Summary
Background
Some countries fortify flour with folic acid to prevent neural tube defects but others do not, partly because of concerns about cancer risks. We aimed to assess the effects of folic acid supplementation on site-specific cancer rates in the randomised trials.
Methods
Meta-analyses of data on each individual in all placebo-controlled trials of folic acid for prevention of cardiovascular disease (10 trials, n=46,969) or colorectal adenoma (3 trials, n=2652) that recorded cancer incidence and recruited >500 participants. All trials were evenly randomised. Risk ratios (RRs) compare those allocated folic acid vs those allocated placebo, giving cancer incidence rate ratios (among those still free of cancer) during, but not after the scheduled treatment period.
Findings
During a weighted mean follow-up duration of 5.5 years, allocation to folic acid quadrupled plasma folate, but had no statistically significant effect on overall cancer incidence (1904 vs 1809 cancers, RR=1.06 [95%CI 0.99–1.13], p=0.10; trend with duration of treatment p=0.46). There was no significant heterogeneity between the results of individual trials (p=0.23), or between the cadiovascular prevention trials and the adenoma prevention trials (p=0.13). Moreover, there was no significant effect of folic acid supplementation on the incidence of cancer of the large intestine, prostate, lung, breast or any other specific site.
Interpretation
Folic acid supplementation does not substantially increase or decrease site-specific cancer incidence during the first 5 years of treatment.
Funding
British Heart Foundation, Medical Research Council, Cancer Research UK, Food Standards Agency.
Introduction
After epidemiological studies found that higher intakes of folate and plasma levels of folate during pregnancy were inversely associated with neural tube defects,1–3 non-randomised4,5 and randomised intervention trials6,7 confirmed a protective effect, and folate is now routinely recommended as a supplement before and during pregnancy.8 Furthermore, population-wide folate fortification of flour for prevention of neural tube defects has been mandatory since 1998 in North America, resulting in a two-fold increase in population plasma folate levels.9,10 It is also mandatory in some other countries,9 including Chile, Argentina, Brazil, South Africa and Australia;11–13 but in New Zealand and in Western European countries it is not mandatory, partly due to concerns about possible adverse effects on cancer incidence and prognosis.13–15
Epidemiological studies in adult populations have found inverse (ie, apparently protective) associations of folate intake and of plasma folate levels with risk of cardiovascular disease16,17 and colorectal cancer.18 To test whether there is any real protective effect against cardiovascular disease, placebo-controlled trials of about 5 years of folic acid were undertaken among a total of some 47,000 adults at high risk of vascular disease, and a collaborative meta-analysis of individual patient data on cancer incidence from each these trials was agreed prospectively in 2004, before any results emerged.19,20 These trials did not suggest any protective effect of folic acid supplementation against cardiovascular disease or against mortality from any cause during the scheduled trial treatment period.20
To test whether there is any real protective effect against colorectal adenomas, another 3 such trials were undertaken among a total of some 3000 patients. One of these, the Aspirin and Folic Acid Polyp Prevention Study (AFPPS), reported in 2007 unexpected increases in advanced colorectal adenomas and prostate cancer after 7 years of treatment with folic acid.21 Also in 2007, it was suggested that transient increases in colorectal cancer incidence in the United States (US) and Canada during 1996–98 might have been due to the 1996–98 introduction of folic acid fortification in North America.22 Taken together, these reports (and awareness that anti-folate drugs such as methotrexate are used for the treatment of certain cancers) prompted regulatory concerns about possible risks of cancer associated with folic acid supplements and folic acid fortification. Animal models had previously suggested the possibility that high intakes of folate could suppress the development of early lesions in normal tissue, but enhance the growth of established neoplasms.23
To see whether, in aggregate, the randomised trials of folic acid show an increase or decrease in cancer risk over a period of just a few years, we present collaborative meta-analyses of site-specific cancer incidence during the scheduled treatment period among a total of 50,000 individuals from all available large cardiovascular and adenoma trials. We do not address the question of whether any effects on cancer incidence will emerge among the participants some years or decades after the trials ended.
Methods
Trial eligibility
Trials were identified by electronic searches using PUBMED with search terms for “randomized trials”, ”folic acid”, “B-vitamins” or “homocysteine-lowering treatment” and by scanning reference lists of trial reports: webappendix p2. Trials were eligible for inclusion if (i) at least one randomized comparison was folic acid vs placebo with scheduled treatment duration at least one year (irrespective of whether any other treatment was tested factorially); (ii) there were 500 or more participants; and (iii) data on cancer incidence had been recorded. Unpublished trials were sought through electronic searches and discussions with other experts in the field, but none was found. Individual participant datasets were obtained for 49 621 participants in 13 trials completed by the end of 2010: webappendix p7.21,24–35 Information on cancer incidence was not recorded in two other trials involving a total of 5992 participants.36,37 (Inclusion of the few cancer deaths recorded in such trials would not materially alter the present meta-analyses of cancer incidence in all other trials.) The protocol for trial identification, analysis and involvement of trialists was agreed following discussion with all collaborators before any results emerged, and published elsewhere.19,20
Baseline and follow-up data
For each participant, information was sought on characteristics recorded prior to randomization, allocated treatment, and the type and date (or time from randomization) of any cancer incidence or mortality during the scheduled treatment period. Information on hospitalizations and cancer incidence was collected in each trial at 3 to 6 month intervals during the scheduled treatment period. In addition to self-reported cancer, additional information on cancer incidence was, where possible, obtained from national cancer registries.26–28, 33 In one trial,29 the site of cancer onset was not recorded in the primary database, so we searched all relevant databases of adverse events to identify incident cancers. The other trials had all sought verification of incident cancers from hospital electronic records or by writing to hospital or family physicians, and in them sites of cancer onset were available for more than 92% of cases.
