Abstract
Background
Trauma centers are caring for increased proportions of elderly patients. While age and ISS are independently associated with mortality, trauma centers were originally designed to care for seriously injured patients without age-specific guidelines. We hypothesized that elderly patients would have different complication patterns than their younger counterparts.
Methods
The trauma registry of an ACS-verified level I trauma center was queried for all patients > 14 years of age admitted between 1/2005 and 12/2008. Mechanism, mortality, and complications were evaluated after dividing patients into eight age groups.
Results
Of the 15,223 patients, 13% were elderly (≥ 65), and 86% were injured via a blunt mechanism. Increasing age correlated with fatality (all ISS scores), end-organ failure and thrombo-embolic complications (DVT and coagulopathy). Analysis revealed a significant breakpoint at 45 years of age for mortality, decubiti and renal failure (all p-values < 0.05). Infectious complications (sepsis, wound infection and abscess) all peaked between 45–65 years old, and then declined with increasing age.
Conclusions
We document that elderly trauma patients suffer the same complications as their younger counterparts, albeit at a different rate. More importantly, we identified a “breakpoint” of increased risk of complications and mortality at greater than 45 years of age. While the mechanisms behind these observations remain unknown, understanding their unique patterns may allow appropriate allocation of resources and focus research efforts on interventions that should improve outcomes.
Keywords: Elderly, trauma, complication, mortality, ISS
INTRODUCTION
As the US population ages, trauma centers have seen an increased proportion of elderly patients presenting with major injury. This change correlates with US Census figures projecting that the > 65 year old population will nearly double in size over the next 20 years from 46 million, estimated for the 2010 census, to 81 million estimated for the 2030 census, which would then represent approximately 22% of the overall total population [1]. This will cause an important change in the demographics seen in trauma admissions throughout the country. Due to the well-known relationship between increased age, ISS and increased mortality [2], trauma centers will be faced with additional challenges to prevent morbidity and mortality in these patients. Additionally, this has financial implications for the future of health care, as the rapidly increasing elderly trauma population will further burden the health care system due to increased cost associated with increased length of hospital stay required for care of elderly patients [3,4].
Most of America’s trauma centers were originally designed to care for seriously injured patients with a wide variety of ages and mechanisms, without tailored treatment protocols for specific age groups. This began to change with the evolution of pediatric trauma centers and the creation of resuscitation protocols specifically designed for the unique needs of injured children to improve their outcomes [5]. Similarly, it is becoming recognized that age-specific considerations of elderly patients are not well addressed in our current trauma system [6]. Accordingly, there has been a surge of interest from both trauma physicians and geriatricians regarding specific risks and needs that the elderly patients face in the post-injury period, accompanied by realization that this group is physiologically different than younger adults, with a decline in baseline function and decreased reserve for handling stress, and therefore may require modification of standardized trauma guidelines to optimize their care [7]. At our Level I Trauma Center, we have noted a significant increase in elderly trauma admissions, and prior to modifying our treatment protocols wanted to better understand how complications differ between age groups. We hypothesized that elderly patients would have different complications than do their younger counterparts.
MATERIALS AND METHODS
The Memorial Hermann Hospital (MHH) is the flagship hospital and trauma center for the University of Texas Health Science Center in Houston, Texas. MHH is one of only two level I trauma centers covering the city of Houston and Harris County, serving a population of over six million people. We queried the MHH trauma registry to capture consecutive adult (> 14 years of age) patients admitted to our academic center between January 1, 2005 and December 31, 2008. We divided the patients into eight age groups by decade, to correlate with categories of data analysis in the National Trauma Data Bank (NTDB) [8]: 15–24, 25–34, 35–44, 45–54, 55–64, 65–74, 75–84, and ≥ 85 years of age. “Elderly” was defined for this study as individuals ≥ 65 years old. We analyzed the relationship between age and Injury Severity Score (ISS), mechanism of injury, mortality, hospital length of stay (LOS) and incidence of complications.
Our database is prospectively collected by concurrent chart review, and undergoes a regular review for accuracy by health care professionals. Trained, dedicated data abstractors collect and enter variables into our trauma registry on a continuous basis. A less than ten percent error rate was accepted.
Organ failures were defined using the Denver scoring system [9, 10]. However, organ dysfunction occurring in the first 48 hours of admission was not considered to be organ failure. This is in keeping with the concept put forth by Moore and colleagues that organ dysfunction (not organ failure) occurring within the first 48 hours of injury represents reversible physiological responses to injury and resuscitation that have the potential for resolution once resuscitation is complete [11]
Systemic inflammatory response syndrome (SIRS) was defined as two or more of the following variables in the absence of an infectious source: (1) core body temperature of more than 38°C or less than 36°C, (2) heart rate of more than 90 beats per minute (3) respiratory rate of more than 20 breaths per minute or a PaCO2 level of less than 32 mm Hg, or (4) abnormal white blood cell count (>12,000/μL or <4,000/μL or >10% bands). Infectious complications were defined as clinical or culture positive diagnosis of ventilator-associated pneumonia, bacteremia, surgical site infection, or intra-abdominal infection, in accordance with the guidelines of the American College of Chest Physicians and the Society of Critical Care Medicine [12]. As well, severe sepsis and septic shock were defined according to standard accepted criteria from a consensus statement of the American College of Chest Physicians and the Society of Critical Care Medicine [12, 13]. Hospital length of stay (in days), ICU length of stay (in days), and ventilator days, are expressed in calendar days.
