Abstract
Previously we reported the optimization of antiviral scaffolds containing benzimidazole and related heterocycles possessing activity against a variety of arenaviruses. These series of compounds were discovered through an HTS campaign of a 400,000 small molecule library using lentivirus-based pseudotypes incorporated with the Lassa virus envelope glycoprotein (LASV GP). This screening also uncovered an alternate series of very potent arenavirus inhibitors based upon an acylhydrazone scaffold. Subsequent SAR analysis of this chemical series involved various substitutions throughout the chemical framework along with assessment of the preferred stereochemistry. These studies led to an optimized analog (ST-161) possessing subnanomolar activity against LASV and submicromolar activity against a number of other viruses in the Arenaviridae family.
Keywords: Lassa fever, Lassa virus, arenavirus, SAR, antiviral
The viral family Arenaviridae encompasses a number of viruses that have been implicated in hemorrhagic fever. These enveloped RNA viruses are found globally and are broadly categorized into two lineages, Old World and New World.1 Of the large number of viruses belonging to this family, five in particular have garnered more interest due to the ability to cause hemorrhagic fever and their classification as Category A pathogens.2 These are comprised of Old World arenavirus Lassa and four New World arenaviruses Machupo (MACV), Junín (JUNV), Guanarito, and Sabiá viruses. Currently, prevention and treatment options are very limited for hemorrhagic fever viruses.
Previous work performed by our group led to the optimization of a high-throughput screening (HTS) hit based upon a benzimidazole scaffold with potent, broad-spectrum arenavirus activity.3 Further expansion of this scaffold led to additional analogs built on related heterocycles, many of which possessed equipotent or enhanced antiviral activities.4 The previously described HTS campaign yielded additional hits with distinct structures and a particular scaffold emerged that was built upon a central cyclopropyl N-acylhydrazone linker shown in Figure 1. Structurally similar analogs containing this linkage have recently been disclosed as possessing phosphodiesterase-10 inhibition activity for use in the treatment of CNS disorders.5 More generally, the acylhydrazone scaffold has been associated with a number of activities including potential treatment of the following: hypertension,6 tuberculosis,7 human immunodeficiency virus type 1,8 leukemia,9 muscle tissue diseases,10 hyperglucagonemia/hyperglycemia,11 neuropathic pain,12 along with inflammatory disorders such as asthma and chronic obstructive pulmonary disease through phosphodiesterase-4 inhibition.13 The acylhydrazone group has also been utilized as an acid-sensitive linker for applications such as antibody conjugation14 and gene delivery.15 As we discovered, the acylhydrazone moiety possessed stability characteristics suitable for our in vitro antiviral assays and this group is certainly more stable than a regular imine due to resonance contributions.16
Figure 1.
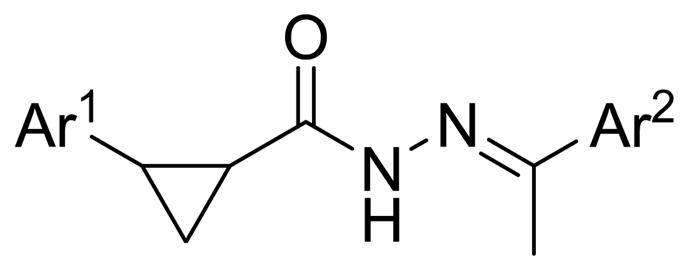
Cyclopropyl N-acylhydrazone-linked scaffold
With the acylhydrazone-based HTS hit (Fig. 2), we were presented with an advantageous starting point given the low nanomolar antiviral potency (25 nM) observed in the LASV pseudotype assay and the degree of selectivity (EC50 > 50 μM for non-arenavirus pseudotypes). In fact, all of the antiviral potency data provided herein were derived from assays utilizing lentivirus-based pseudotypes incorporating LASV GP for which descriptions and methods have previously been disclosed.17 The use of a pseudotype assay was necessary since working with live LASV requires a Biosafety Level 4 (BSL-4) facility, exceeding our BSL-2 rated laboratory. As will be mentioned, live LASV in BSL-4 facilities at The United States Army Medical Research Institute for infectious diseases (USAMRIID) was used to confirm antiviral activity against authentic virus and to follow up on results of the pseudotype-based assay in order to compare in vitro antiviral potency for our lead molecule. SAR analysis involved all areas of the acylhydrazone scaffold along with the distal rings to determine the groups vital to antiviral potency. These studies also included a number of various substitutions along with stereochemical analysis of the cyclopropyl ring. All of the EC50 values provided are averages (geometric mean) of at least three experiments unless otherwise noted.18
Figure 2.