Analyses of the individual participant data were checked for consistency with any published reports and with the trialists to help ensure that the data were incorporated correctly into the meta-analysis. Investigators were asked to confirm summary data for each treatment group on the number of randomized patients, on plasma levels of folate and homocysteine before and after starting treatment, and on numbers who developed each of the pre-defined outcomes.
The main outcome was incident cancer, defined as the first occurrence after randomization but during the scheduled treatment period of any non-fatal cancer (except non-melanoma skin cancer) or fatal cancer. Where cancer was diagnosed only at death and no other information was available, the date of diagnosis was taken as the date of death. Where cancer site was available only for mortality, the data were used for analyses of incidence as well as mortality. Cancers were subdivided into the 18 most common types, based on the International Classification of Disease (ICD-10), with the aggregate of all other types as a 19th category and missing ICD codes as a 20th. Having individual participant data from each trial facilitated uniform categorization of cancer types (and permitted analyses of treatment effects in pre-specified subgroups).
Statistical analyses
Comparisons of cancer rates by allocated treatment were based on intention-to-treat analyses of first events during the scheduled treatment period. The logrank observed minus expected (o-e) statistics from each trial and their variances (v) were separately summed to produce, respectively, a grand total observed minus expected statistic (G) and its variance (V).38 The one-step estimate of the log of the event rate ratio is then G/V with variance 1/V (and 95% CI G/V ± 1.96/√V). For n trials, a chi-squared statistic for heterogeneity with n−1 degrees of freedom (χ2n−1) is S-G2/V, where S is the sum over all trials of (o-e)2/v.
The effects on cancer incidence were assessed in pre-defined subgroups of year of follow-up (first 3 years or later), age, sex, plasma folate, plasma homocysteine, and whether or not there was nationwide folic acid fortification. Heterogeneity of the rate ratios (RR) among these subgroups was investigated by a global chi-squared test to reduce the chance of misinterpreting any false positive results arising from multiple comparisons.39 99% CIs were used for individual trials or subgroups (again to avoid misinterpreting false positive results), but 95% CIs were used for the overall findings. To correct for multiple comparisons, p-values for particular types of cancer were multiplied by the number of types investigated (to a maximum corrected p-value of 1.0).40,41
To help re-assess the hypotheses of increased risk of colorectal adenoma and prostate cancer raised by AFPPS,21 the present meta-analyses assessed the effects of folic acid on colorectal and prostate cancer with and without exclusion of the AFPPS trial.40,41 The provision of (o-e) for each trial facilitates sensitivity analyses that exclude or include particular trials.
Folate reduces homocysteine, and the mean reduction in all trials was the weighted mean of study-specific percent homocysteine reductions, with weights proportional to the variances of the logrank statistics for overall cancer incidence. Statistical Analysis System (SAS) version 9.2 was used.
Role of the funding sources
The funders of the individual trials had no role in study design, data collection, data analysis, data interpretation, or writing or submission of the report. The CTSU authors had full access to all the data and analyses and accept responsibility for this report. Final analyses and a draft report were circulated to all authors, revised and re-circulated. All authors are responsible for the decision to submit for publication.
Results
Individual participant data were obtained from all 13 trials of at least 500 participants and one year of scheduled treatment that had recorded cancer incidence, involving in total 49,621 participants (2652 from 3 trials in patients with a prior colorectal adenoma and 46,969 from 10 trials in people with or at high risk of cardiovascular disease: Table 1). Two-thirds of the participants were men and the mean age at entry was 64 (SD 10) years: webappendix p7. The daily doses of folic acid ranged from 0.5 to 5 mg, except in one trial of 40 mg daily.25 All trials compared the effects of folic acid versus placebo, except one trial29 that compared analyses of 2.5 mg versus 0.02 mg (only 5% of the recommended dietary intake; approximately equivalent to placebo). Treatment duration varied from 2.0 to 7.7 years, with weighted average 5.5 years.
Table 1.
