Abstract
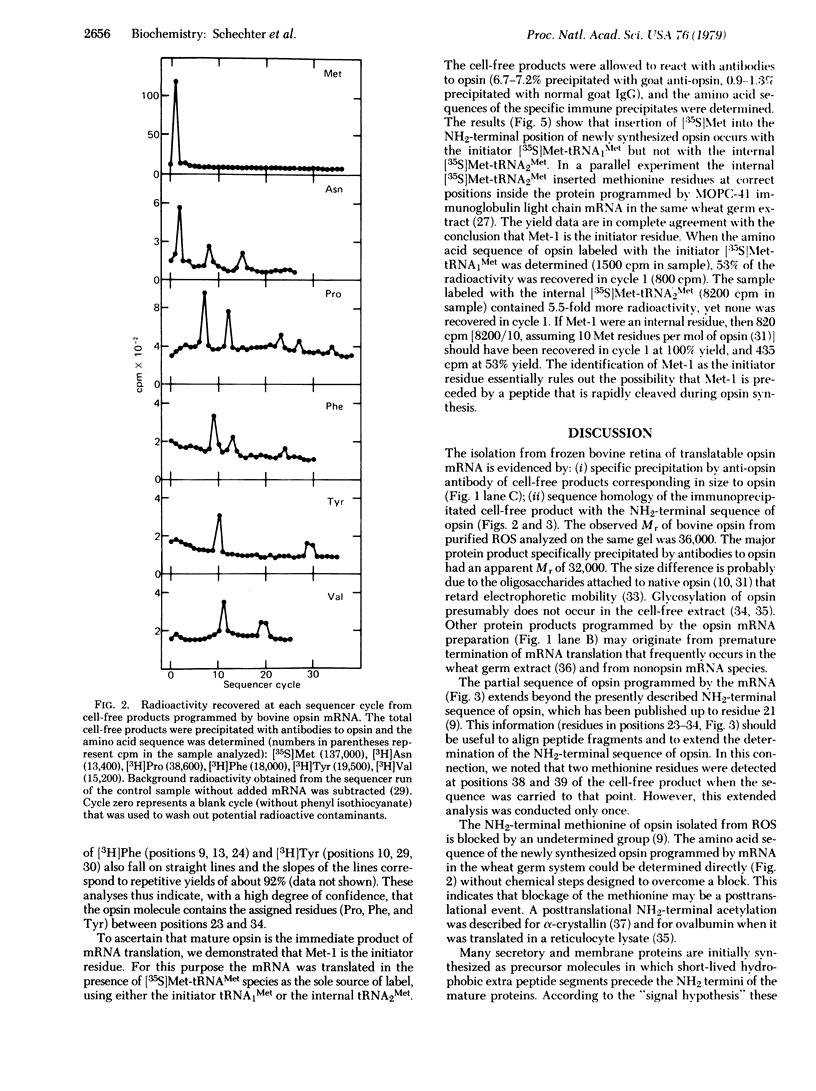
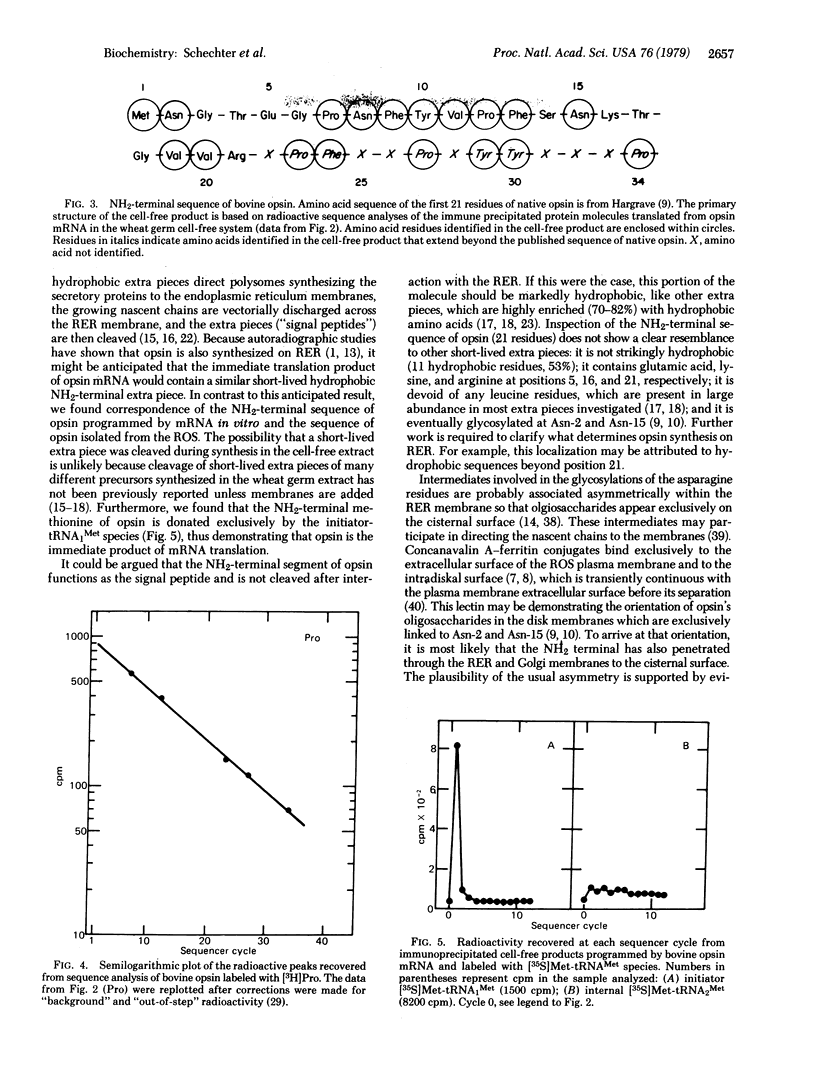
Opsin, the apoprotein of the visual pigment rhodopsin, is synthesized on membranes of the rough endoplasmic reticulum and subsequently passes through the Golgi apparatus to the rod outer segment. This pathway parallels the early stages of biosynthesis of some secretory proteins and viral membrane glycoproteins. Most of these proteins are initially synthesized as precursor molecules with a short-lived hydrophobic extra peptide segment at the NH2 terminus. Therefore we investigated whether or not the immediate translation product of opsin mRNA contains a similar short-lived NH2-terminal extra peptide. The mRNA coding for opsin was isolated from bovine retina polysomes precipitated by antibodies to opsin. The mRNA directed the cell-free synthesis of a protein comparable in size to opsin that was specifically precipitated by anti-opsin antibodies. Sequence analyses of the immunoprecipitated protein labeled with six radioactive amino acids (Met, Asn, Pro, Phe, Tyr, Val) provided the following result: [Formula: see text] (X is unknown). This partial sequence of the cell-free product corresponds exactly to the published NH2-terminal segment of native opsin (21 residues long) and extends beyond this region. Met-1 was shown to be the initiator methionine residue, because only the initiator [35S]Met-tRNA1Met—not the internal [35S]Met-tRNA2Met—donated the NH2-terminal methionine. This finding essentially rules out the possibility that Met-1 was preceded by a peptide that was rapidly cleaved. Thus opsin, and not a precursor, is the immediate product of opsin mRNA translation.
