Abstract
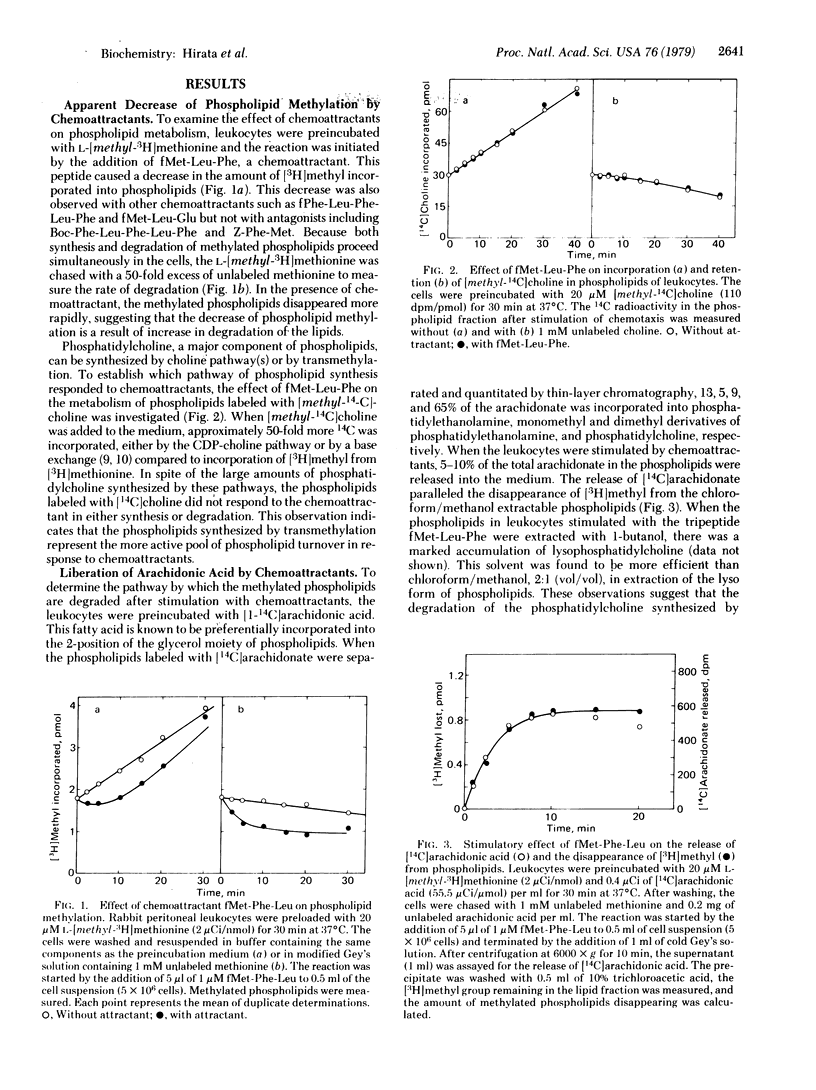
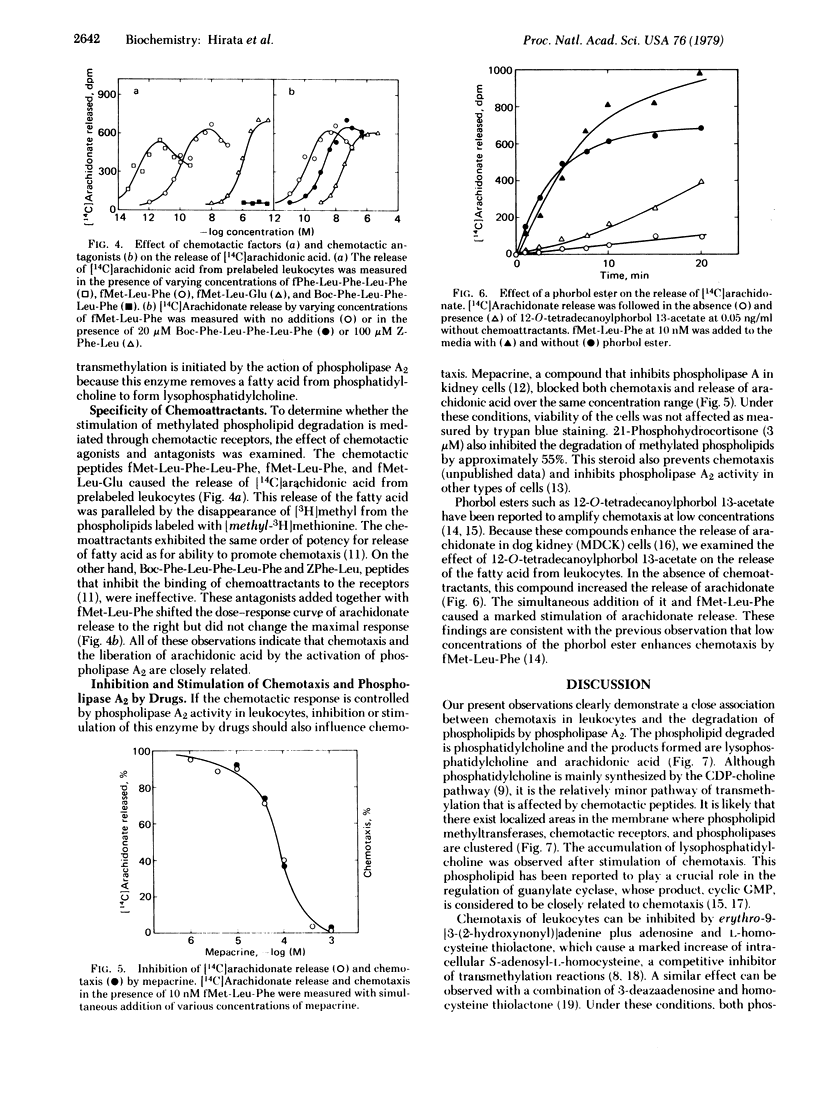
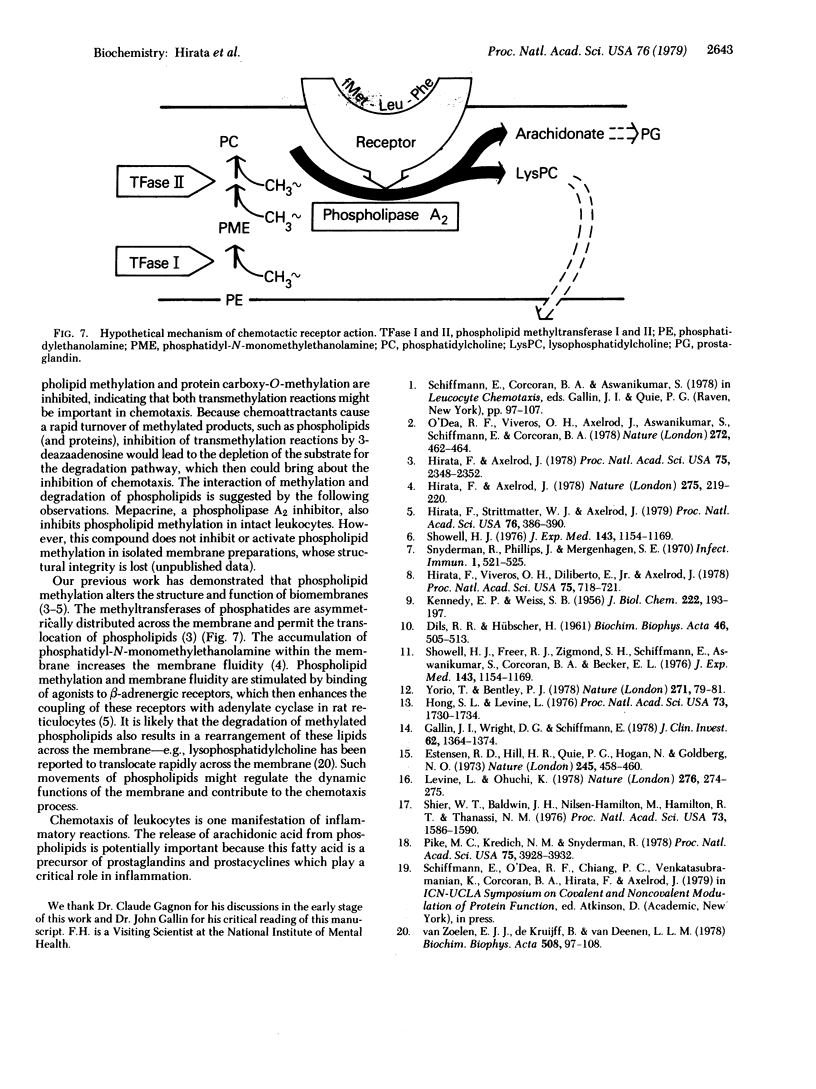
When rabbit peritoneal leukocytes were treated with chemoattractants such as fMet-Leu-Phe, an apparent decrease of [3H]methyl incorporation into the lipid fraction from L-[methyl-3H]methionine was observed. This decrease was a result of increased degradation of methylated phospholipids, not of decreased synthesis. Chemotactic peptides did not affect the metabolism of the phospholipids in which [methyl-14C]choline was incorporated. The disappearance of the [3H]methyl group was associated with the release of [1-14C]arachidonic acid from phospholipids prelabeled with these compounds. These findings suggested the activation by chemoattractants of phospholipase A2, an enzyme that removes an unsaturated fatty acid from phospholipids. The order of potency of chemoattractants for the stimulated degradation of phospholipids was in good agreement with that for chemotaxis. Mepacrine (quinacrine) and hydrocortisone inhibited and a phorbol ester enhanced both chemotaxis and phospholipase A2 activity. These results, taken together, suggest close association of the metabolism of methylated phospholipids with chemotaxis in rabbit peritoneal leukocytes.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach M. L., Lacroute F., Botstein D. Evidence for transcriptional regulation of orotidine-5'-phosphate decarboxylase in yeast by hybridization of mRNA to the yeast structural gene cloned in Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):386–390. doi: 10.1073/pnas.76.1.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DILS R. R., HUBSCHER G. Metabolism of phospholipids. III. The effect of calcium ions on the incorporation of labelled choline into rat-liver microsomes. Biochim Biophys Acta. 1961 Jan 29;46:505–513. doi: 10.1016/0006-3002(61)90581-9. [DOI] [PubMed] [Google Scholar]