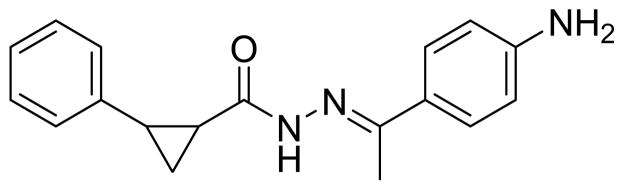
HTS hit
The majority of the analogs to be presented in this letter were purchased from commercial sources. For these molecules, the stereochemistry for the cyclopropyl ring was not specified by the corresponding vendor. Accordingly, the cis/trans configuration is not defined in the structures below with the exception of the lead molecule, ST-161, and the corresponding cis isomer (5g). We believe the remaining compounds all share the trans configuration based upon antiviral activity and the patterns observed during SAR analysis of this series. The definitive stereochemical designation for ST-161 and cis isomer 5g was made possible by custom synthesis which was initiated with the proper configuration already in place for a one-step procedure (Scheme 1). ST-161 was readily isolated from the reaction of trans-hydrazide 1 and methyl ketone 2 in 78% yield. 19 This same reaction sequence was used for 5g starting with cis-configured hydrazide 1.
Scheme 1.
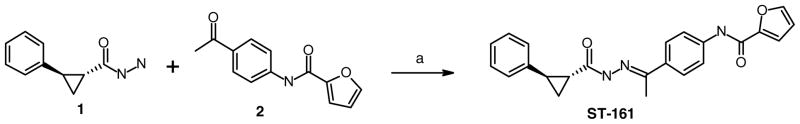
Reagents and conditions: (a) EtOH, rt, 18 h, reflux, 4 h (78%).
The SAR study encompassing the largest number of analogs involved substitution of the Ar2 phenyl ring and included groups with various electronic and steric attributes (Table 1). The goal was to find a preferred group type and also the favored position on the phenyl ring. Disubstitution and additional ring incorporation were also explored. To this end, analogs were procured and several groups of compounds emerged in which substitution ranged from simple halogen groups to amide linked heterocycles. Single digit nanomolar EC50 values were achieved with substitution in the para-position of the phenyl ring with the following smaller moieties: hydroxyl (3j), methoxy (3l) and methyl (3n). Concerning these particular analogs, a decrease in potency of 6- to 40-fold was observed when the same groups were moved from the para-position (3j, 3l, 3n) to the meta-position (3i, 3k, 3m). Albeit a small data set, substitution at the ortho-position proved to be the most disfavored location on the Ar2 phenyl ring (3b and 3h). This may be due to an alternate geometry that is adopted which results from steric interactions of the ortho-group and the methylhydrazone moiety. In most cases the di-substituted analogs did not outperform their mono-substituted counterparts except for the 3,4-dimethyl substituted analog (3q), which was about twice as potent as the para-substituted analog (3n). Substitution of the Ar2 phenyl ring was also made with larger groups including cyclohexyl (3t), phenyl (3u) and various amide-linked moieties (3v–z), mostly at the para-position with the exception of meta-substituted analog 3x. This particular study led to the emergence of two major trends. Firstly, it appeared that the suspected hydrophobic pocket corresponding to this region of the scaffold (Ar2) was rather sizeable. This observation was based upon the high antiviral potency among the sterically bulky analogs containing a planar aromatic ring substituent (3u, 3y–z). Secondly, increased activity tracked well with the presence of an amide-linked group at the para-position (3v–w, 3y–z) which may serve as a hydrogen bond donor and acceptor. With the combination of these two structural modifications, we were able to achieve subnanomolar potency as observed with 3y and 3z. The reduced antiviral activity of the cyclohexyl ring analog (3t) may be due to the preferred conformation of this saturated ring and subsequent fit at the site of interaction. Overall, we found that substitution in the para-position was favored more than all other patterns and disubstitution typically resulted in a detrimental effect in terms of antiviral activity. We were able to achieve subnanomolar potency through the combination of optimal positioning on the phenyl ring, increased steric bulk, and addition of an amide linker.
Table 1.
