Abstract
The value of a positron emission tomography and X-ray computed tomography (PET/CT) combined service in terms of diagnostic accuracy, cost-effectiveness and impact on clinical decision-making is well-documented in the literature. Its role in the management of patients presenting with cancer is shifting from early staging and restaging to the early assessment of the treatment response. Currently, the application of PET/CT has extended to non-oncological specialties—mainly neurology, cardiology and rheumatology. A further emerging application for PET/CT is the imaging of infection/inflammation. This article illustrates some of the PET/CT applications in both oncological and non-oncological disorders. In view of the absence of this modality in Oman, this article aims to increase the awareness of the importance of these imaging modalities and their significant impact on diagnosis and management in both oncological and non-oncological specialties for patients of all age groups as well as the decision-makers.
Keywords: Positron Emission Tomography, X-Ray Computed Tomography, Medical Oncology, Oman
The Minstry of Health in Oman was established in August 1970 by a Royal Decree that stated that all Omani citizens shall get free of charge health services. Today, four decades later the Omani health sector has developed immensely. This is evidenced by the increases in the number of hospitals; the number of hospital beds; the purchasing of new equipment; the number, type and complexity of therapies; the technical proficiency of the staff, including those who are national, and the variety of procedures performed. Above all, the quality of the health service in the country has vastly improved. Four decades ago, there was only a very basic health service which provided, for instance, life-saving treatments only to those fortunate few who could reach a health centre or hospital. Nowadays highly complicated and skilled procedures and surgery are available to everyone. Currently, both those living in urban areas and those living in very remote areas can access the health services they need.1
The inauguration of the National Oncology Centre (NOC) at the Royal Hospital (RH), Muscat, Oman, in December 2004 was one of the biggest recent additions to health services in the country. It included a well-equipped radiotherapy department with two linear accelerators, the first in the country. This meant that oncology patients no longer needed to travel to nearby countries for radiotherapy treatments. The NOC also includes departments of medical and paediatric oncology, haemato-oncology and nuclear medicine. However, an important contemporary key modality is missing in this centre and in the country as a whole: that of a PET/CT service. This article highlights the importance of PET/CT in managing cancer. By illustrating some of the applications of PET/CT, we hope to raise awareness of its importance and cost-effectiveness.
Positron emission tomography (PET) was introduced in the 1970s as a research device in neurosciences and cardiology. Two decades later, X-ray computed tomography (CT) technology was added to the PET scanner, producing so-called fusion, or hybrid, imaging. A PET/CT combined scanner was first introduced in 1998. Since then, patients can be scanned both with PET and CT at the same time without having to move. The aim of this hybrid imaging was to combine the functional molecular imaging of PET with the high-contrast anatomical images provided by CT.
The value of combining PET and CT (PET/CT) in terms of diagnostic accuracy, cost-effectiveness and the impact on clinical decision-making and health outcomes are well-documented in the literature.2 PET/CT imaging with the main radiopharmaceutical used, 18F-fluorodeoxyglucose (18F-FDG), is considered today, in most of the developed world, to be essential in the management of a range of malignancies. Table 1 lists the major malignancies or cancers where PET/CT provides a unique clinical value. In addition, the role of PET/CT in the management of patients presenting with cancer is shifting from early staging and restaging after recurrence to the early assessment of treatment response.3,4
Table 1:
Optimal indications of positron emission tomography/X-ray computed tomography in various malignancies
| Malignancy | Tracer(s) | Role |
|---|---|---|
| Lung cancers/mesotheliomas | 18F-FDG | Diagnosis; staging; recurrent disease; EBRT planning; therapy response. |
| Colorectal cancers | 18F-FDG | Staging of distant metastases; recurrent disease. |
| Breast cancers | 18F-FDG; 18F-FLT | Staging of distant metastases; recurrent disease; re-staging; therapy response. |
| Lymphomas | 18F-FDG; 11C-MET | Staging; therapy response. |
| Multiple myelomas | 18F-FDG | Staging; diagnosis; therapy response. |
| Gynaecological cancers | 18F-FDG | Staging; recurrent disease; therapy response; re-staging. |
| Melanomas | 18F-FDG | Staging of distant metastases; therapy response; recurrent disease. |
| Sarcomas | 18F-FDG | Staging; re-staging; therapy response. |
| Primary brain tumours | 18F-FDG; 11C-MET; 18F-FLT | Grading/prognosis; guide for biopsy; recurrence versus scar post-therapy. |
| Head and neck cancers | 18F-FDG; 18F-MISO | Staging; therapy response; cancer of unknown primary; EBRT planning; recurrent disease. |
| Oesophageal/gastric cancers | 18F-FDG; 18F-FLT | Staging of distant metastases; therapy response. |
| Biliary tract cancers | 18F-FDG | Staging of distant metastases. |
| Pancreatic cancers | 18F-FDG | Staging of distant metastases; recurrent disease; therapy response. |
| Prostate cancers | 11C-choline; 18F-choline | Staging of distant metastases; recurrent disease. |
| Follicular thyroid cancers | 18F-FDG | Re-staging of radioiodide-negative, thyroglobulin-positive cancer. |
| Medullary thyroid cancers | 18F-FDG; 18F-DOPA | Staging. |
| Neuroendocrine tumours | 68Ga-DOTA-TOC | Re-staging; selection for PRRT. |
18F-FDG = 18F-fluorodeoxyglucose; EBRT = external beam radiation therapy; 18F-FLT = 18F-fluorothymidine; 11C-MET = 11C-methionine; 18F-MISO = 18F-fluoromisonodazol; 18F-DOPA = 18F-dihydroxyphenylalanine; 68Ga-DOTA-TOC = gallium-68-DOTA-TOC; PRRT = peptide radio-receptor therapy (with somatostatin analogues).