Design and eligibility criteria of included trials
| Number randomized | Prior disease | Main country | Median duration of treatment (yrs) | Folic acid daily dose (mg/day) | Total number of incident cancers | Number(%) with known site of origin | |
|---|---|---|---|---|---|---|---|
| Colorectal adenoma trials | |||||||
| Harvard | 692 | Adenoma | USA | 4.0 | 1.0 | 49 | 48 (98.0) |
| UK CAP | 939 | Adenoma | UK | 2.9 | 0.5 | 27 | 27 (100.0) |
| AFPPS | 1021 | Adenoma | USA | 7.7 | 1.0 | 92 | 89 (96.7) |
| Subtotal | 2652 | - | - | 168 | 164 (97.6) | ||
| Cardiovascular disease trials | |||||||
| VITRO | 701 | CVD | Netherlands | 2.5 | 5.0 | 19 | 18 (94.7) |
| HOST | 2056 | Renal | USA | 3.2 | 40.0 | 137 | 135 (98.5) |
| SU-FOL-OM3 | 2501 | CVD | France | 4.4 | 0.6 | 171 | 170 (99.4) |
| WENBIT | 3090 | CHD | Norway | 3.2 | 0.8 | 144 | 141 (97.9) |
| VISP | 3680 | Stroke | USA/Canada | 2.0 | 2.5 | 187 | 88 (47.1) |
| NORVIT | 3749 | CHD | Norway | 3.4 | 0.8 | 149 | 135 (90.6) |
| WAFACS | 5442 | CVD | USA | 7.3 | 2.5 | 414 | 384 (92.8) |
| HOPE-2 | 5522 | CVD/DM | Canada/USA | 5.0 | 2.5 | 662 | 650 (98.2) |
| VITATOPS | 8164 | CVD | Australia/India/UK | 3.7 | 2.0 | 345 | 317 (91.9) |
| SEARCH | 12064 | CHD | UK | 7.0 | 2.0 | 1317 | 1244 (94.5) |
| Subtotal | 46969 | - | - | 3545 | 3282 (92.6) | ||
|
| |||||||
| Total | 49621 | *5.5 | **2.0 | 3713 | 3446 (92.8) | ||
CHD: Coronary heart disease
CVD: Prior cardiovascular disease or increased risk of cardiovascular disease
DM: Diabetes mellitus
Weighted average, weighted by trial-specific variances of observed (O–E) for cancer incidence
Median value
Allocation to folic acid was associated with quadrupling median plasma folate levels (57.3 vs 13.5 nmol/L) and with reduction by a quarter in plasma homocysteine levels (9.3 vs 12.3 μmol/L): webappendix p6. As expected, effects on homocysteine levels were greater in non-fortified than in fortified populations (27% and 20% reductions, respectively).20
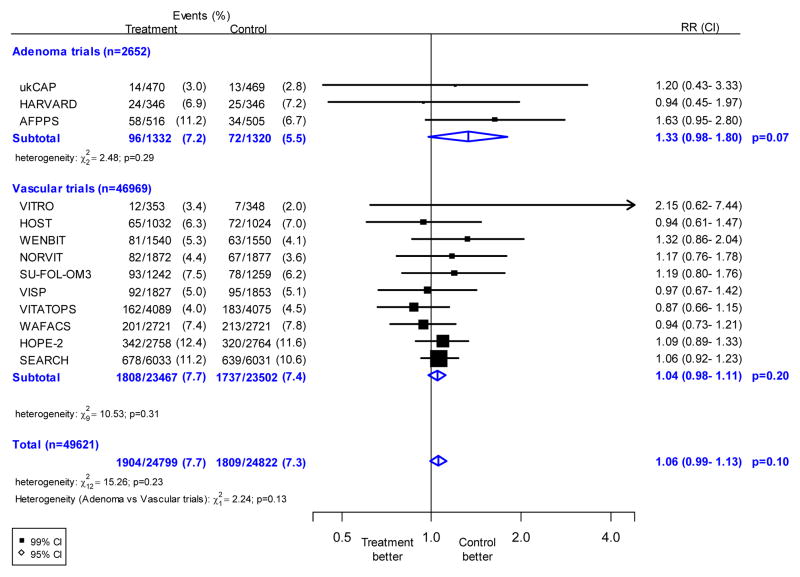
Information was available on 3713 incident cancers during the scheduled treatment period. Allocation to folic acid treatment did not have any statistically significant effect on overall cancer incidence, with 1904 (7.7%) first events in 24,799 participants allocated folic acid versus 1809 (7.3%) in 24,822 allocated control (RR 1.06; 95% CI 0.99–1.13, p=0.10: Figure 1). There was no significant heterogeneity between the results of individual trials (χ212=15.3; p=0.23), or between the adenoma trials (96 vs 72 cancers, RR 1.33; 95% CI 0.98–1.80) and vascular trials (1808 vs 1737 cancers, 1.04; 95% CI 0.98–1.11) (χ21 = 2.2; p=0.13).
Figure 1. Effects of folic acid allocation on overall first cancer incidence.
The black squares denote the rate ratios (RRs) and horizontal lines the 99% confidence intervals (CIs). Each square has area inversely proportional to the variance of the log of the rate ratio. The diamonds represent the summary estimates and their corresponding 95% CIs.
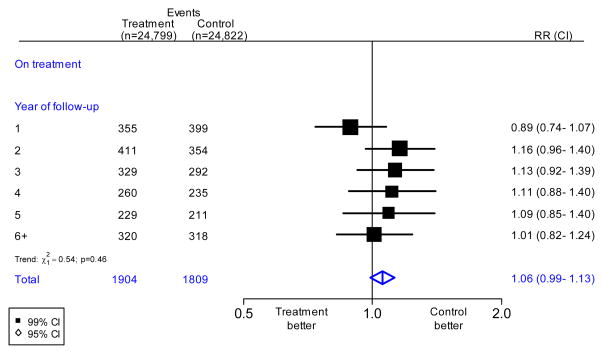
Importantly, there was no evidence of an increasing effect of folic acid with increasing duration of treatment (Figure 2), although only 3 of the trials lasted more than 5 years.21,31,33 Nor were there any significant differences by sex, age, pre-treatment blood levels of folate, pre-treatment blood levels of homocysteine, folic acid fortification in the population, folic acid dose or percent homocysteine reduction: webappendix pp3–4. Even in the trial of 40 mg/day of folic acid there was no apparent increase in overall cancer incidence (65 vs 72 cancers, RR=0.94; 99% CI 0.61–1.47).25
Figure 2. Effects of folic acid on cancer incidence in all available trials, by year of follow-up.
The black squares denote the rate ratios (RRs) and horizontal lines the 99% confidence intervals (CIs). Each square has area inversely proportional to the variance of the log of the rate ratio. The diamonds represent the summary estimates and their corresponding 95% CIs.