For the purposes of aggregate evaluation, pulmonary complications were documented in the trauma registry and were defined as the development of acute respiratory distress syndrome, pleural effusions, pulmonary edema, or respiratory failure (by Denver score [9]). Similarly, cardiac complications were defined as the development of cardiac arrhythmia requiring pharmacological intervention, pacing or direct cardio-version, congestive heart failure, cardiac arrest, or cardiac tamponade. Coagulation complications were also retrieved through the registry and were defined as the development of hypocoagulable state after admission (international normalized ratio greater than 1.5 without documented pre-hospital warfarin use), venous or arterial thrombo-embolic event, or stroke. Integumentary complications were defined as documented sacral decubiti and or skin breakdown.
Complications were then analyzed individually and in categories of single system organ failure (cardiac, respiratory, renal and integument), coagulation complications (hypo-coagulable, deep venous thrombosis-DVT, pulmonary embolism-PE, and stroke), and infectious complications (pneumonia, sepsis/shock, surgical site infection and deep tissue infection / abscess).
Continuous data are presented as medians with comparisons between groups performed using the Wilcoxon rank sum (Mann-Whitney U test). Categorical data are reported as proportions and, where appropriate, tested for significance using χ2 or Fisher exact tests. Each decade group was compared to the immediately preceding decade group, and complication analysis was made by univariate as well as non-parametric trend analysis. After analysis of groups by decade, we found that the complication graphs had visually obvious changes in the slopes at the 45–54 decade group which univariate analysis failed to demonstrate as significant differences. Therefore we dichotomized at this break point and re-analyzed the data in 2 groups: patients < 45years and those ≥ 45 years of age. A p-value of 0.05 was considered significant. Statistical analysis was conducted in Stata® 11.1 IC (2009 StatCorp, College Station, TX).
RESULTS
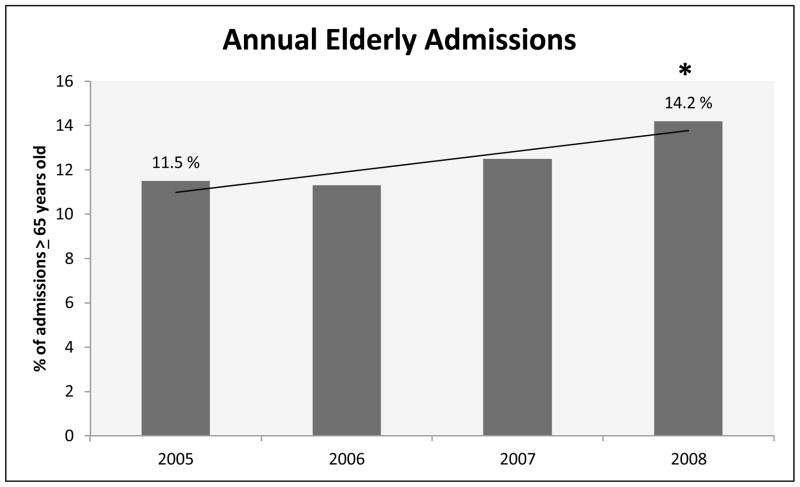
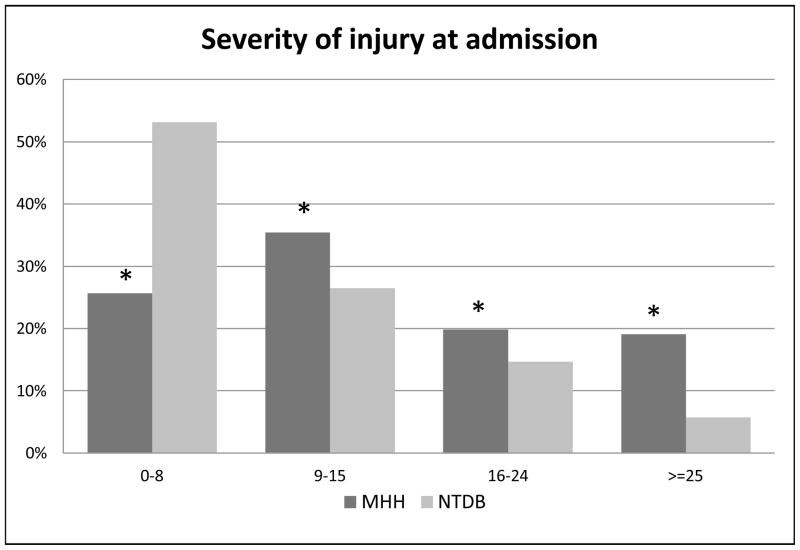
There were 15,223 adult trauma patients admitted during the four year study period (Table 1), of which 13% were ≥ 65 years old, and 38% were ≥ 45 years old. Overall, our study population was 71% male, however when divided to young versus elderly, with elderly defined as ≥ 65 years, males were 75% of the younger patients, and only 50% of the elder patients. Comparing the first year of the study to the last, we demonstrate a nearly three percent significant increase in the number of elderly patients admitted to our trauma center from 2005–2008 (Figure 1). The mechanism of injury experience by our patients was 86% blunt in the overall population, but varied with the highest percentage of penetrating trauma in 20–35 year old groups (19%) and an age-associated trend down to three to five percent in the ≥ 75 year old groups (p<0.001). Overall, the severity of injury (as measured by ISS) treated at our facility is greater than the averages recorded nationally within the NTDB, with 40% of our patients being admitted with ISS scores over 16 (Table 2), compared to 21% of patients in the NTDB with ISS ≥16 [8] (Figure 2).