Favored positioning of Ar2 substituent(s)
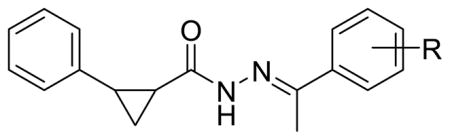
| ||
|---|---|---|
| Compound | R | LASV EC50 (nM) |
| 3a | H | 47 |
| 3b | 2-Cl | 320 |
| 3c | 4-Cl | 20 |
| 3d | 3-Br | 56 |
| 3e | 4-Br | 470 |
| 3f | 3-NO2 | 250 |
| 3g | 4-NO2 | 62 |
| 3h | 2-OH | 570 |
| 3i | 3-OH | 94 |
| 3j | 4-OH | 2.3 |
| 3k | 3-OMe | 42 |
| 3l | 4-OMe | 4.0 |
| 3m | 3-Me | 58 |
| 3n | 4-Me | 9.0 |
| 3o | 2,3-diMe | 800 |
| 3p | 2,4-diMe | 170 |
| 3q | 3,4-diMe | 3.9 |
| 3r | 3,4-diCl | 72 |
| 3s | 3,4-diOMe | 450 |
| 3t | 4-cyclohexyl | 24 |
| 3u | 4-phenyl | 1.3 |
| 3v | 4-NHAc | 2.6 |
| 3w |
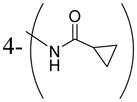
|
2.1 |
| 3x |
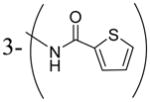
|
6300 |
| 3y |
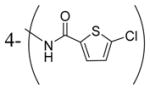
|
0.95 |
| 3z |
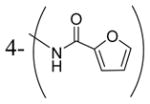
|
0.34 |
Although the majority of the modifications performed during the SAR studies involved the Ar2 portion of the scaffold, some alternate structural explorations were performed including placement of a tert-butyl substituent in the Ar1 ring. The effect of this bulky, lipophilic moiety was compared to the unsubstituted phenyl ring and results are shown in Table 2. Substitution was also altered in the Ar2 phenyl ring in order to expand the data set with groups selected from chloro, fluoro and 2-fluorobenzamide. An unsubstituted phenyl ring in Ar2 was also included. Overall, a three- to twelvefold shift in potency was observed and increased activity tracked with the addition of the t-butyl moiety. Similarly found for the Ar2 ring, a sizeable hydrophobic pocket may be present at the site of biological interaction for Ar1 as evidenced by improved antiviral activity for this bulky t-butyl substituent.
Table 2.
Effect of bulky Ar1 substituent
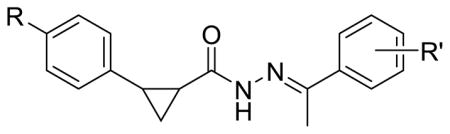
| |||
|---|---|---|---|
| Compound | R | R′ | LASV EC50 (nM) |
| 3a | H | H | 47 |
| 4a | t-Bu | H | 15 |
| 3c | H | 4-Cl | 20 |
| 4b | t-Bu | 4-Cl | 6.6 |
| 4c | H | 4-F | 52 |
| 4d | t-Bu | 4-F | 16 |
| 4e | H |
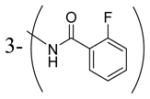
|
9100 |
| 4f | t-Bu |
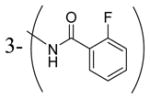
|
770 |
A few of the other SAR studies were of a limited scope and encompassed only one or two analogs derived from structural modifications of the acylhydrazone core and attached phenyl rings. Stereochemical analysis of the cyclopropyl ring was also performed and all of these results are summarized in Table 3. The importance of the cyclopropyl-substituted phenyl ring (Ar1) was apparent with the complete loss of activity for analog 5a at a concentration of 50 μM, compared to an EC50 value of 42 nM for the parent compound (3k). The addition of a second phenyl ring, as in analog 5b, was found to be highly detrimental with a greater than 700-fold decrease in potency observed in the LASV pseudotype assay when compared to the parent analog (5c). It is possible that the suspected hydrophobic pocket occupied by the Ar1 phenyl ring may not be large enough to accommodate the increased steric bulk. Nearly a 30-fold improvement in the EC50 value was observed when lipophilic character was introduced to a simple amine functionality at Ar2, switching from NH2 (5c) to NMe2 (5d). The effect of the methyl group substituent of the acylhydrazone linker was determined through hydrogen replacement or expansion of steric bulk with an isopropyl moiety. The analog with hydrogen replacement (5e) was inactive at the highest concentration tested (50 μM) while the isopropyl analog (5f) resulted in an eightfold reduction in antiviral potency compared to parent compound 3a. The most potent analog in this acylhydrazone series was discovered during a study to determine the preferred stereochemistry of the cyclopropyl substituents. It was found that the trans configuration was far superior to the cis isomer as observed with analogs ST-161 (0.34 nM) and 5g (850 nM), separated by over three orders of magnitude with respect to antiviral potency.