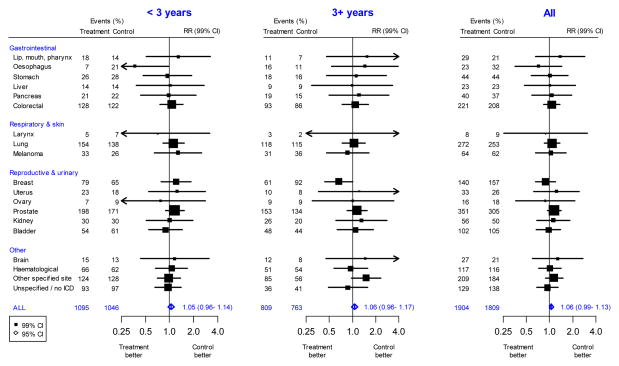
The 3713 cancers were classified into 18 main types. There was no statistically significant effect of folic acid allocation on the incidence of colorectal cancer (221 vs 208; RR 1.07; 99%CI 0.83–1.37), lung cancer (272 vs 253; 1.08, 0.86–1.35), breast cancer (140 vs 157; 0.89, 0.66–1.20), prostate cancer (351 vs 305; 1.15, 0.94–1.41), the less common types, or cancer of an unknown type, either overall or by treatment duration (Figure 3, Table 2, webappendix p7).
Figure 3. Effects of folic acid on first cancer incidence, by type and duration of treatment.
The black squares denote the rate ratios (RRs) and horizontal lines the 99% confidence intervals (CIs). Each square has area inversely proportional to the variance of the log of the rate ratio. The diamonds represent the summary estimates and their corresponding 95% CIs.
Table 2.
Number of people with incident cancers at specific sites, by duration of treatment
| Cancer site | Incidence of first cancer after randomisation | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <3 years
|
3+ years
|
All years
|
||||||||||||
| Folic acid | Control | Uncorrected p-value1 | Corrected p-value2 | Folic acid | Control | Uncorrected p-value1 | Corrected p-value2 | Folic acid | Control | RR | (CI)4 | Uncorrected p-value1 | Corrected p-value2 | |
| Numbers at start of time period | 24799 | 24822 | 17292 | 17363 | 24799 | 24822 | ||||||||
| Lip, mouth, pharynx | 18 | 14 | 0.47 | 1.00 | 11 | 7 | 0.35 | 1.00 | 29 | 21 | 1.38 | (0.66–2.86) | 0.26 | 1.00 |
| Esophagus | 7 | 21 | 0.01 | 0.15 | 16 | 11 | 0.34 | 1.00 | 23 | 32 | 0.72 | (0.36–1.44) | 0.22 | 1.00 |
| Stomach | 26 | 28 | 0.82 | 1.00 | 18 | 16 | 0.73 | 1.00 | 44 | 44 | 1.01 | (0.58–1.75) | 0.97 | 1.00 |
| Liver or gall bladder | 14 | 14 | 0.96 | 1.00 | 9 | 9 | 0.98 | 1.00 | 23 | 23 | 1.01 | (0.47–2.15) | 0.98 | 1.00 |
| Pancreas | 21 | 22 | 0.91 | 1.00 | 19 | 15 | 0.59 | 1.00 | 40 | 37 | 1.07 | (0.59–1.93) | 0.78 | 1.00 |
| Colorectal | 128 | 122 | 0.66 | 1.00 | 93 | 86 | 0.58 | 1.00 | 221 | 208 | 1.07 | (0.83–1.37) | 0.49 | 1.00 |
| Larynx | 5 | 7 | 0.56 | 1.00 | 3 | 2 | 0.64 | 1.00 | 8 | 9 | 0.89 | (0.25–3.11) | 0.81 | 1.00 |
| Lung | 154 | 138 | 0.35 | 1.00 | 118 | 115 | 0.78 | 1.00 | 272 | 253 | 1.08 | (0.86–1.35) | 0.37 | 1.00 |
| Melanoma | 33 | 26 | 0.35 | 1.00 | 31 | 36 | 0.55 | 1.00 | 64 | 62 | 1.04 | (0.66–1.64) | 0.84 | 1.00 |
| Non-melanoma skin | 53 | 52 | 0.91 | 1.00 | 33 | 31 | 0.82 | 1.00 | 86 | 83 | 1.04 | (0.70–1.55) | 0.80 | 1.00 |
| Breast | 79 | 65 | 0.25 | 1.00 | 61 | 92 | 0.01 | 0.18 | 140 | 157 | 0.89 | (0.66–1.20) | 0.30 | 1.00 |
| Uterus | 23 | 18 | 0.49 | 1.00 | 10 | 8 | 0.68 | 1.00 | 33 | 26 | 1.23 | (0.63–2.41) | 0.43 | 1.00 |
| Ovary | 7 | 9 | 0.60 | 1.00 | 9 | 9 | 0.99 | 1.00 | 16 | 18 | 0.88 | (0.37–2.15) | 0.72 | 1.00 |
| Prostate | 198 | 171 | 0.16 | 1.00 | 153 | 134 | 0.26 | 1.00 | 351 | 305 | 1.15 | (0.94–1.41) | 0.07 | 1.00 |
| Kidney | 30 | 30 | 1.00 | 1.00 | 26 | 20 | 0.37 | 1.00 | 56 | 50 | 1.12 | (0.68–1.85) | 0.56 | 1.00 |
| Bladder | 54 | 61 | 0.52 | 1.00 | 48 | 44 | 0.69 | 1.00 | 102 | 105 | 0.97 | (0.68–1.39) | 0.83 | 1.00 |
| Brain | 15 | 13 | 0.69 | 1.00 | 12 | 8 | 0.41 | 1.00 | 27 | 21 | 1.27 | (0.60–2.69) | 0.40 | 1.00 |
| Hematological | 66 | 62 | 0.74 | 1.00 | 51 | 54 | 0.80 | 1.00 | 117 | 116 | 1.01 | (0.72–1.42) | 0.94 | 1.00 |
| Other sites | 124 | 128 | 0.90 | n/a | 85 | 56 | 0.02 | n/a | 209 | 184 | 1.15 | (0.88–1.49) | 0.18 | n/a |
| Missing ICD/Unspecified | 93 | 97 | 0.78 | n/a | 36 | 41 | 0.55 | n/a | 129 | 138 | 0.94 | (0.68–1.28) | 0.58 | n/a |
|
| ||||||||||||||
| Total5 | 1095 | 1046 | 0.26 | n/a | 809 | 763 | 0.23 | n/a | 1904 | 1809 | 1.06 | (0.99–1.13) | 0.10 | n/a |
P-values are two-sided, from log-rank analyses with continuity correction.