Table 1.
Demographics
| Patients | 15,223 |
| ≥ 45 years | 5,816 |
| ≥ 55 years | 3,392 |
| ≥ 65 years | 1,921 |
| Male Gender | 71% |
| Blunt Mechanism | 86% |
| ISS | |
| 0–8 | 3,966 (26%) |
| 9–15 | 5,483 (35%) |
| 16–24 | 3,064 (20%) |
| ≥ 25 | 2,949 (19%) |
| Case Fatality Rate | 6.8 % |
Description of demographics of all trauma patients within study. ISS: Injury Severity Score
Figure 1. Annual Elderly Admissions.
Percentage of admissions ≥ 65 years old, including all ISS groups, separated by year, noting significant increase of 2.7% from 2005 to 2008. * p< 0.001.
Table 2.
Admission numbers by Age & ISS
| Age (years) | ISS 0–15 | ISS 16–24 | ISS ≥ 25 |
|---|---|---|---|
| 15–24 | 2394 | 680 | 789 |
| 25–34 | 1896 | 560 | 505 |
| 35–44 | 1626 | 473 | 484 |
| 45–54 | 1435 | 513 | 476 |
| 55–64 | 869 | 320 | 282 |
| 65–74 | 488 | 194 | 163 |
| 75–84 | 357 | 179 | 156 |
| ≥ 85 | 224 | 101 | 59 |
Distribution of patients within each age and ISS category. ISS: Injury Severity Score
Figure 2. Severity of Injury at Admission.
Graph comparing ISS scores of admissions to Memorial Hermann Hospital (MHH) versus those recorded in the National Trauma Data Bank (NTDB), stratified by ISS group. Significantly lower percentage of MHH admissions in the 0–8 ISS group versus NTDB; significantly higher percentage of admissions in the 9–15, 16–24 and ≥ 25 ISS groups at MHH versus NTDB. All p values < 0.001.
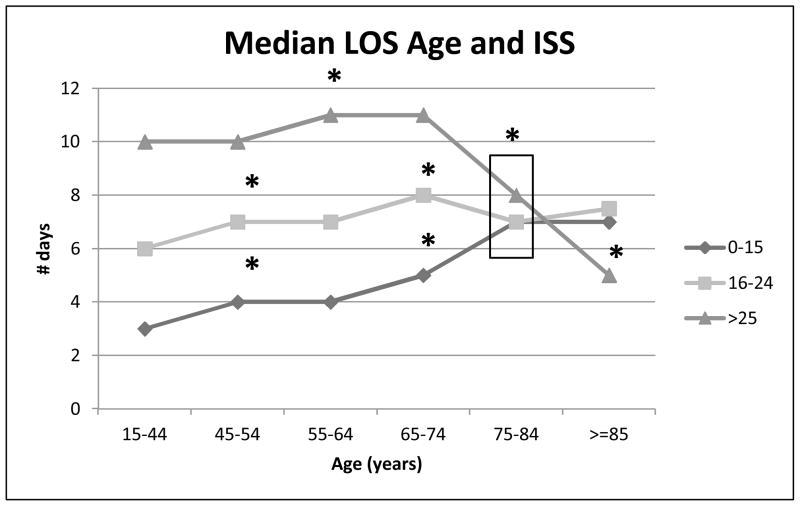
When examined overall, there was no significant difference in either ISS or length of stay (LOS) across the age groups (not shown), however once stratified by ISS there were significant differences found at multiple points. For patients with 0 – 15 ISS, there was a significant increase seen in median length of stay at 45–54 years, 65–74 years and 75–84 years of age, when each was compared to the preceding age group. For the 16 – 24 ISS patients, there was a significant increased in median LOS at 45–54 and 65–74, when compared to the age group below them (35 – 44 and 55 – 64, respectively), followed by a significant decrease in the median LOS seen in the 75–84 year age group. The most severely injured patients (ISS ≥ 25) had significant increase in median LOS seen at 55–64, followed by a significant decrease seen at 75–84 (Figure 3). Due to a lack of variation, age groups 15–24, 25–34 and 35–44 were condensed to one age group (15–44) for this graph. The LOS was 3, 3 and 3 days for the individual groups at ISS 0–15, 5, 6 and 6 at ISS 16–24, and 9, 10 and 10 for those with an ISS ≥ 25.