Table 3.
Diverse substitutions and stereochemical analysis
| Cmpd | Structure | LASV EC50 (nM) | Cmpd | Structure | LASV EC50 (nM) |
|---|---|---|---|---|---|
| 5a |
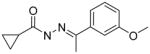
|
>50,000 | 5b |
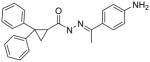
|
19,000 |
| 5c |
|
25 | 5d |
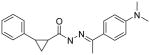
|
0.90 |
| 5e |
|
>50,000 | 5f |
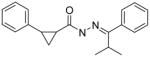
|
390 |
| 5g |
|
850 | ST-161 |
|
0.34 |
Since all of the data presented above was derived from the LASV pseudotype assay, we decided to follow up on in vitro potency with live virus for our most potent inhibitor, ST-161. To this end, a plaque reduction assay utilizing live LASV was performed within the BSL-4 facilities at USAMRIID. We were gratified to find that the previously observed antiviral activity of ST-161 was confirmed with live virus.
The previously reported benzimidazole-based arenavirus inhibitor ST-193 was found to retain high potency against a number of viruses in the Arenaviridae family of both New World and Old World lineages.3 Therefore, a similar study was done with our most potent analog in the acylhydrazone series, ST-161, to determine the spectrum of antiviral activity against other arenaviruses. The potency data was collected from alternate pseudotype assays incorporating envelopes from MACV, JUNV, Sabiá virus, Guanarito and Tacaribe viruses (Table 4). While the high potency of ST-161 observed in the LASV pseudotype assay was not observed for the other viruses, this compound still retains respectable submicromolar activity against these New World arenaviruses. The propensity of ST-161 for LASV-associated antiviral activity, rather than New World arenaviruses, was explained by previous studies which showed specificity for LASV GP in cell-cell fusion assays and reduced activity observed with JUNV GP.20
Table 4.
Broad-spectrum arenavirus activity for compound ST-161
| Cmpd | LASV EC50 (nM) | MACV EC50 (nM) | JUNV EC50 (nM) | Sabiá EC50 (nM) | Guanarito EC50 (nM) | Tacaribe EC50 (nM) |
|---|---|---|---|---|---|---|
| ST-161 | 0.34 | 730 | 800 | 130 | 120a | 630 |
Average value from only two experiments.
Through the optimization of a HTS hit containing a cyclopropyl acylhydrazone linker, lead compound ST-161 was discovered which possessed very high potency against LASV in the pseudotype assay, which was later confirmed with live virus. As a result of these efforts, antiviral activity was increased over 70-fold compared to the original hit while also removing a potentially genotoxic para-substituted aniline moiety. This molecule was also found to have antiviral activity against five New World arenaviruses warranting the potential use as a broad-spectrum countermeasure.
Acknowledgments
We gratefully acknowledge the financial support from NIH grants 5R44AI056525 and 1R01AI093387. We thank Phil Ferro and Mary Guttieri for their work with live LASV at USAMRIID BSL-4 facilities. We also thank Natasha Cerruti for technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.Buchmeier MJ, de la Torre J-C, Peters CJ. Arenaviridae: the viruses and their replication. In: Fields BN, Knipe DM, Howley PM, Roizman B, Straus SE, editors. Fields Virology. 5. Lippincott, Williams and Wilkins; Philadelphia: 2007. pp. 1791–1827. [Google Scholar]
- 2.NIAID biodefense research agenda for CDC category A agents. Feb, 2002. Publication No. 03-5308. [Google Scholar]
- 3.Dai D, Burgeson JR, Gharaibeh DN, Moore AL, Larson RA, Cerruti NR, Amberg SM, Bolken TC, Hruby DE. Bioorg & Med Chem Lett. 2013;23:744–749. doi: 10.1016/j.bmcl.2012.11.095. [DOI] [PubMed] [Google Scholar]
- 4.Burgeson JR, Moore AL, Gharaibeh DN, Larson RA, Cerruti NR, Amberg SM, Hruby DE, Dai D. Bioorg & Med Chem Lett. 2013;23:750–756. doi: 10.1016/j.bmcl.2012.11.093. [DOI] [PubMed] [Google Scholar]