Adapted from: Strauss HW, Mariani G, Volterrani D, Larson SM. Nuclear Oncology: Pathophysiology and Clinical Applications. New York: Springer Verlag, 2013.
With PET evaluation, the term “staging” refers to parameters N and M, since parameter T is best staged with morphological imaging such as computed tomography and/or magnetic resonance imaging. Furthermore, a common feature of positron emission tomography imaging with 18F-FDG is the prognostic information it provides, based on the intensity of uptake, as an indirect parameter of tumour aggressiveness.
Currently, the use of 18F-FDG PET/CT has extended to non-oncological applications as well. The main non-oncological use of 18F-FDG PET is in neurology (mostly for diagnosing patients with dementia, or for localising the ictal focus in patients with drug-refractory epilepsy) and in cardiology (for assessing the myocardial viability). A further emerging indication for 18F-FDG PET/CT includes imaging of infection and inflammation.
Regarding the applications in cardiology, scintigraphy with technetium (99mTc)-sestamibi evaluates the semi-quantitative myocardial blood flow, and therefore underestimates the myocardial viability.5 Different PET imaging agents can be used to assess myocardial perfusion (such as 13N-ammonia, 15O-water and, more recently, rubidium-82 [82Rb]-chloride), while 18F-FDG uptake reflects the metabolic activity. A positive 18F-FDG uptake in the region with reduced perfusion indicates myocardial viability, thus predicting that the patient will benefit from revascularisation procedures. On the other hand, a reduced 18F-FDG uptake in the region, with reduced perfusion as well (i.e., matched perfusion/metabolic defects), indicates an irreversibly-damaged myocardium.6 Of special interest to cardiology is a new agent for myocardial perfusion labelled with 18F, the 18F-BMS-747158-02, which is in an advanced stage of clinical validation.
As mentioned above, important non-oncological applications include neurological disorders, mainly to differentiate the various types of dementia. In addition, 18F-FDG PET may have a role in the pre-surgical assessment of patients with partial complex seizures, where magnetic resonance imaging (MRI) is either normal or equivocal.7 The use of 18F-FDG in rheumatology has been documented, with applications including the assessment of disease activity in arthritis, based on evaluating the metabolic activity in synovitis; this parameter helps to measure disease activity in patients with rheumatoid arthritis.8 Additionally, 18F-FDG-PET is employed to evaluate the disease extent/activity in arteritis.9 Recently, 18F-FDG has also been used to evaluate the response to therapy, especially when using expensive biological drugs such as anti-tumour-necrosis factor (anti-TNF) drugs, as an early prediction of the clinical outcome reduces the overall cost of treatment.10
An emerging use of PET/CT is to assess infection and inflammation. PET has both high sensitivity and specificity in imaging osteomyelitis. In addition, it has been used to evaluate sarcoidosis and inflammatory bowel disease.11 Furthermore, 18F-FDG PET/CT has an important role in evaluating patients with a fever of unknown origin (FUO), as it helps in the precise localisation and early identification of the underlying cause of this clinical condition. In most patients with FUO, the main cause will turn out to be due to an infection, autoimmune disease or malignancy; using PET/CT as a single modality can diagnose and assess those diseases with a high level of sensitivity.12
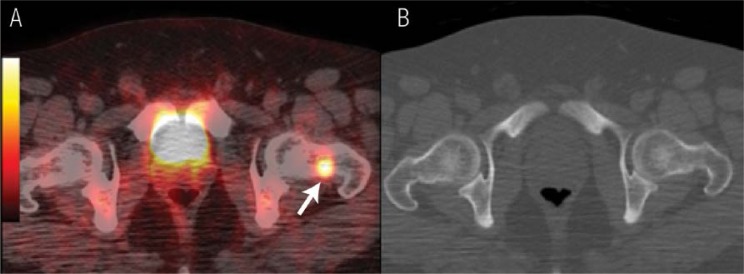
In spite of the wide range of applications mentioned above, however, the vast majority of clinical PET scans performed today worldwide are in the setting of cancer staging and restaging, in which PET/CT often ‘upstages’ many patients [Figure 1].
Figure 1 A & B.
18F-fluorodeoxyglucose positron emission tomography (PET) / X-ray computed tomography (CT) for a patient diagnosed with nasopharyngeal carcinoma in which the PET/CT imaging upstaged the disease by revealing a bone lesion that was not detected by CT. A: The fused PET/CT image revealed a focal uptake in the neck of the left femur (arrow). B: The CT image, bone window, did not show any bone abnormalities. The technetium (99mTc)-methylene diphosphonate (MDP) bone scan of this patient was also negative (images not included).