Figure 3. Median Length of Stay by ISS and Age.
Relationship between age and median length of hospital stay, stratified by ISS scores: 0–15, 16–24 and ≥ 25. Age groups combined from 15–44 years due to lack of variation: ISS 0–15 (3, 3 and 3), ISS 16–24 (5, 6 and 6) and ISS ≥25 (9, 10 and 10). Significant increase in LOS from age 65–74 to 75–84 for 0–15 ISS group. Significant decrease in LOS between ages 65–74 to 75–84 for 16–24 and ≥ 25 ISS group. All * p < 0.05.
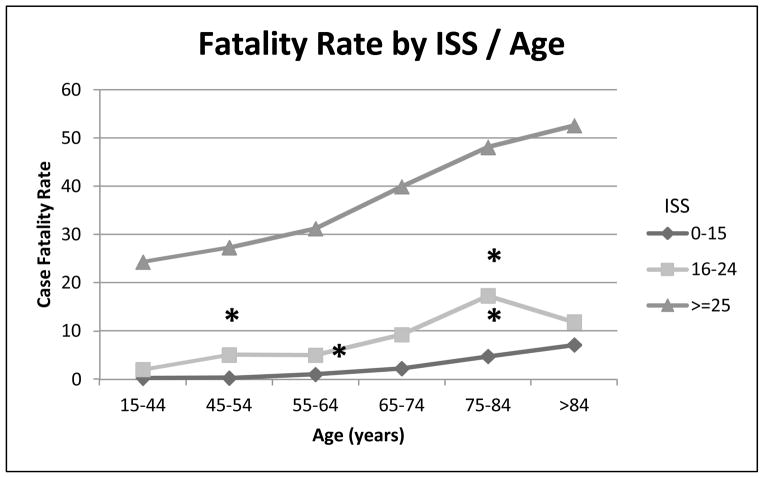
As has been shown in previous studies [2], increasing age correlated with increased fatality for all ISS groups in our study. Direct comparison of individual age groups revealed significant differences at multiple ages. As shown in Figure 4, patients in the mild-moderate severity of injury group (ISS 0–15) had significant increases in case fatality rates at 45–54 (p<0.001), 55–64 (p=0.023) and 75–84 (p=0.044) years of age compared to the preceding age bracket. In the severe injury group (ISS 16–24), there were significant increases at 45–54 (p<0.001) and 75–84 (p=0.022) years of age compared to the preceding age bracket. The increase in case fatality rate seen from 55–64 to 65–74 years was close but not significant (p=0.058). For the very severe ISS ≥ 25 group, there were no significant differences between individual age groups identified. When patients were dichotomized into two groups either greater than or less than 45 years of age, and then re-analyzed, there was a significant difference seen at each ISS level (p<0.001). Additionally, analysis of patients in groups of greater than or less than 65 years of age showed significant differences (p<0.001) for each stratification of ISS.
Figure 4. Fatality Rate by ISS/Age.
Relationship between age and case fatality rate, stratified by ISS groups: 0–15, 16–24 and ≥ 25. Age groups were combined from 15–44 years due to lack of variation: ISS 0–15 (0.2, 0.2 and 0.4), ISS 16–24 (2, 2 and 1) and ISS ≥25 (23, 25 and 26). Significant increase in fatality from age 15–44 to 45–54, and 65–74 to 75–84, within ISS 16–24. Significant increased in mortality from 45–54 to 55–64, and 65–74 to 75–84 within ISS 0–15. No significant differences between individual age groups for ISS ≥ 25. All * p < 0.05.