Corrected p-values have been multiplied by the number of tests (18 sites) to allow for making multiple comparisons.
Excludes non-fatal non-melanoma skin cancer.
All confidence intervals are 99%, except for “all cancers”, which is a 95% confidence interval
Does not include non-fatal non-melanoma cancers
n/a = not applicable
Although there were conventionally significant protective effects against oesophagus cancer during the first 3 years (7 vs 21) but not later (16 vs 11), and against breast cancer later (61 vs 92) but not during the first 3 years (79 vs 65), these unanticipated period-specific protective effects ceased to be significant when corrected for multiple comparisons. For adverse effects, however, even before any such corrections there were no conventionally significant findings on any type of cancer in either time period.
Results for colorectal and prostate cancer are presented separately for each individual trial (webappendix pp8–9), and the analyses were repeated after exclusion of the AFPPS trial,21 which had reported a statistically significant excess risk of colorectal adenomas and prostate cancer. Overall, the remaining trials did not support a significant adverse effect on colorectal cancer (1.08; 95%CI: 0.89–1.30; heterogeneity [AFPPS versus the other trials] χ21=0.28, p=0.6) or prostate cancer (1.11; 95%CI: 0.95–1.30; heterogeneity [AFPPS versus the other trials] χ21=4.46, p=0.03). The provision of (o-e) for each trial (webappendix pp8–10) facilitates additional sensitivity analyses that exclude particular trials.
Discussion
Both the hopes for cancer prevention and the concerns about rapidly increased cancer risk from folic acid supplementation are not confirmed by this meta-analysis of the large folic acid trials. Although the point estimate for overall cancer incidence was slightly increased, this apparent increase was compatible with a chance effect, and there was no apparent increase in risk with increasing duration of treatment or dose of folic acid. Taking all studies together, allocation to folic acid for an average duration of 5 years had no significant short-term effect on overall or site-specific cancer incidence during the trials. In particular, supplementation had no significant effect on the incidence of cancers of the large intestine (despite the epidemiological evidence of protection),18 prostate, lung, breast or any other specific site, even in the period more than 3 years after randomization, although the power to detect differences for cancer at particular sites at varying intervals of follow-up is limited. The results of the present meta-analysis, involving 49,621 participants, are unlikely to be biased by the lack of availability of data on cancer incidence from two small trials of 1882 and 4110 participants that were treated with folic acid for 2 and 4 years, respectively.36,37
A previous meta-analysis using summary data from a sub-set of these trials suggested a marginally significant excess of prostate cancer.42 The present meta-analysis, however, which used individual participant data in a time-to-event analysis from all large trials, found no significant excess of prostate cancer or of any other type of cancer. Thus, any excess risk of prostate cancer from folic acid supplementation was most likely exaggerated by the play of chance in the AFPPS results.43 Appropriate interpretation of such findings requires avoidance of unduly selective emphasis on particular trials by analyzing all trials, and by hypothesis testing after excluding the results of the hypothesis-generating trial.40,41 For, an apparent excess or shortfall of some types of cancer in individual trials can be expected by chance alone when many different types of cancer are analyzed separately.
Although many of the trials used combinations of B-vitamins (vitamin B12 and vitamin B6 in addition to folic acid), it is unlikely that this would have concealed any effects of folic acid alone on cancer rates. The median daily dose of folic acid in the trials was 2.0 mg, which is much greater than in most widely used vitamin supplements (0.1 – 0.8 mg) and an order of magnitude greater than the dose of 0.1 – 0.2 mg typically delivered by flour fortification programs.8
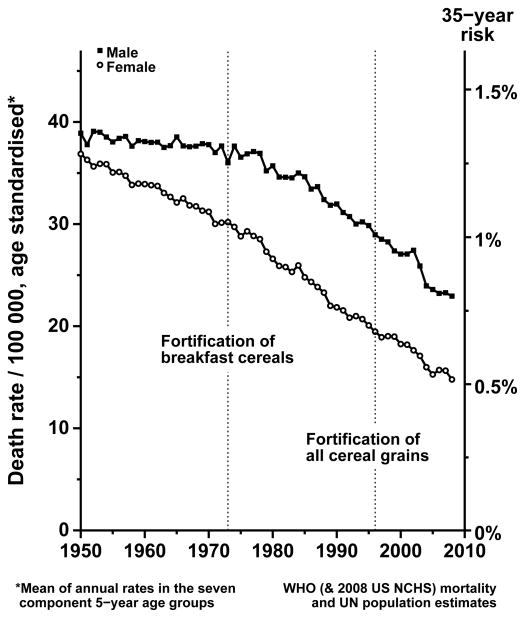
Despite the low doses provided by fortification, it had been suggested that transient increases in colorectal cancer incidence in the US and Canada in 1996–98 (during the 3-year period in which nationwide introduction of folate fortification was being established) might have been due to fortification.22 Although the observed increases in colorectal cancer incidence during 1996–98 are unexplained, they occurred too soon to be plausibly ascribable to the introduction of folate fortification during 1996–98 and did not persist after 1998. Moreover, national trends in mortality from colorectal cancer in the US at ages 35–69 years (which are less likely to be influenced by artefactual trends in cancer detection or registration rates) show no evidence of any new hazard after the introduction of fortification: Figure 4. Likewise, examination of US mortality rates at ages 35–69 years from the other main types of cancer (data not shown) provides no good evidence of any hazard following fortification.