- Estensen R. D., Hill H. R., Quie P. G., Gogan N., Goldberg N. D. Cyclic GMP and cell movement. Nature. 1973 Oct 26;245(5426):458–460. doi: 10.1038/245458a0. [DOI] [PubMed] [Google Scholar]
- Gallin J. I., Wright D. G., Schiffmann E. Role of secretory events in modulating human neutrophil chemotaxis. J Clin Invest. 1978 Dec;62(6):1364–1374. doi: 10.1172/JCI109257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic methylation of phosphatidylethanolamine increases erythrocyte membrane fluidity. Nature. 1978 Sep 21;275(5677):219–220. doi: 10.1038/275219a0. [DOI] [PubMed] [Google Scholar]
- Hirata F., Axelrod J. Enzymatic synthesis and rapid translocation of phosphatidylcholine by two methyltransferases in erythrocyte membranes. Proc Natl Acad Sci U S A. 1978 May;75(5):2348–2352. doi: 10.1073/pnas.75.5.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S. L., Levine L. Inhibition of arachidonic acid release from cells as the biochemical action of anti-inflammatory corticosteroids. Proc Natl Acad Sci U S A. 1976 May;73(5):1730–1734. doi: 10.1073/pnas.73.5.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- Levine L., Ohuchi K. Retinoids as well as tumour promoters enhance deacylation of cellular lipids and prostaglandin production in MDCK cells. Nature. 1978 Nov 16;276(5685):274–275. doi: 10.1038/276274a0. [DOI] [PubMed] [Google Scholar]
- O'Dea R. F., Viveros O. H., Axelrod J., Aswanikaumar S., Schiffmann E., Corcoran B. A. Raipid stimulation of protein carboxymethylation in leukocytes by a chemotatic peptide. Nature. 1978 Mar 30;272(5652):462–464. doi: 10.1038/272462a0. [DOI] [PubMed] [Google Scholar]