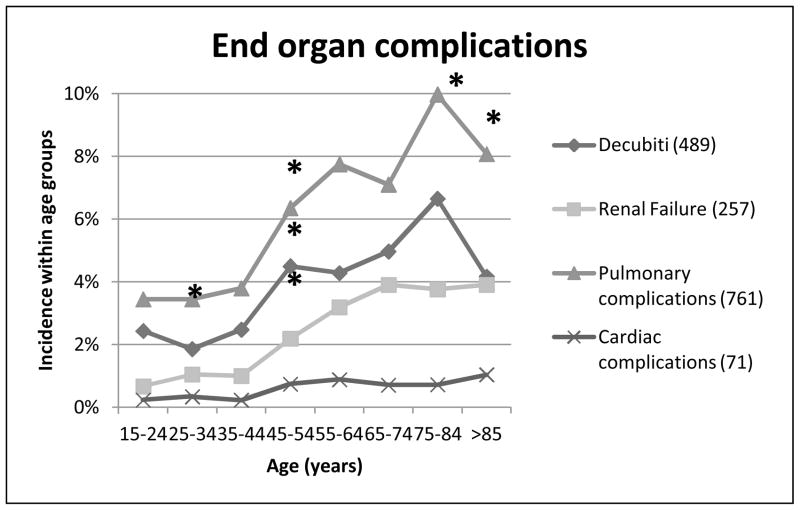
Next we looked at the morbidity experienced by our trauma patients. Complications relating to single end-organ failure were evaluated for integument failure as evidenced by decubiti ulcers, and renal, pulmonary and cardiac complications (Figure 5). The incidence of decubiti in our patients rose significantly at the 45–54 year age group (p<0.001), trended up at the 65–74 year group (p=0.093), then rose significantly for the 75–84 year old group (p<0.001). The incidence then dropped significantly for the >85 year olds (p<0.001). Changes occurred at earlier time points for pulmonary failure, which had a significantly increased incidence at 25–34 year olds (p<0.001), and again at 45–54 year olds (p<0.001). There was again a downturn between the last two age groups, with a trend to decreased incidence in >85 year olds (p=0.09). Renal failure only had a significant increase at 45–54 year old group, although there were trends to increased incidence at both 25–34 years (p=0.098) and 55–64 years (p=0.056). Cardiac failure had a much lower incidence for all age groups, and only trended toward a change at the 45–54 year olds (p=0.08).
Figure 5. End-organ complications.
Relationship between age and end organ complications, including integumentary complications (decubiti skin ulcers), renal failure, pulmonary complications (ARDS, pleural effusion, pulmonary edema, respiratory failure), and cardiac complications (arrhythmia, congestive heart failure, cardiac arrest, tamponade, myocardial infarction). *p < 0.01.
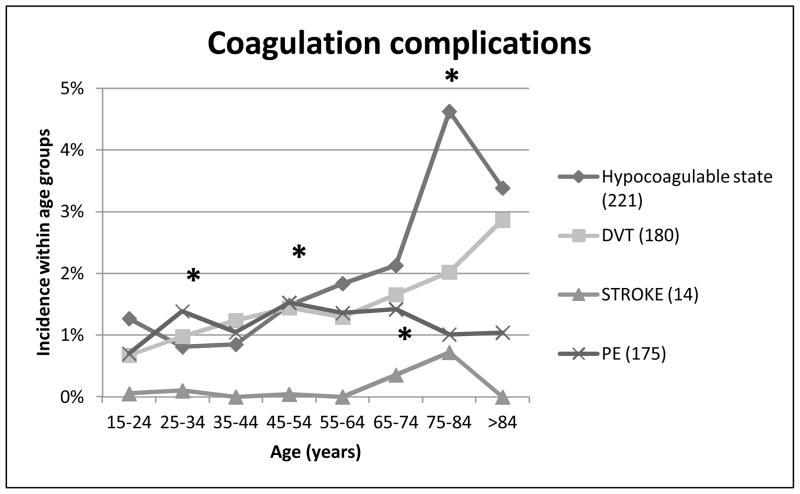
Next we examined coagulation complications, including hypocoagulable state, DVT, pulmonary embolism, and stroke (Figure 6). The incidence of hypocoagulopathy had significant increases at 45–54 year age group (p=0.035) and at 75–84 year olds (p=0.006). There were no significant differences in incidence of DVT’s across the age groups, however the incidence of pulmonary embolism was significantly increased in the 25–34 year olds. There was a low overall incidence of strokes in our trauma population, however there was a significant increase seen at the 65–74 year old group.
Figure 6. Coagulation complications.
Relationship between age and thrombo-embolic and coagulation complications, including hypocoagulable state, DVT, PE and stroke. * p < 0.05
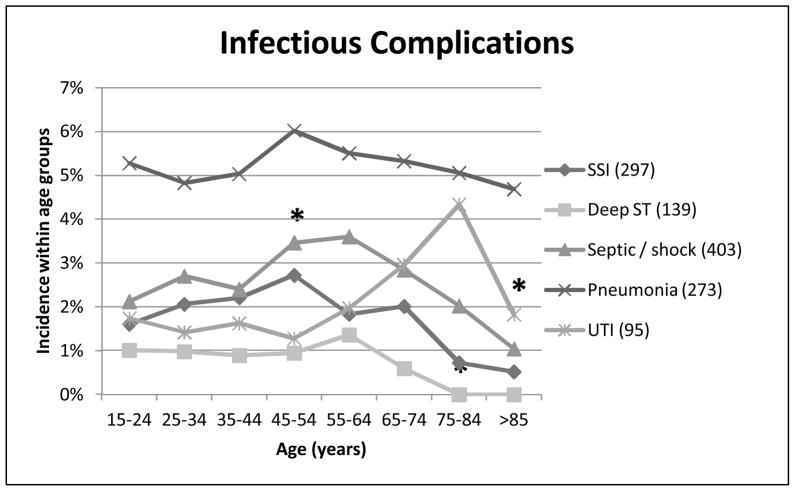
Lastly, we analyzed the incidence of infectious complications, including pneumonia, urinary tract infections (UTI), sepsis / septic shock, surgical site infections and deep-space tissue infections (Figure 7). Pneumonia had the highest incidence across all the age groups, however there were no significant differences in comparison between different ages. The incidence of sepsis / septic shock had a significant increase at 45–54 years of age (p=0.025), followed by a consistent trend of age-related decreased incidence. Surgical site infections had a peak incidence at 45–54 years of age, followed by a trending decrease, which reached significance only at the 75–84 year olds (p=0.034). Similarly, deep soft tissue infections and abscesses peaked at 55–64 years of age, then declined, reaching significance at the 75–84 year old group. The peak incidence of urinary tract infections was seen later, with the highest percentage seen in 75–84 year olds, followed by a significant decrease to the ≥ 85 year olds (p=0.029).