Future Uses of 18F-Labelled Radiotracers
Among the 4 short-lived cyclotron-produced PET isotopes, 18F has the longest half-life (110 mins), making it the most suitable isotope to label PET radiotracers. It can be commercially produced, hence reducing the necessity of in-house cyclotron production in each PET/CT institute. This has resulted in a wide range of research projects worldwide to develop new 18F-labelled PET radiotracers. One example of a newly developed PET radiotracer is 3′-deoxy-3′-18F-fluorothymidine (18F-FLT). It is a thymidine analogue and its uptake detects cellular proliferation.13 It shows a good uptake in a number of tumours: lung cancer, lymphoma, breast cancer and glioma. The sensitivity and specificity of FLT compared to that of FDG in those tumours is, however, beyond the scope of this article. Another 18F-labelled PET radiotracer is 2-18F-fluoro-L-tyrosine (8F-TYR), a marker of amino acid transport. It has shown good uptake in meningiomas even after irradiation. It can clearly aid in the visualisation and follow-up of such tumours.14
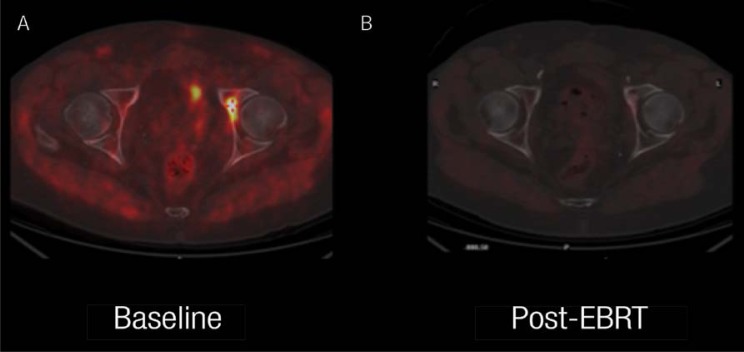
Imaging of Alzheimer’s disease (AD) has developed over the last decade. By using PET, it has been possible to detect the deposition of amyloid B in the human brain. Recently, 18F-labelled amyloid PET radiotracers have been approved for clinical trials aiming to assess the effect of drugs for AD therapy. The latest so far is 18F-florbetapir; this agent was approved by the United States Food & Drug Administration (FDA) in April 2012. It also received marketing authorisation in October 2012 from the European Medicines Agency’s Committee for Medicinal Products for Human Use.15 Another recently addition to the 18F PET radiotracer family is 18F-fluorocholine. This agent is used for imaging prostate cancer, where its main applications are to evaluate local recurrence or metastatic disease. It is being increasingly used in Europe and Japan [Figure 2].16
Figure 2 A & B.
18F-fluorocholine positron emission tomography (PET)/X-ray computed tomography (CT) scan in a patient with prostatic adenocarcinoma. This imaging was done to assess the efficacy of therapy in this patient with a single bone metastasis, appearing one year after primary treatment (serum prostate-specific antigen was 2.4 ng/ml). The images are of the fused PET/CT transaxial section at the level of the femoral heads. A: The baseline images showed focal accumulation of the tracer in the left acetabulum, the only site of metastatic disease. B: The favorable response after external beam radiation therapy (EBRT) is shown in this image.
Last but not least, is the radiotracer 18F-sodium fluoride (NaF). It has been known for decades that 18F-fluoride has a high affinity for bone, yet it was not widely used. This was mainly due to its high energy (511 Kilo-electron volts [keV]) which limited its use with the existing gamma cameras due to the scintillation employed—sodium iodide activated with thallium [NaI(Tl)]—being too low in density and of insufficient thickness, and hence having a low stopping power. Furthermore, 99mTc-methylene diphosphonate (MDP) was readily available and suitable for use with the gamma camera. Since the introduction of modern PET and PET/CT scanners, 18F-fluoride has been more frequently used for evaluating bone abnormalities that are due to both benign and malignant diseases. There are a number of studies that have compared 99mTc-MDP bone scanning to 18F-fluoride PET and found that the latter has a higher diagnostic accuracy in detecting skeletal diseases.17
PET Imaging Agents Other than 18F-Fluorodeoxy-glucose
The use of 18F-FDG suffers from important limitations in certain oncological conditions, due either to the physiological biodistribution (for instance, very high uptake in the brain cortex or at the excretion sites) that hampers identification of the tumour lesions, or to the low expression of the glucose transporter system in certain cancers (such as in prostate cancers, mucinous cancers, hepatocellular carcinomas and bronchioloalveolar lung cancers, among others). Alternative PET tracers can be employed in these conditions, for instance radiolabelled amino acids (such as tyrosine or choline analogues) or radiolabelled precursors of deoxyribonucleic acid (DNA) synthesis (such as thymidine analogues). Perhaps the best established occurrence of this type is the use of 11C-choline or 18F-fluorocholine in patients with prostate cancer, with tumours that are generally characterised by the low expression of the glucose transporter proteins. 11C-acetate is instead most frequently employed in patients with a hepatocellular carcinoma, another tumour characterised by the low expression of the glucose transporter proteins. Proximity with the very high uptake in the brain cortex can hamper the detection of primary brain tumours; in these patients, discrimination of tumour recurrence from post-radiotherapy scar/necrosis (which is not an easy task with conventional CT/MRI) is better achieved by using alternative PET tracers such as 11C-methionine or 11C-thymidine/18F-fluoroethyl-L-tyrosine (FET). Still another imaging alternative is to use somatostatin analogues labelled with gallium-68 [68Ga] (i.e., 68Ga-DOTA-TOC/NOC/TATE). This radionuclide is especially attractive for PET centres since, despite its short physical half-life of only 68 mins, it is available even without an in-house cyclotron as the product of a germanium-68 [68Ge]/68Ga generator. In fact, the physical half-life of the parent radionuclide 68Ge (approximately 9 months) enables a single generator to meet clinical needs over at least 6 months. The 68Ga-labelled somatostatin analogues mentioned above are especially useful for PET imaging in patients with neuroendocrine tumours, another oncological condition where 18F-FDG imaging is suboptimal. Additionally, the 68Ga PET label on these compounds can be substituted with a therapeutic radionuclide, such as yttrium-90 (90Y) or lutetium-177 (177Lu), to deliver treatment to 68Ga-DOTA-TATE-positive tumours.