- 5.Ripka A, Shapiro G, Chesworth R. WO2010006130. PCT Application. 2010 Jan 14;
- 6.Leal CM, Pereira SL, Kuemmerle AE, Leal DM, Tesch R, de Sant’Anna CMR, Fraga CAM, Barreiro EJ, Sudo RR, Zapata-Sudo G. Eur J Med Chem. 2012:5549–57. doi: 10.1016/j.ejmech.2012.06.056. [DOI] [PubMed] [Google Scholar]
- 7.Jordao AK, Sathler PC, Ferreira VF, Campos VR, de Souza MCBV, Castro HC, Lannes A, Lourenco A, Rodrigues CR, Bello ML, Lourenco MCS, Carvalho GSL, Almeida MCB, Cunha AC. Bioorg & Med Chem. 2011;19:5605–5611. doi: 10.1016/j.bmc.2011.07.035. [DOI] [PubMed] [Google Scholar]
- 8.Gong Q, Menon L, Ilina T, Miller LG, Ahn J, Parniak MA, Ishima R. Chem Bio & Drug Design. 2011;77:39–47. doi: 10.1111/j.1747-0285.2010.01052.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cui Z, Li Y, Ling Y, Huang J, Cui J, Wang R, Yang X. Eur J Med Chem. 2010;45:5576–5584. doi: 10.1016/j.ejmech.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Fraga CAM, Takashi Sudo R. WO2006017921. PCT Application. 2006 Feb 23;
- 11.Madsen P, Ling A, Plewe M, Sams CK, Knudsen LB, Sidelmann UG, Ynddal L, Brand CL, Andersen B, Murphy D, Teng M, Truesdale L, Kiel D, May J, Kuki A, Shi S, Johnson MD, Teston KA, Feng J, Lakis J, Anderes K, Gregor V, Lau J. J Med Chem. 2002;45:5755–5775. doi: 10.1021/jm0208572. [DOI] [PubMed] [Google Scholar]
- 12.Diaz P, Xu J, Astruc-Diaz F, Pan HM, Brown DL, Naguib M. J Med Chem. 2008;51:4932–4947. doi: 10.1021/jm8002203. [DOI] [PubMed] [Google Scholar]
- 13.Kummerle AE, Schmitt M, Cardozo SVS, Lugnier C, Villa Pl, Lopes AB, Romeiro NC, Justiniano H, Martins MA, Fraga CAM, Bourguignon J-J, Barreiro EJ. J Med Chem. 2012;55:7525–7545. doi: 10.1021/jm300514y. [DOI] [PubMed] [Google Scholar]
- 14.Mueller BM, Wrasildo WA, Reisfeld RA. Bioconjugate Chem. 1990;1:325–330. doi: 10.1021/bc00005a005. [DOI] [PubMed] [Google Scholar]
- 15.Aissaoui A, Martin B, Kan E, Oudrhiri N, Hauchecorne M, Vigneron JP, Lehn JM, Lehn P. J Med Chem. 2004;47:5210–5223. doi: 10.1021/jm0408159. [DOI] [PubMed] [Google Scholar]
- 16.Carey FA, Sundberg RJ. Advanced Organic Chemistry. 5. A. Springer; New York: 2008. pp. 650–651. [Google Scholar]
- 17.Larson RA, Dai D, Hosack VT, Tan Y, Bolken TC, Hruby DE, Amberg S. J Virol. 2008;82:10768. doi: 10.1128/JVI.00941-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Each pseudotype inhibition assay was performed multiple times, with a minimum of three trials (the one exception, as pointed out in the text, was ST-161 against Guanarito, which was only done twice). The median relative standard error of the EC50 value was 0.22, and in all cases except one was 0.3 or less (this exception was Compound 5g against Lassa, which had an RSE of 0.65 after eight tests). Each experiment used multiple replicates (three or four) of each test concentration (usually 7-to-14 different concentrations separated by increments of 0.25-to-0.7 logs) to generate a single EC50 value using XLfit (IDBS) software with a four-parameter one-site dose-response curve fit. EC50 calculations from multiple experiments were compiled by generating an average (geometric mean). Relative standard error is calculated as the standard error (standard deviation of the individual EC50 calculations divided by the square root of the number of experiments) relative to the average.
- 19.Spectra data for ST-161: 1H NMR (300 MHz, DMSO-d6): δ 10.60 (d, 1H), 10.29 (d, 1H), 7.95 (s, 1H), 7.75 (m, 4H), 7.26 (m, 6H), 6.71 (dd, 1H), 2.71 (m, 1H), 2.34 (m, 1H), 2.25 (d, 3H), 1.52 (m, 2H); LRMS: 388.3 (M+H)+
- 20.York J, Dai D, Amberg SM, Nunberg JH. J Virol. 2008;82:10932–10939. doi: 10.1128/JVI.01140-08. [DOI] [PMC free article] [PubMed] [Google Scholar]