Figure 7. Infectious complications.
Relationship between age and infectious complications, including pneumonia, sepsis/shock (septicemia, shock, SIRS), surgical site infection, deep-space infections and intra-abdominal infection. * p < 0.05
DISCUSSION
The demographics of the trauma population are changing, with increasing numbers of elderly patients being admitted after major injury. In the four years of our study, we discovered a nearly three percent increase in percentage of elderly trauma admissions. US Census figures estimate that in 2010 15% of the population is over 65 years of age, and project that this will increase to over 20% of the population by the year 2030. Age has been shown to be an independent predictor of a mortality following trauma, and care for the elderly trauma patient is characterized by increased cost due to longer hospital stays and increased complication rates [3,4]. As the number of elderly trauma admissions increases, improvement of care and complication prevention will become an increasingly important issue.
For this study, we chose to define ‘elderly’ as individuals ≥ 65 years of age, however there is no consensus in the literature for this definition. This lack of uniformity of definition was noted in a 2003 EAST guidelines literature review of geriatric trauma which cited a range from 55 to 80 years of age as a cutoff for the definition of “elderly” or “geriatric” patients. They even noted papers which argued that increased mortality was seen as early as 45 years of age [14]. Our results confirmed previous findings of a strong association between increased age and ISS with an increase in mortality [2,3]. Interestingly, we also found the “breakpoint” for the increasing complications and associated mortality started at 45 years of age, as some of the papers in the EAST review had intimated. Specifically, Holcomb, et al analyzed patients by age, number of rib fractures and outcomes, and found that patients > 45 years of age with more than four rib fractures were at increased risk of adverse outcomes [15]. Furthermore, Todd, et al implemented a multidisciplinary clinical pathway to improve the outcomes of elderly patients with multiple rib fractures, and demonstrated a decline in ventilator days, hospital LOS, infectious morbidity and mortality [16].
We also examined age in relation to hospital LOS. When examined by age alone, there was no difference in median hospital LOS between the age groups (not shown), however when stratified by ISS, there was a significant age-associated increase in hospital stay in the lower severity injuries (ISS 0–15) which began trending upward after 45 years of age. In the higher severity injuries (ISS 16 – 24 and ISS ≥ 25) there was an increase in median hospital LOS until the age of 65–74, followed by a significant decreased. This decline likely is associated with the increasing mortality seen in these age groups (Figure 3).
We demonstrated an age-dependent increase in renal failure, as well as respiratory, cardiac and integumentary complications (decubiti ulcers), each of which began to increase after the age of 45 in our study. Much of this may be explained by decreasing baseline function and pre-existing medical conditions, as well as decreased reserve for response to stress in these older patients. The respiratory system has well-documented age-related changes, including decreased alveolar surface area of four percent per decade beginning after 30 years of age, reducing the area for gas exchange by 0.5% per year. Multiple rib fractures in particular are associated with significant morbidity, including atelectasis, pneumonia, ventilator dependence and mortality [17]. Renal failure is seen with increasing age due to progressive sclerosis of glomeruli as a natural aging process, which results in a loss of functioning nephrons by ten percent per decade after 40 years of age and a corresponding decline in glomerular filtration rate. As such, the elderly patient is less able to concentrate urine, making urine output a less reliable surrogate for organ perfusion [17]. Skin atrophy with aging is due to decreased tissue water, subcutaneous fat and tonicity, making the elderly immobilized trauma patient far more vulnerable to pressure ulcers [17]. Indeed, the incidence of skin decubiti was the most pronounced age-related organ failure we found, with increases starting at age 45 and more than doubling by age 75.
Increase in cardiac failure was less impressive in our study population, although cardiovascular comorbidities are the most frequent in the geriatric population. Decreased ability to respond to catecholamines limits maximal heart rate, and coronary artery disease reduces auto-regulation of blood flow to the heart, thus increasing occult or overt ischemia in times of increased demand [17]. Despite this decreased physiologic reserve, elderly patients are still able to mount a hyperdynamic response to severe stress, and therefore resuscitation in this population is not futile [18]. The results from the analysis of coagulation complications were less impressive, but increases in hypocoagulopathy were seen beginning after 45 years of age, with a peak at 75–84 years (Figure 6). Age alone is the most significant risk factor for DVT, and both immobility and medication increase the risk, but were not measured in this study. Pre-existing medications could also partially explain the correlation seen with hypocoagulopathy and age, however this information was not available within our database. A 2008 study by Williams, et al evaluated pre-injury anticoagulation and found it to be independently associated with mortality, and therefore advocated early coagulopathy assessment and aggressive reversal of anticoagulation in this population [19].