International Atomic Energy Agency Expert Missions to Oman: Evaluating the status of nuclear medicine and recommendations regarding a PET/X-ray CT and cyclotron facility
Since Oman became a member of the International Atomic Energy Agency (IAEA) in February 2009, a number of expert missions have taken place. The ones that concern us in this article are those undertaken to evaluate the status of nuclear medicine in the country and their feedback reports and recommendations. The first of these was in December 2009. It concluded that “Oman has currently a capacity to fully utilize two PET/CT cameras to be located in Muscat region. A single PET/CT should be introduced as soon as possible with a second one to follow.”18 This was followed shortly with another expert mission in March 2010 which recommended: “A national PET/CT Scanner Centre including a cyclotron should be planned and built. The logical location for this would be the Royal Hospital as it has a large Oncology Program.”19 This was shortly followed by another expert mission that took place in October 2010. In the feedback report the expert wrote: “The level of NM practice in Oman is of a remarkably high quality. The country looks very well prepared for PET. The medical doctors would only require a limited period of very focused training for PET practice. However, it would still require many of the other professionals needed to run a complex facility such as a cyclotron/PET centre, namely radiopharmacists/chemists and medical physicists. Their preparation would be longer and training should start as soon as a positive decision is taken about the implementation of a cyclotron in the country.”20
Two years later in 2012, the IAEA undertook an external audit of the practice of nuclear medicine in Oman. One of the recommendations of this expert mission was “consider introduction of PET/CT in the country. This will have positive return in the management of cancer patients and saving in the use of high cost chemotherapeutic drugs”.21 In the same year, another expert mission took place in September. It concluded: “There is a need for PET/CT in Royal Hospital and Sultan Qaboos University Hospital (SQUH). Cyclotron facility in Muscat region is needed to supply radiopharmaceuticals for both centers. Such a project will take time and in view of the urgent need for PET/CT, exporting the radioactivity as a temporary solution should be considered. This is not cost-effective for long-term practice. The best option to establish such centre is to do it as a turnkey project.”22 The last expert mission that took place at the time of writing this paper was in March 2013 and concluded: “There is an urgent need to (establish) two PET/CT scanners in Royal Hospital and SQUH, which is fully justified. There is also a need to establish a National Cyclotron Facility that can produce and supply the PET tracers to the local centers”.23
How many Scanners are Needed to Provide Appropriate Access to PET/CT?
A detailed derivation of the population need is difficult to ascertain from the literature, but the most quoted current figures suggest that the appropriate number of PET scanners to provide adequate care for the population’s clinical needs is approximately 1 per 1–1.5 million people, although the geographical spread of a nation’s population may skew this.24,25 As the clinical utility and benefits to patient management of PET have been increasingly demonstrated in the most recent literature, even greater access to PET is likely in the future with a 20% annual growth rate of PET procedures expected after the year 2014.26
In a recent review of PET access in Europe, it was recommended that one PET camera is needed per 1.2 million people to meet the population’s needs,24 and the report of the Department of Health in the UK recommended one camera per 1–1.5 million people.25 The population number per PET scanner is much lower when it comes to the USA and other European countries, where there is one PET camera per 300,000–900,000 people [Table 2];27 a similar distribution of PET cameras exists in some of the Gulf Cooperation Council (GCC) countries.28 In the Australian PET report, the State Health Departments of Queensland and Victoria recommended 4 scanners in each state, hence assuming a ratio of one camera per 900,000 people and one camera per 1.2 million people, respectively.29 However, Australia is not a densely populated country and by the end of 2012 there were 36 PET/CT cameras serving the population of 22.5 million. A more useful metric may be that for every one radiation therapy linear accelerator, a PET/CT camera is required.