Figure 4. Colorectal cancer mortality trends, 1950–2008, in the United States at ages 35–69.
Male (upper line) and female annual mortality rates: rates at ages 35–69 are calculated as the mean of the annual rates in the seven component 5-year age groups. Source: WHO mortality database and UN population tables. Mortality rates at ages 35–69 years were standardised for age by averaging the seven component 5-year age groups. If the annual rate per 100,000, standardised in this way, is R, then the 35-year death rate from colorectal cancer, ignoring competing risks, is 1−exp(−35R/100,000).
Although our meta-analyses included all large trials of folic acid, the power to exclude beneficial or adverse effects on cancer at individual sites was limited by the number of cancers and the relatively short duration of follow-up in these trials. While the present meta-analyses addresses the effects of high doses of folic acid on cancer during the scheduled trial treatment period, they do not address the question of whether any beneficial or harmful effects on cancer incidence will eventually emerge among the participants many years after the trials ended. Follow-up for decades after the trials ended may be feasible, especially in populations with automated record linkage to cancer registries and causes of death, but again it will be important not to place unduly data-dependent emphasis on the results for specific types of cancer in individual trials after particular follow-up durations.
Nevertheless, the human evidence about folic acid and cancer that has impeded folic acid fortification in the UK and some other countries involved apparent increases in incidence of colorectal and prostate cancer within just the first few years of starting treatment which, if real, should have been detectable during the trials. The present meta-analyses (which include the hypothesis-generating trial) address this issue directly, showing that in aggregate the trials provide no statistically significant evidence of short-term effects of folic acid supplementatation on overall cancer incidence, or on the incidence of any particular type of cancer. The present meta-analysis rules out moderate increases in overall cancer incidence from folic acid supplementation during the trials. Large increases in common cancer types are similarly unlikely. Since fortification involves folic acid doses an order of magnitude lower, its effects on cancer risk are likely to be even smaller.
Supplementary Material
Webfigure 1: Screening and selection of included trials
Webfigure 2: Effects of folic acid on cancer incidence, in pre-specified groups. Symbols and conventions as in Figure 1.
Webfigure 3: Effects of folic acid on overall first cancer incidence by percentage reduction in homocysteine or dose of folic acid. Symbols and conventions as in Figure 1.
Research in context.
Previous
Regulatory concerns about possible adverse effects of folic acid on cancer have delayed the introduction of folic acid fortification of flour for the prevention of neural tube defects in the United Kingdom and many other countries. The human evidence underlying these concerns involved apparent increases in cancer incidence within only a few years of starting treatment.
Now
Evidence from some trials that folic acid substantially increased site-specific cancer incidence within just a few years in certain trials is not supported by the other trials, or by the overall results from all trials.
Acknowledgments
The main acknowledgement is to the many participants in the trials, and to the trialists who cared for them and shared the trial data. We thank Jill Boreham for Figure 4.
B-Vitamin Treatment Trialists’ (BVTT) Collaboration
SEARCH: J Armitage, L Bowman, R Clarke, S Parish, R Peto, R Collins; HOPE-2: E Lonn, S Yusuf; WAFACS: JE Manson, R Glynn, F Grodstein, CM Albert, NR Cook; VITATOPS: G Hankey, J W Eikelboom; VISP: J Toole, MR Malinow, LE Chambless, JD Spence, LC Pettigrew, VJ Howard, EG Sides, CH Wang, M Stampfer; SU.FOL.OM3: P Galan, S Hercberg; WENBIT: O Nygård, M Ebbing, JE Nordrehaug, DWT Nilsen, PM Ueland, H Refsum, SE Vollset; NORVIT: KH Bønaa, I Njølstad; HOST: R Jamison, JM Gaziano, P Guarino; AFPPS: J A Baron; HARVARD: EA Giovannucci; ukCAP: RFA Logan; VITRO: M denHeijer, H Blom, G Bos. BVTT secretariat: R Clarke, J Halsey, S Lewington, D Bennett, R Collins, R Peto.
Footnotes
Contributions The authors accept full responsibility for the content of this paper. All authors contributed to either the collection or analysis of the data, or both, and to preparation of the report. All authors had an opportunity to contribute to the interpretation of the results and to a critical review of the final draft of the manuscript.
Conflicts of interest Sources of funding for individual trials are described in their separate publications. The Clinical Trial Service Unit and Epidemiological Studies Unit (CTSU), where the BVTT secretariat is located, has a policy of not accepting fees, honoraria, or paid consultancies directly or indirectly from any industry. It receives its core funding from the British Heart Foundation, UK Medical Research Council and Cancer Research UK. Support for this project was also provided by a grant from the UK Food Standards Agency (N05072).