The most intriguing results were in the infectious complication analysis, with initial upward trend followed by a decline in incidence of infections after the age of 65. The reason for this has not yet been elucidated, and is an area of ongoing research. The incidence of UTI followed a different pattern, with the peak incidence not occurring until 75–84 years of age. One possible explanation is that the diagnosis of infection is impaired in this population. Girard, et al noted that elderly patients do not always mount the typical physiological response to infection, which could lead to delayed or missed diagnoses [20]. Perhaps there were incidents of infection in our elderly patients manifested only by delirium, and not recognized by the clinicians.
In healthy individuals, the response to trauma is an upregulated pro-inflammatory cascade. In severely injured patients who experience a ‘second hit’, this response can overcome normal regulatory signals and progress into a dysfunctional and protracted immune response leading to multi-system organ failure. A recent publication from Valente, et al explored the innate differences in the immune response of elderly trauma patients, defined as > 65 years, compared to non-injured controls. Their analysis of serum samples for markers of neutrophil activation found that elderly patients had a delayed or muted immune system response to injury [21]. Although this report did not compare elderly injured to young injured patients, and they have yet to correlate their preliminary data with clinical findings of infection, it does raise an interesting potential explanation of our pattern of infectious complications.
There are a few limitations in this study that should be mentioned. This was a retrospective review of a clinical database, not a prospective analysis of data specifically related to the geriatric population. Additionally, we have no data on pre-existing disease in the population that can be correlated with their incidence of complications. Previous studies have noted a 9–20% incidence of pre-existing conditions, increasing to nearly 70% in patients greater than 75 years old [14, 17]. The existence of age-associated co-morbidities or dysfunction likely contributes to some of the end-organ failure demonstrated within our study. The DNR status of these patients was not assessed as part of this study, and details on whether or not supportive care was withdrawn was not available in our database during this time period. These data are now part of current chart reviews to provide insight in the future to end-of-life decisions in our patients.
Conclusions
The increasing age of the trauma population and the increased morbidity and mortality experienced by these patients is an issue of real and growing concern to all trauma surgeons. There have been recent publications noting improvement in outcomes of geriatric trauma patients when a geriatrician became involved in their care. Whether through management of pre-existing disease, suggestions for medications better suited for the elderly, or recognition of new-onset delirium as an early sign of sepsis, involvement of geriatricians reduced many hospital acquired complications and improved their outcomes [22]. In light of the data from our study, we plan to work in conjunction with our geriatric colleagues to further investigation the unique complication patterns and physiological differences seen in our elderly trauma patients. We believe that an interdisciplinary team can help prevent complications and improve outcomes, returning this vulnerable population to a healthy functional status, on par to their lives before injury.
Footnotes
Financial disclosure: This research was supported by the University of Texas Medical School-Houston and NIGMS funding (T-32 GM008792 and P-50 GM38529)
Reprints: John B. Holcomb, MD, 6410 Fannin Street, Suite 1100, Houston, TX 77030, Phone: 713-500-5493, Fax: 713-512-7135
Presented at the Regional Resident paper competition, ACS-COT, November 7, 2010; Presented at the Western Trauma Association Annual Meeting, March 3, 2010.
Contributor Information
Sasha D Adams, Email: sasha.d.adams@uth.tmc.edu.
Bryan A Cotton, Email: bryan.a.cotton@uth.tmc.edu.
Mary F McGuire, Email: mary.f.mcguire@uth.tmc.edu.
Edmundo Dipasupil, Email: edmundo.dipasupil@memorialhermann.org.
Jeanette M Podbielski, Email: jeanette.m.podbielski@uth.tmc.edu.
Adrian Zaharia, Email: adrian.zaharia@uth.tmc.edu.
Drue N Ware, Email: drue.n.ware@uth.tmc.edu.
Brijesh S Gill, Email: brijesh.s.gill@uth.tmc.edu.
Rondel Albarado, Email: rondel.albarado@uth.tmc.edu.
Rosemary A Kozar, Email: rosemary.a.kozar@uth.tmc.edu.
James R Duke, Email: james.h.duke@uth.tmc.edu.
Philip R Adams, Email: pradamsmd@yahoo.com.
Carmel B Dyer, Email: carmel.b.dyer@uth.tmc.edu.
John B Holcomb, Email: john.holcomb@uth.tmc.edu.
References
- 1.U.S. Census Bureau. Population estimates. http://www.census.gov/popest/estimates.html.