Table 2:
Number of positron emission tomography/computed tomography units per population in some developed countries and the Gulf Cooperation Council countries19,20
| Country | PET/CT units | Units per population |
|---|---|---|
| USA | 1,000 | 1 per 297,000 |
| Austria | 17 | 1 per 477,000 |
| Belgium | 21 | 1 per 490,000 |
| Germany | 85 | 1 per 970,000 |
| Switzerland | 7 | 1 per 1,000,000 |
| Japan | 100 | 1 per 1,270,000 |
| Sweden | 7 | 1 per 1,290,000 |
| Denmark | 4 | 1 per 1,350,000 |
| Netherlands | 10 | 1 per 1,600,000 |
| France | 36 | 1 per 1,650,000 |
| Spain | 19 | 1 per 2,100,000 |
| KSA | 12 | 1 per 2,400,000 |
| UAE | 3 | 1 per 1,800,000 |
| Kuwait | 3 | 1 per 857,000 |
| Bahrain | 2 | 1 per 500,000 |
| Qatar | 1 | 1 per 1,853,563 |
PET = positron emission tomography; CT = computed tomography; KSA = Saudi Arabia; UAE = United Arab Emirates.
Current Utilisation of PET/CT Services in Oman
In Oman the importance of PET/CT imaging has been acknowledged for nearly a decade, especially for cancer patients. Due to the lack of PET/CT facilities in the country, patients are being sent abroad at the expense of the Ministry of Health.
The cases sent abroad are specially selected, and PET/CT is used in such cases as a problem-solving tool in cases where both the existing imaging modalities and the histopathology reports are inconclusive. Those patients are mainly from the medical oncology, haemato-oncology, paediatric oncology and endocrinology departments. Other medical specialties began sending patients with benign conditions for PET/CT imaging, primarily to evaluate viability in patients with coronary artery disease, or rheumatology patients (mainly those with vasculitis in whom the available imaging modalities, namely CT and MRI, were inconclusive).
So far, PET/CT imaging has not been fully utilised, particularly for oncology patients. Although experts in medical oncology and nuclear medicine realise the importance of using PET/CT in staging, mid-therapy evaluation, end-of-therapy evaluation, and restaging, they are not able to use PET/CT in this way. The fact that such a modality is not available in Oman places several important logistical limitations on the treatment options, as it is not cost-effective to send all of those patients abroad.
Would PET/CT be a Cost-Effective Modality in Oman?
Reviewing the latest available cancer incidence statistics in Oman shows that there were 950 new cases diagnosed in 2010.30 Additionally, the top 10 most common cancers in Oman are similar to the rest of the world. Breast cancer and cervical cancer represent the top two cancers in women, while prostate cancer is the top ranking cancer in men, followed by non-Hodgkin’s lymphoma.30 Bearing in mind the ideal utilisation of PET/CT imaging, there will be about 800–900 patients per year needing to be scanned for primary staging; about one-third of those patients will have early evaluations of the response to chemotherapy/radiotherapy by a second early scan, and later on a third scan will be needed to evaluate the response at completion of treatment. The above considerations will add to the current practice in Oman for cancer patients, who will benefit from restaging using PET/CT imaging; alternatively, as is currently the case, PET/CT can be used as a problem-solving tool when other investigations are inconclusive.
Using the above figure, it is calculated that more than 2,000 patients in departments of adult and paediatric oncology will benefit from PET/CT if it is available in the country. Also, it should be noted that these numbers were published two years ago and that there is an expected increase in the number of new cancer cases worldwide over the coming two decades, more so in developing countries. There will be an expected increase in the incidence of cancer cases from 12.7 million new cases in 2008 to 22.2 million cases in 2030.31 This is further exacerbated in Oman by the rapidly increasing mean age of the population; more cancers are to be expected as the population lives longer. Furthermore, there are several instances in which patients with benign conditions will benefit from in-house PET/CT imaging, such as patients with coronary artery disease being evaluated for myocardial viability as well as patients with infectious diseases, and rheumatological and neurological conditions.
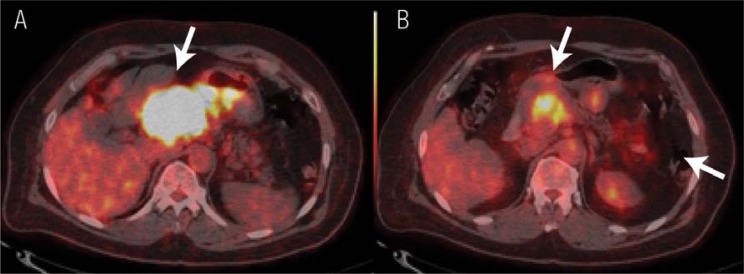
In the long term, it would be neither affordable nor cost-effective to send such a large number of patients abroad for PET/CT scans. Furthermore, the time factor in some of these cases is even more critical than the cost, especially for those patients who require re-evaluation half-way through treatment. In this regard, mid-treatment PET/CT scans to evaluate the response to chemotherapy have proven to be very useful for clinical decision-making regarding early changes in the treatment regimens [Figure 3]. This approach helps in the efficient use of certain expensive chemotherapy drugs. For example, PET/CT imaging after 3 chemotherapy cycles in patients with non-Hodgkin lymphomas has helped save EUR 1,879 per patient in Belgium.32 In this manner, PET/CT has the potential to enable personalised patient management.5 This is the real future challenge for physicians in general, and not solely oncologists.
Figure 3 A & B.