Keywords: rough endoplasmic reticulum, precursor protein, hydrophobic NH2-terminal extra piece, initiator methionine
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A. J., Tanaka M., Shichi H. Concanavalin A binding to rod outer segment membranes: usefulness for preparation of intact disks. Exp Eye Res. 1978 Nov;27(5):595–605. doi: 10.1016/0014-4835(78)90144-6. [DOI] [PubMed] [Google Scholar]
- Besharse J. C., Hollyfield J. G., Rayborn M. E. Photoreceptor outer segments: accelerated membrane renewal in rods after exposure to light. Science. 1977 Apr 29;196(4289):536–538. doi: 10.1126/science.300504. [DOI] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Amino acid-sequence variability at the N-terminal extra piece of mouse immunoglobulin light-chain precursors of the same and different subgroups. Biochem J. 1976 Jul 1;157(1):145–151. doi: 10.1042/bj1570145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein Y., Schechter I. Primary structures of N-terminal extra peptide segments linked to the variable and constant regions of immunoglobulin light chain precursors: implications on the organization and controlled expression of immunoglobulin genes. Biochemistry. 1978 Jun 13;17(12):2392–2400. doi: 10.1021/bi00605a022. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Fung B. K., Hubbell W. L. Organization of rhodopsin in photoreceptor membranes. 2. Transmembrane organization of bovine rhodopsin: evidence from proteolysis and lactoperoxidase-catalyzed iodination of native and reconstituted membranes. Biochemistry. 1978 Oct 17;17(21):4403–4410. doi: 10.1021/bi00614a008. [DOI] [PubMed] [Google Scholar]
- Habener J. F., Rosenblatt M., Kemper B., Kronenberg H. M., Rich A., Potts J. T., Jr Pre-proparathyroid hormone; amino acid sequence, chemical synthesis, and some biological studies of the precursor region. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2616–2620. doi: 10.1073/pnas.75.6.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrave P. A., Fong S. L. The amino- and carboxyl-terminal sequence of bovine rhodopsin. J Supramol Struct. 1977;6(4):559–570. doi: 10.1002/jss.400060409. [DOI] [PubMed] [Google Scholar]
- Hargrave P. A. The amino-terminal tryptic peptide of bovine rhodopsin. A glycopeptide containing two sites of oligosaccharide attachment. Biochim Biophys Acta. 1977 May 27;492(1):83–94. doi: 10.1016/0005-2795(77)90216-1. [DOI] [PubMed] [Google Scholar]
- Hirano H., Parkhouse B., Nicolson G. L., Lennox E. S., Singer S. J. Distribution of saccharide residues on membrane fragments from a myeloma-cell homogenate: its implications for membrane biogenesis. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2945–2949. doi: 10.1073/pnas.69.10.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye S., Wang S., Sekizawa J., Halegoua S., Inouye M. Amino acid sequence for the peptide extension on the prolipoprotein of the Escherichia coli outer membrane. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1004–1008. doi: 10.1073/pnas.74.3.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irving R. A., Toneguzzo F., Rhee S. H., Hofmann T., Ghosh H. P. Synthesis and assembly of membrane glycoproteins: presence of leader peptide in nonglycosylated precursor of membrane glycoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1979 Feb;76(2):570–574. doi: 10.1073/pnas.76.2.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamine J., Buchanan J. M. Processing of 60,000-dalton sarc gene protein synthesized by cell-free translation. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4399–4403. doi: 10.1073/pnas.75.9.4399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kean E. L., Plantner J. J. Biosynthesis of mannose-containing heteropolymers by cell-free preparations of bovine retina. Exp Eye Res. 1976 Aug;23(2):89–104. doi: 10.1016/0014-4835(76)90193-7. [DOI] [PubMed] [Google Scholar]
- Lingappa V. R., Shields D., Woo S. L., Blobel G. Nascent chicken ovalbumin contains the functional equivalent of a signal sequence. J Cell Biol. 1978 Nov;79(2 Pt 1):567–572. doi: 10.1083/jcb.79.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein C., Brownlee G. G., Harrison T. M., Mathews M. B. A possible precursor of immunoglobulin light chains. Nat New Biol. 1972 Sep 27;239(91):117–120. doi: 10.1038/newbio239117a0. [DOI] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Gagnon J., Walsh K. A. Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A. 1978 Jan;75(1):94–98. doi: 10.1073/pnas.75.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papermaster D. S., Converse C. A., Siuss J. Membrane biosynthesis in the frog retina: opsin transport in the photoreceptor cell. Biochemistry. 1975 Apr 8;14(7):1343–1352. doi: 10.1021/bi00678a001. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Converse C. A., Zorn M. Biosynthetic and immunochemical characterization of large protein in frog and cattle rod outer segment membranes. Exp Eye Res. 1976 Aug;23(2):105–115. doi: 10.1016/0014-4835(76)90194-9. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Dreyer W. J. Rhodopsin content in the outer segment membranes of bovine and frog retinal rods. Biochemistry. 1974 May 21;13(11):2438–2444. doi: 10.1021/bi00708a031. [DOI] [PubMed] [Google Scholar]