- Pike M. C., Kredich N. M., Snyderman R. Requirement of S-adenosyl-L-methionine-mediated methylation for human monocyte chemotaxis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3928–3932. doi: 10.1073/pnas.75.8.3928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Baldwin J. H., Nilsen-Hamilton M., Hamilton R. T., Thanassi N. M. Regulation of guanylate and adenylate cyclase activities by lysolecithin. Proc Natl Acad Sci U S A. 1976 May;73(5):1586–1590. doi: 10.1073/pnas.73.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Showell H. J., Freer R. J., Zigmond S. H., Schiffmann E., Aswanikumar S., Corcoran B., Becker E. L. The structure-activity relations of synthetic peptides as chemotactic factors and inducers of lysosomal secretion for neutrophils. J Exp Med. 1976 May 1;143(5):1154–1169. doi: 10.1084/jem.143.5.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyderman R., Phillips J., Mergenhagen S. E. Polymorphonuclear leukocyte chemotactic activity in rabbit serum and Guinea pig serum treated with immune complexes: evidence for c5a as the major chemotactic factor. Infect Immun. 1970 Jun;1(6):521–525. doi: 10.1128/iai.1.6.521-525.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yorio T., Bentley P. J. Phospholipase A and the mechanism of action of aldosterone. Nature. 1978 Jan 5;271(5640):79–81. doi: 10.1038/271079a0. [DOI] [PubMed] [Google Scholar]
- van Zoelen E. J., de Kruijff B., van Deenen L. L. Protein-mediated transbilayer movement of lysophosphatidylcholine in glycophorin-containing vesicles. Biochim Biophys Acta. 1978 Mar 21;508(1):97–108. doi: 10.1016/0005-2736(78)90191-8. [DOI] [PubMed] [Google Scholar]