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography (PET)/X-ray computed tomography (CT) performed as a mid-therapy scan for the early assessment of treatment response. The PET/CT images are of a patient with a gastrointestinal stromal tumour. A: The image revealed intense 18F-FDG uptake in the primary tumour (arrow). A follow-up scan at the mid-therapy stage to assess treatment response revealed a mild reduction in the tumour size in the CT image (not included). B: The PET/CT image revealed a significant reduction in the 18F-FDG uptake, indicating a good response to treatment (arrow).
How many PET/CT Scanners and Cyclotrons are Needed in Oman?
The ratio of PET/CT scanners per population varies worldwide: the numbers vary from one scanner per population of 300,000 to one scanner per one million. At the time of writing, the population in Oman is approaching 3 million; hence, two PET/CT scanners would be fully utilised, provided they were acquired soon. If, on the other hand, the approval/planning phase takes longer and 2–3 additional years are needed to begin building the facility (with an anticipated clinical introduction of 5 years), then 3 PET/CT scanners will be needed. This is due to both the increase in population and the number of newly diagnosed cancer cases, as well as the additional numbers of older cancer patients. Further delay in starting this project will only increase expenditure in the health sector, both by increasing the number of patients sent abroad for PET/CT and by the inappropriate usage of expensive, and potentially futile, chemotherapy.
The cyclotron, on the other hand, is a different issue. There is a need to install a national medium energy cyclotron. The two feasible options at present would be for the facility to be based either at the RH or SQUH. This cyclotron should be capable of producing all PET tracers for use within the same institutition and, additionally, 18F-FDG or other 18F-labelled radiotracers that can be transported to other PET/CT scanners located elsewhere. One of these facilities needs to be properly planned and then implemented immediately. Once it is fully functional, a second cyclotron could be planned for the other institute; in the long term, both the RH and SQUH need to have their own cyclotrons. This is because those two centres should be able to utilise PET/CT technology fully by using all radiotracers. Whereas 18F-FDG (with a half-life of 110 mins) can be transported between the two centres, the other short-lived radiotracers, 15O, 11C and 13N, have a very short half-life ranging between 2–20 mins. They can only be used in the institute where the cyclotron is installed.
It is envisaged that a minimum of 3 years is required to install a fully operational cyclotron, with this sometimes taking up to 5 years. One might ask if Oman should wait for the cyclotron before starting the PET/CT facility. The answer would be no; the best way to proceed would be to start planning for a comprehensive, integrated PET/CT and cyclotron facility. Installing a PET/CT scanner requires less time and it can be started in one to two years; until the cyclotron is functional, the scans can be perfomed using imported radiotracers, although in this case only 18F-labelled tracers such as 18F-FDG could be used. This will be a useful temporary solution until the cyclotron starts functioning. It would also provide a good back-up if the cyclotron has technical problems or needs maintenance. It is worth mentioning that importing 18F-FDG should only be considered as a temporary solution. It will not be cost-effective in the long term and, additionally, will not allow the full utilisation of PET/CT.
Building the national capacity is desirable and should be established in parallel with the planning of the PET/CT and cyclotron facility. A team of highly trained and qualified staff is needed to run such a facility efficiently and safely. This team should include qualified medical physicists, radiochemists, radiopharmacists and nuclear medicine technologists.
Planning for the Future in Oman
Any Omani PET/CT initiative should address these key issues prior to introducing a local service:
A long-term plan is needed to consider the number of PET/CT scanners required, the location of the facilities and a possible timeline for their introduction.
A consideration of the need for a cyclotron within Oman versus a supply of radiotracers from other GCC states should be undertaken. Even with an internal source of positron-emitting tracers, consideration should be given to external backup facilities to allow for continued service during scheduled maintenance and unexpected outages.
Taking into consideration the complexity of such facilities and international requirements, particularly the Good Manufacturing Practice (GMP) guidelines, a turnkey project would be the best option for building such facilities.
The education and training of physicians/radiologists, scientists (physicists and radiochemists) and technologists for the operation of the equipment is absolutely mandatory. Some of this should be undertaken abroad in facilities with PET experience and working practices similar to those in Oman.
The education of referrers as to the appropriate use of PET imaging in the management of their patients is essential.
Clear evidence-based guidelines should be developed as to the appropriate use of 18F-FDG-PET scanning.
A funding model should be developed, allowing for flexibility in addressing cancer issues of key importance in Oman. These may be slightly different to those in developed countries.
Conclusion
This article illustrates some of the applications of PET/CT in oncological and non-oncological patients. In view of the increase in the number of newly diagnosed cancer patients, in addition to the long-term follow-up needed for oncological patients in general, the demand for PET/CT will definitely increase in the future. It is important to consider that the number of new cancer patients mentioned is based on a Ministry of Health publication of new cancer cases registered in 2008. First, it should be noted that these figures do not include those patients who went abroad for diagnosis and/or treatment, either at private or government expense. Second, these numbers also do not include benign, non-oncological patients who received PET/CT scans abroad, either at government expense or privately sought second opinions. Therefore, if 2,000 oncological patients could have benefited from a PET/CT service 5 years ago and a further two years are required before PET/CT service will be available—assuming planning for such a service begins immediately—it is likely that, 7 years later, this number will definitively have doubled, if not tripled.