References
- 1.Hibbard BM. The role of folic acid in pregnancy with particular reference to anaemia, abruption and abortion. J Obstet Gynaecol Br Common W. 1964;71:529–42. doi: 10.1111/j.1471-0528.1964.tb04317.x. [DOI] [PubMed] [Google Scholar]
- 2.Molloy AM, Kirke P, Hillary I, Weir DG, Scott JM. Maternal serum folate and vitamin B12 concentrations in pregnancies associated with neural tube defects. Arch Dis Child. 1985;80:660–65. doi: 10.1136/adc.60.7.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bower C, Stanley FJ. Dietary folate as a risk factor for neural tube defects: evidence from a case-control study in Western Australia. Med J Aust. 1989;150:613–19. doi: 10.5694/j.1326-5377.1989.tb136723.x. [DOI] [PubMed] [Google Scholar]
- 4.Smithells RW, Sheppard S, Schorah CJ, et al. Possible prevention of neural tube defects by periconceptional vitamin supplementation. Lancet. 1980;1:339–40. doi: 10.1016/s0140-6736(80)90886-7. [DOI] [PubMed] [Google Scholar]
- 5.Berry RJ, Li Z, Erickson JD, Li S, Moore CA, Wang H, Mulinare J, Zha P, Wong LY, Gindler J, Hong SX, Correa A. Prevention of neural-tube defects with folic acid in China. China-U.S. Collaborative Project for Neural Tube Defect Prevention. N Engl J Med. 1999;341:1485–90. doi: 10.1056/NEJM199911113412001. [DOI] [PubMed] [Google Scholar]
- 6.Prevention of neural tube defects: results of the Medical Research Council Vitamin Study. MRC Vitamin Study Research Group. Lancet. 1991;338:131–37. [PubMed] [Google Scholar]
- 7.Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N Engl J Med. 1992;327:1832–35. doi: 10.1056/NEJM199212243272602. [DOI] [PubMed] [Google Scholar]
- 8.Scientific Advisory Committee on Nutrition. Folate and disease prevention. London: The Stationery Office; 2006. [Google Scholar]
- 9.Centres for Disease Control and Prevention (CDC) Trends in wheat flour fortification with folic acid and iron-worldwide, 2004 and 2007. Morb Mortal Wkly Rep. 2008;57:8–10. [PubMed] [Google Scholar]
- 10.Jacques PF, Selhub J, Bostom AG, Wilson PW, Rosenberg IH. The effect of folic acid fortification on plasma folate and total homocysteine concentrations. N Engl J Med. 1999;340:1449–54. doi: 10.1056/NEJM199905133401901. [DOI] [PubMed] [Google Scholar]
- 11.Sayed AR, Bourne D, Pattinson R, Nixon J, Henderson B. Decline in the prevalence of neural tube defects following folic acid fortification and its cost-benefit in South Africa. Birth Defects Res Clin Mol Teratol. 2008;82:211–6. doi: 10.1002/bdra.20442. [DOI] [PubMed] [Google Scholar]
- 12.López-Camelo JS, Castilla EE, Orioli IM. INAGEMP (Instituto Nacional deGenética Médica Populacional); ECLAMC (Estudio Colaborativo Latino Americano de Malformaciones Congénitas). Folic acid flour fortification: impact on the frequencies of 52 congenital anomaly types in three South American countries. Am J Med Genet. 2010;152:2444–58. doi: 10.1002/ajmg.a.33479. [DOI] [PubMed] [Google Scholar]
- 13.Australian Institute of Health and Welfare (AIHW) Mandatory folic acid and iodine fortification in Australia and New Zealand: baseline report for monitoring. Canberra: AIHW; 2011. [Google Scholar]
- 14.Folic acid: an update on scientific developments. European Union Publications Office; 2009. FFSA Meeting summary report. [Google Scholar]
- 15.Scientific Advisory Committee on Nutrition. Folic acid and colorectal cancer risk: review of recommendations for folic acid mandatory fortification. London: The Stationery Office; 2009. [Google Scholar]
- 16.Clarke R, Daly L, Robinson K, Naughten E, Cahalane S, Fowler B, et al. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991;324:1149–55. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- 17.Boushey CJ, Beresford SA, Omenn GS, Motulsky AG. A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA. 1995;274:1049–57. doi: 10.1001/jama.1995.03530130055028. [DOI] [PubMed] [Google Scholar]
- 18.Sanjoaquin MA, Allen NA, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. 2005;113:825–38. doi: 10.1002/ijc.20648. [DOI] [PubMed] [Google Scholar]
- 19.B-Vitamin Treatment Trialists’ Collaboration. Homocysteine-lowering trials for prevention of cardiovascular events: a review of the design and power of the large randomized trials. Am Heart J. 2006;151(2):282–87. doi: 10.1016/j.ahj.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 20.Clarke R, Halsey J, Lewington S, et al. Effects of lowering homocysteine levels with B vitamins on cardiovascular disease, cancer, and cause-specific mortality. Meta-analysis of 8 randomized trials involving 37485 individuals. Arch Intern Med. 2010;170:1622–31. doi: 10.1001/archinternmed.2010.348. [DOI] [PubMed] [Google Scholar]
- 21.Cole BF, Baron JA, Sandler RS, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–59. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 22.Mason JB, Dickstein A, Jacques PF, et al. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16:1325–29. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]
- 23.Kim YI. Will mandatory folic acid fortification prevent or promote cancer? Am J Clin Nutr. 2004;80:1123–28. doi: 10.1093/ajcn/80.5.1123. [DOI] [PubMed] [Google Scholar]