- Papermaster D. S., Schneider B. G., Zorn M. A., Kraehenbuhl J. P. Immunocytochemical localization of opsin in outer segments and Golgi zones of frog photoreceptor cells. An electron microscope analysis of cross-linked albumin-embedded retinas. J Cell Biol. 1978 Apr;77(1):196–210. doi: 10.1083/jcb.77.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantner J. J., Kean E. L. Carbohydrate composition of bovine rhodopsin. J Biol Chem. 1976 Mar 25;251(6):1548–1552. [PubMed] [Google Scholar]
- Roberts B. E., Paterson B. M. Efficient translation of tobacco mosaic virus RNA and rabbit globin 9S RNA in a cell-free system from commercial wheat germ. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2330–2334. doi: 10.1073/pnas.70.8.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lenard J. Membrane asymmetry. Science. 1977 Feb 25;195(4280):743–753. doi: 10.1126/science.402030. [DOI] [PubMed] [Google Scholar]
- Röhlich P. Photoreceptor membrane carbohydrate on the intradiscal surface of retinal rod disks. Nature. 1976 Oct 28;263(5580):789–791. doi: 10.1038/263789a0. [DOI] [PubMed] [Google Scholar]
- Saibil H., Chabre M., Worcester D. Neutron diffraction studies of retinal rod outer segment membranes. Nature. 1976 Jul 22;262(5566):266–270. doi: 10.1038/262266a0. [DOI] [PubMed] [Google Scholar]
- Schechter I., Burstein Y. Identification of N-terminal methionine in the precursor of immunoglobulin light chain. Initiation of translation of messenger ribonucleic acid in plants and animals. Biochem J. 1976 Mar 1;153(3):543–550. doi: 10.1042/bj1530543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I., Burstein Y. Marked hydrophobicity of the NH2-terminal extra piece of immunoglobulin light-chain precursors: possible physiological functions of the extra piece. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3273–3277. doi: 10.1073/pnas.73.9.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schechter I. Partial amino acid sequence of the precursor of immunoglobulin light chain programmed by messenger RNA in vitro. Science. 1975 Apr 11;188(4184):160–162. doi: 10.1126/science.803715. [DOI] [PubMed] [Google Scholar]
- Schechter I. Use of antibodies for the isolation of biologically pure messenger ribonucleic acid from fully functional eukaryotic cells. Biochemistry. 1974 Apr 23;13(9):1875–1885. doi: 10.1021/bi00706a016. [DOI] [PubMed] [Google Scholar]
- Schmeckpeper B. J., Cory S., Adams J. M. Translation of immunoglobulin mRNAs in a wheat germ cell-free system. Mol Biol Rep. 1974 Mar;1(6):355–363. doi: 10.1007/BF00309570. [DOI] [PubMed] [Google Scholar]
- Schubert D. Immunoglobulin biosynthesis. IV. Carbohydrate attachment to immunoglobulin subunits. J Mol Biol. 1970 Jul 28;51(2):287–301. doi: 10.1016/0022-2836(70)90143-9. [DOI] [PubMed] [Google Scholar]
- Shaper J. H., Stryer L. Accessibility of the carbohydrate moiety of membrane-boound rhodopsin to enzymatic and chemical modification. J Supramol Struct. 1977;6(3):291–299. doi: 10.1002/jss.400060302. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Berns T. J., van Westreenen H., Bloemendal H. Synthesis of lens protein in vitro. Role of methionyl-tRNAs in the synthesis of calf-lens -crystallin. Eur J Biochem. 1972 Oct 17;30(1):48–52. doi: 10.1111/j.1432-1033.1972.tb02070.x. [DOI] [PubMed] [Google Scholar]
- Tabas I., Kornfeld S. The synthesis of complex-type oligosaccharides. III. Identification of an alpha-D-mannosidase activity involved in a late stage of processing of complex-type oligosaccharides. J Biol Chem. 1978 Nov 10;253(21):7779–7786. [PubMed] [Google Scholar]
- Young R. W. The renewal of photoreceptor cell outer segments. J Cell Biol. 1967 Apr;33(1):61–72. doi: 10.1083/jcb.33.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemell R., Burstein Y., Schechter I. Initiator methionine residues at the NH2-termini of the two precursors of MOPC-41 immunoglobulin light chain. Studies with the initiator and internal tRNAMet species. Eur J Biochem. 1978 Aug 15;89(1):187–193. doi: 10.1111/j.1432-1033.1978.tb20911.x. [DOI] [PubMed] [Google Scholar]