In conclusion, thousands of patients would benefit from an in-house PET/CT and cyclotron facility and thousands of Omani riyals would be saved in the long term. However, it important to remember that hypothetical calculations of the number of patients or money spent are easy, but the same is not true of calculations regarding the quality of the service. In a country like Oman, where there have been tremendous improvements in the health services over the last four decades, and where there is good will towards further improvement, it is the authors’ opinions that PET/CT is no longer an option, but a necessity.
Declaration
The second and third authors were IAEA experts who visited Oman in 2009 as part of the technical cooperation programme with the IAEA. Also part of the data in this paper was presented as a white paper to MOH in 2010 written by the first author and Dr Zahid Al-Mandhari, National Oncology Centre, Royal Hospital, Muscat.
References
- 1.Alshishtawy MM. Four Decades of Progress: Evolution of the health system in Oman. SQU Med J. 2010;10:12–22. [PMC free article] [PubMed] [Google Scholar]
- 2.Krause BJ, Schwarzenböck S, Souvatzoglou M. FDG PET and PET/CT. Recent Results Cancer Res. 2013;187:351–69. doi: 10.1007/978-3-642-10853-2_12. [DOI] [PubMed] [Google Scholar]
- 3.Vranjesevic D, Filmont JE, Meta J, Silverman DH, Phelps ME, Roa J, et al. Whole-body 18F-FDG PET and conventional imaging for predicting outcome in previously treated breast cancer patients. J Nucl Med. 2002;43:325–9. [PubMed] [Google Scholar]
- 4.Ben-Haim S, Ell P. 18F-FDG PET and PET/CT in the evaluation of cancer treatment response. J Nucl Med. 2009;50:88–99. doi: 10.2967/jnumed.108.054205. [DOI] [PubMed] [Google Scholar]
- 5.Altehoefer C, vom Dahl J, Biedermann M, Uebis R, Beilin I, Sheehan F, et al. Significance of defect severity in technetium-99m-MIBI SPECT at rest to assess myocardial viability: Comparison with fluorine-18-FDG PET. J Nucl Med. 1994;35:569–74. [PubMed] [Google Scholar]
- 6.Hess B, Tägil K, Cuocolo A, Anagnostopoulos C, Bardiés M, Bax J, et al. EANM/ESC procedural guidelines for myocardial perfusion imaging in nuclear cardiology. Eur J Nucl Med Mol Imaging. 2005;32:855–97. doi: 10.1007/s00259-005-1779-y. [DOI] [PubMed] [Google Scholar]
- 7.Barrington S, Scarsbrook A. Evidence-Based Indications for the use of PET/CT in the United Kingdom 2012. London: The Royal College of Physicians and Royal College of Radiologists; 2012. [Google Scholar]
- 8.Beckers C, Ribbens C, André B, Marcelis S, Kaye O, Mathy L, et al. Assessment of disease activity in rheumatoid arthritis with (18)F-FDG PET. J Nucl Med. 2004;45:956–64. [PubMed] [Google Scholar]
- 9.Kobayashi Y, Ishii K, Oda K, Nariai T, Tanaka Y, Ishiwata K, et al. Aortic wall inflammation due to Takayasu arteritis imaged with 18F-FDG PET coregistered with enhanced CT. J Nucl Med. 2005;46:917–22. [PubMed] [Google Scholar]
- 10.Elzinga EH, van der Laken CJ, Comans EF, Boellaard R, Hoekstra OS, Dijikmans BA, et al. 18F-FDG PET as a tool to predict the clinical outcome of infliximab treatment of rheumatoid arthritis: An explorative study. J Nucl Med. 2011;52:77–80. doi: 10.2967/jnumed.110.076711. [DOI] [PubMed] [Google Scholar]
- 11.Gotthardt M, Bleeker-Rovers CP, Boerman OC, Oyen WJ. Imaging of inflammation by PET, conventional scintigraphy, and other imaging techniques. J Nucl Med. 2010;51:1937–49. doi: 10.2967/jnumed.110.076232. [DOI] [PubMed] [Google Scholar]
- 12.Keidar Z, Gurman-Balbir A, Gaitini D, Israel O. Fever of unknown origin: The role of 18F-FDG PET/CT. J Nucl Med. 2008;49:1980–5. doi: 10.2967/jnumed.108.054692. [DOI] [PubMed] [Google Scholar]
- 13.van Waarde A, Cobben DC, Suurmeijer AJ, Maas B, Vaalburg W, de Vries EF, et al. Selectivity of 18F-FLT and 18F-FDG for differentiating tumor from inflammation in a rodent model. J Nucl Med. 2004;45:695–700. [PubMed] [Google Scholar]
- 14.Rutten I, Cabay J-E, Withofs N, Lemaire C, Aerts J, Baart V, Hustinx R. PET/CT of skull base meningiomas using 2–18F-fluoro-L-tyrosine: Initial report. J Nucl Med. 2007;48:720–5. doi: 10.2967/jnumed.106.038216. [DOI] [PubMed] [Google Scholar]