- 2.Tornetta P, 3rd, Mostafavi H, Riina J, et al. Morbidity and mortality in elderly trauma patients. J Trauma. 1999;46(4):702–6. doi: 10.1097/00005373-199904000-00024. [DOI] [PubMed] [Google Scholar]
- 3.Taylor MD, Tracy JK, Meyer W, et al. Trauma in the elderly: intensive care unit resource and outcome. J Trauma. 2002;53(3):407–14. doi: 10.1097/00005373-200209000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Newell MA, Rotondo MF, Toschlog EA, et al. The elderly trauma patient: An investment for the future? J Trauma. 2009;67(2):337–340. doi: 10.1097/TA.0b013e3181add08b. [DOI] [PubMed] [Google Scholar]
- 5.Potoka DA, Schall LC, Gardner MJ, et al. Impact of pediatric trauma centers on mortality in a statewide system. J Trauma. 2000;49(2):237–45. doi: 10.1097/00005373-200008000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs DG. Special considerations in geriatric injury. Curr Opin Crit Care. 2003;9(6):535–9. doi: 10.1097/00075198-200312000-00012. [DOI] [PubMed] [Google Scholar]
- 7.Campbell JW, Degolia PA, Fallon WF, et al. In harm’s way: Moving the older trauma patient toward a better outcome. Geriatrics. 2009;64(1):8–13. [PubMed] [Google Scholar]
- 8.American College of Surgeons National Trauma Data Bank. Report. 2009 http://www.facs.org/trauma/ntdb/docpub.html.
- 9.Moore FA, Sauaia A, Moore EE, et al. Post-injury multiple organ failure: a bimodal phenomenon. J Trauma. 1996;40:501–510. doi: 10.1097/00005373-199604000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Sauaia A, Moore FA, Moore EE, et al. Multiple organ failure can be predicted as early as 12 hours after injury. J Trauma. 1998;45(2):291–301. doi: 10.1097/00005373-199808000-00014. [DOI] [PubMed] [Google Scholar]
- 11.Ciesla DJ, Moore EE, Johnson JL, et al. Multiple organ dysfunction during resuscitation is not post-injury multiple organ failure. Arch Surg. 2004;139(6):590–594. doi: 10.1001/archsurg.139.6.590. [DOI] [PubMed] [Google Scholar]
- 12.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–874. [PubMed] [Google Scholar]
- 13.Bone RC, Sprung CL, Sibbald WJ. Definitions for sepsis and organ failure. Crit Care Med. 1992;20(6):724–726. doi: 10.1097/00003246-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs DG, Plaisier BR, Barie PS, et al. Practice management guidelines for geriatric trauma: The EAST practice management guidelines work group. J Trauma. 2009;54(2):391–416. doi: 10.1097/01.TA.0000042015.54022.BE. [DOI] [PubMed] [Google Scholar]
- 15.Holcomb JB, McMullin NR, Kozar RA, et al. Morbidity from rib fractures increases after age 45. J Am Coll Surg. 2003;196(4):549–55. doi: 10.1016/S1072-7515(02)01894-X. [DOI] [PubMed] [Google Scholar]
- 16.Todd SR, McNally MM, Holcomb JB, et al. A multidisciplinary clinical pathway decreases rib-fracture associated infectious morbidity and mortality in high-risk trauma patients. Am J Surg. 2006;192(6):806–11. doi: 10.1016/j.amjsurg.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 17.Schulman CI, Alouidor R, McKenney MG. Geriatric Trauma. In: Moore EE, Feliciano DV, Mattox KL, editors. Trauma. 6. McGraw-Hill Companies; 2008. pp. 1003–1018. [Google Scholar]
- 18.McKinley BA, Marvin RG, Cocanour CS, et al. Blunt trauma resuscitation: the old can respond. Arch Surg. 2000;135(6):688–93. doi: 10.1001/archsurg.135.6.688. [DOI] [PubMed] [Google Scholar]
- 19.Williams TM, Sadjadi J, Harken AH, et al. The necessity to assess anticoagulation status in the elderly injured patients. J Trauma. 2008;65(4):772–6. doi: 10.1097/TA.0b013e3181877ff7. [DOI] [PubMed] [Google Scholar]
- 20.Girard TD, Opal SM, Ely EW. Insights into Severe sepsis in older patients: from epidemiology to evidence based management. Aging and infectious diseases. 2005;40:719–725. doi: 10.1086/427876. [DOI] [PubMed] [Google Scholar]
- 21.Valente SA, Fallon WF, Alexander TS, et al. Immunologic function in the elderly after injury – the neutrophil and innate immunity. J Trauma. 2009;67(5):968–974. doi: 10.1097/TA.0b013e3181b84279. [DOI] [PubMed] [Google Scholar]
- 22.Fallon WF, Jr, Rader E, Zyzanski S, et al. Geriatric outcomes are improved by a geriatric trauma consultation service. J Trauma. 2006;61(5):1040–6. doi: 10.1097/01.ta.0000238652.48008.59. [DOI] [PubMed] [Google Scholar]