- 24.den Heijer M, Willems HP, Blom HJ, et al. Homocysteine lowering by B vitamins and the secondary prevention of deep vein thrombosis and pulmonary embolism: a randomized, placebo-controlled, double-blind trial. Blood. 2007;109:139–44. doi: 10.1182/blood-2006-04-014654. [DOI] [PubMed] [Google Scholar]
- 25.Jamison RL, Hartigan P, Kaufman JS, et al. Effect of homocysteine lowering on mortality and vascular disease in advanced chronic kidney disease and end-stage renal disease: a randomized controlled trial. JAMA. 2007;298:1163–70. doi: 10.1001/jama.298.10.1163. [DOI] [PubMed] [Google Scholar]
- 26.Ebbing M, Bønaa KH, Nygård O, et al. Cancer incidence and mortality after treatment with folic acid and vitamin B12. JAMA. 2009;302:2119–26. doi: 10.1001/jama.2009.1622. [DOI] [PubMed] [Google Scholar]
- 27.Bonaa KH, Njolstad I, Ueland PM, et al. Homocysteine lowering and cardiovascular events after acute myocardial infarction. N Engl J Med. 2006;354:1578–88. doi: 10.1056/NEJMoa055227. [DOI] [PubMed] [Google Scholar]
- 28.Galan P, Kesse-Guyot E, Czernichow S, et al. Effects of B-vitamins and omega-3 fatty acids on cardiovascular diseases: a randomised placebo-controlled trial. BMJ. 2010;341:C6273. doi: 10.1136/bmj.c6273. doi:10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toole JF, Malinow MR, Chambless LE, et al. Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. JAMA. 2004;291:565–75. doi: 10.1001/jama.291.5.565. [DOI] [PubMed] [Google Scholar]
- 30.VITATOPS Trial Study Group. B-vitamins in patients with recent transient ischaemic attack or stroke in the vitamins to prevent stroke (VITATOPS) trial: a randomised, double-blind, parallel, placebo-controlled trial. Lancet Neurology. 2010;9:855–65. doi: 10.1016/S1474-4422(10)70187-3. [DOI] [PubMed] [Google Scholar]
- 31.Zhang SM, Cook NR, Albert CM, Gaziano JM, Buring JE, Manson JE. Effect of folic acid, vitamin B6, and vitamin B12 on cancer risk in women: a randomized trial. JAMA. 2008;300:2012–21. doi: 10.1001/jama.2008.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lonn E, Yusuf S, Arnold MJ, et al. Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med. 2006;354:1567–77. doi: 10.1056/NEJMoa060900. [DOI] [PubMed] [Google Scholar]
- 33.Armitage JM, Bowman L, Clarke RJ, et al. Effects of homocysteine-lowering with folic acid plus vitamin B12 vs placebo on mortality and major morbidity in myocardial infarction survivors: a randomized trial. JAMA. 2010;303:2486–94. doi: 10.1001/jama.2010.840. [DOI] [PubMed] [Google Scholar]
- 34.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 35.Wu K, Platz EA, Willett WC, et al. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am J Clin Nutr. 2009;90:1623–31. doi: 10.3945/ajcn.2009.28319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baker F, Picton D, Blackwood S, et al. Blinded comparison of folic acid and placebo in patients with ischaemic heart disease: an outcome trial. Circulation. 2002;106(Suppl 2):741S. [Google Scholar]
- 37.Bostom AG, Carpenter MA, Kusek JW, et al. Homocysteine-lowering and cardiovascular disease outcomes in kidney transplant recipients: primary results from the Folic Acid for Vascular Outcome Reduction In Transplantation trial. Circulation. 2011;123:1763–70. doi: 10.1161/CIRCULATIONAHA.110.000588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta-blockade during and after myocardial infarction: an overview of the randomized trials. Prog in Cardiovasc Dis. 1985;27:335–71. doi: 10.1016/s0033-0620(85)80003-7. [DOI] [PubMed] [Google Scholar]
- 39.Sankoh AJ, Huque MF, Dubey SD. Some comments on frequently used multiple end-point adjustment methods in clinical trials. Stat Med. 1997;16:2529–42. doi: 10.1002/(sici)1097-0258(19971130)16:22<2529::aid-sim692>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 40.Peto R, Emberson J, Landray M, et al. Analyses of cancer data from three ezetimibe trials. N Engl J Med. 2008;359:1357–66. doi: 10.1056/NEJMsa0806603. [DOI] [PubMed] [Google Scholar]
- 41.Baigent C, Peto R, Gray R, Parish S, Collins R. Large-scale randomized evidence: trials and meta-analyses of trials. In: Warrell D, Cox TM, Firth JD, editors. Oxford Textbook of Medicine. 5. Oxford: Oxford University Press; 2011. [Google Scholar]
- 42.Wien TN, Pike E, Wisloff T, Staff A, Smeland S, Kemp M. Cancer risk with folic acid supplements: a systematic review and meta-analysis. BMJ Open. 2012;2:e000653. doi: 10.1136/bmjopen-2011-000653. doi:10.1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Figueiredo JC, Grau MV, Haile RW, Sandler RS, Bresalier RS, Burke CA, McKeown-Eyssen GE, Baron JA. Folic acid and risk of prostate cancer: results from a randomized clinical trial. J Natl Cancer Inst. 2009;101:432–435. doi: 10.1093/jnci/djp019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Webfigure 1: Screening and selection of included trials
Webfigure 2: Effects of folic acid on cancer incidence, in pre-specified groups. Symbols and conventions as in Figure 1.
Webfigure 3: Effects of folic acid on overall first cancer incidence by percentage reduction in homocysteine or dose of folic acid. Symbols and conventions as in Figure 1.