- 15.Johnson KA, Minoshima S, Bohnen NI, Donohoe KJ, Foster NL, Herscovitch P, et al. Appropriate use criteria for amyloid PET: A report of the Amyloid Imaging Task Force, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer’s Association. J Alzheimers Dement. 2013;9:e1–16. doi: 10.1016/j.jalz.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jadvar H. Prostate cancer: PET with 18F-FDG, 18F- or 11C-acetate, and 18F- or 11C-choline. J Nucl Med. 2011;52:81–9. doi: 10.2967/jnumed.110.077941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grant FD, Fahey FH, Packard AB, Davis RT, Alavi A, Treves ST. Skeletal PET with 18F-Fluoride: Applying new technology to an old tracer. J Nucl Med. 2008;49:68–78. doi: 10.2967/jnumed.106.037200. [DOI] [PubMed] [Google Scholar]
- 18.Bailey D, Mariani G. Department of Technical Cooperation (TC). Endof-Mission Report. Vienna: International Atomic Energy Agency; 2010. Fact Finding Mission on Current Status of Clinical Practice of Nuclear Medicine and Diagnostic Imaging (OMA /6/302), 5–9 Dec 2009. [Google Scholar]
- 19.Parker W, Seuntjens J. Department of Technical Cooperation (TC). End of Mission Report. Vienna: International Atomic Energy Agency; 2010. Review and make recommendations on Medical Physics in Oman; Urgent needs for diagnostic imaging and therapeutic Oncology Services in Oman (OMA 6003-0), 6–10 Mar 2010. [Google Scholar]
- 20.Dondi M. Department of Technical Cooperation (TC). End of Mission Report. Vienna: International Atomic Energy Agency; 2011. Project review mission; Fact finding mission on national needs in NM; Upstream work for the next TC cycle (OMA/6002), 24–28 Oct 2010. [Google Scholar]
- 21.Marengo M, Hilson AJ, Descristoforo C. (OMA 6002/04/01), 4–8 Feb 2012. Department of Technical Cooperation (TC). End of Mission Report. Vienna: International Atomic Energy Agency; 2012. Assessment of Quality Assurance practices in Nuclear Medicine in Oman; Strengthening National Capacity in radiation Medicine and Dosimetry. [Google Scholar]
- 22.Vora M, Schlyer D. Department of Technical Cooperation (TC). End of Mission Report. Vienna: International Atomic EnergyAgency; 2013. Technical, Physical and Human Resource Requirements for Cyclotron/PET radiopharmaceutical Facility (OMA/6/401), 1–5 Sep 2012. [Google Scholar]
- 23.Soloviev D, Saeid HM. Department of Technical Cooperation (TC). End of Mission Report. Vienna: International Atomic Energy Agency; 2013. Design of Cyclotron and Associated Facility (OMA/6/004), 9–13 Mar 2013. [Google Scholar]
- 24.Bedford M, Maisey MN. Requirements for clinical PET: Comparisons within Europe. Eur J Nucl Med Mol Imaging. 2004;31:208–21. doi: 10.1007/s00259-003-1351-6. [DOI] [PubMed] [Google Scholar]
- 25.UK Department of Health . A Framework for the Development of Positron Emission Tomography Services in England. London: UK Department of Health; 2005. [Google Scholar]
- 26.Burns M. PET and SPECT Markets to top $6 billion by 2021. From: www.biotechsystems.com/breakingmarketnews/pet-and-spect-markets-to-top-6-billion-by-2021.asp Accessed: Jan 2013.
- 27.Bailey D. Survey conducted Jan–Feb 2003. Nuclear Medicine list serve (nucmed@imaging.robarts.ca). Update 2005 includes data presented at the Society of Nuclear Medicine Annual Meeting, Toronto, Canada, Jun 2005 and at the ANZSNM Annual Meeting, Melbourne, May 2005. From: http://irus.rri.on.ca/mailman/listinfo/nucmed. Accessed: Jan 2013.
- 28.The Cooperation Council for Arab States of the Gulf. Member States. Available at: http://www.gcc-sg.org/eng/indexc64c.html?action=GCC Accessed: Jan 2013.
- 29.Medicare Services Advisory Committee (MSAC) Positron Emission Tomography. Canberra: Commonwealth of Australia; 2001. Publications Approval Number: 2822. [Google Scholar]
- 30.Ministry of Health Sultunate of Oman Cancer Incidence in Oman Report of 2010. Available at: http://www.moh.gov.om/en/reports/Cancer%20Incidence%20in%20Oman%202010.pdf Accessed: Jan 2013.
- 31.Bray F, Jemal A, Grey N, Ferlay J, Forman D. Global cancer transitions according to the Human Development Index (2008–2030): A population-based study. Lancet Oncol. 2012;13:790–801. doi: 10.1016/S1470-2045(12)70211-5. [DOI] [PubMed] [Google Scholar]
- 32.Moulin-Ramsee G, Spaepen K, Stroobants S, Mortelmans Non-Hodgkin lymphoma: Retrospective study on the cost-effectiveness of early treatment response assessment by FDG-PET. Eur J Nucl Med Mol Imaging. 2008;35:1074–80. doi: 10.1007/s00259-007-0690-0. [DOI] [PubMed] [Google Scholar]