J. 2005;19:1009–1011. doi: 10.1096/fj.04-2304fje. [DOI] [PubMed] [Google Scholar]
- 24.Trenerry MK, Carey KA, Ward AC, Cameron-Smith D. STAT3 signaling is activated in human skeletal muscle following acute resistance exercise. J Appl Physiol. 2007;102:1483–1489. doi: 10.1152/japplphysiol.01147.2006. [DOI] [PubMed] [Google Scholar]
- 25.Mason SD, Howlett RA, Kim MJ, et al. Loss of skeletal muscle HIF-1alpha results in altered exercise endurance. PLoS Biol. 2004;2:e288. doi: 10.1371/journal.pbio.0020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mason SD, Rundqvist H, Papandreou I, et al. HIF-1alpha in endurance training: suppression of oxidative metabolism. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2059–69. doi: 10.1152/ajpregu.00335.2007. [DOI] [PubMed] [Google Scholar]
- 27.Rullman E, Rundqvist H, Wagsater D, et al. A single bout of exercise activates matrix metalloproteinase in human skeletal muscle. J Appl Physiol. 2007;102:2346–51. doi: 10.1152/japplphysiol.00822.2006. [DOI] [PubMed] [Google Scholar]
- 28.Haas TL. Molecular control of capillary growth in skeletal muscle. Can J Appl Physiol. 2002;27:491–515. doi: 10.1139/h02-027. [DOI] [PubMed] [Google Scholar]
- 29.Li P, Akimoto T, Zhang M, Williams RS, Yan Z. Resident stem cells are not required for exercise-induced fiber-type switching and angiogenesis but are necessary for activity-dependent muscle growth. Am J Physiol Cell Physiol. 2006;290:C1461–8. doi: 10.1152/ajpcell.00532.2005. [DOI] [PubMed] [Google Scholar]
- 30.Bigard X, Sanchez H, Zoll J, et al. Calcineurin co-regulates contractile and metabolic components of slow muscle phenotype. J Biol Chem. 2000;275:19653–60. doi: 10.1074/jbc.M000430200. [DOI] [PubMed] [Google Scholar]
- 31.Ouchi N, Oshima Y, Ohashi K, et al. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric oxide synthesis-dependent mechanism. J Biol Chem. 2008;283:32802–11. doi: 10.1074/jbc.M803440200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Papetti M, Herman IM. Mechanisms of normal and tumor-derived angiogenesis. Am J Physiol Cell Physiol. 2002;282:C947–70. doi: 10.1152/ajpcell.00389.2001. [DOI] [PubMed] [Google Scholar]