Abstract
This research evaluated the neural correlates of implicit associative memory processes (habit-based processes) through the imaging (fMRI) of a marijuana Implicit Association Test. Drug-related associative memory effects have been shown to consistently predict level of drug use. To observe differences in neural activity of associative memory effects, this study compared 13 heavy marijuana users and 15 non-using controls, ranging in age from 18 to 25, during performance of a marijuana Implicit Association Test (IAT). Group by condition interactions in the putamen, caudate, and right inferior frontal gyrus were observed. Relative to non-users, marijuana users showed greater bilateral activity in the dorsal striatum (caudate and putamen) during compatible trials focused on perceived positive outcomes of use. Alternatively, relative to the marijuana-using group, the non-users showed greater activity in the right inferior frontal gyrus during incompatible trials, which require more effortful processing of information. Further, relative to fixation, heavy users showed bilateral activity in the caudate and putamen, hippocampus and some frontal regions during compatible trials and no significant activity during incompatible trials. The non-using group showed greater activity in frontal regions during incompatible trials relative to fixation and no significant activity during compatible trials. These findings are consistent with a dual process framework of appetitive behaviors proposing that (1) implicit associations underlying habit are mediated through neural circuitry dependent on the striatum, and (2) deliberative/controlled behaviors are mediated through circuitry more dependent on the prefrontal cortex.
Keywords: fMRI, Marijuana IAT, habit, associative processes, memory associations, implicit cognition
1. Introduction
Following voluntary repetitive drug use, changes in the brain occur that affect control over continued use. For instance, complex associative learning and memory processes influenced by repetitive drug use result in neurobiological consequences that include the strengthening of associative memories. These associative memories in turn can affect subsequent drug use. The influence of these associative memory effects is now well documented in numerous behavioral studies on appetitive behaviors, showing predictive utility in a range of populations and for several drugs of abuse (for reviews, see Ames et al., 2006; Rooke et al., 2008; Stacy and Wiers, 2010). Implicit associative memory effects appear to be, at least in part, responsible for some of the irrational decision making associated with continued drug use. Although much has been learned about neuroadaptive changes that occur in the brain from substance abuse (e.g., Bechara, 2005; Bechara et al., 2002; Everitt et al., 2001, 2008; Robinson and Berridge, 2001; Volkow and Fowler, 2000; also see Adinoff, 2004), implicit associative drug-related memory processes relevant to habit are under-studied processes of addictive behavior.
While these associative memories form one of the processes that maintain drug use, they are not the only relevant process. Other relevant processes include prefrontal cortical regions (e.g., dorsolateral, orbital frontal sub-regions, and inferior frontal gyrus; Aron, Robbins & Poldrack, 2004) involved in deliberative/inhibitory control processes that often modify or inhibit automatic processes thought to be mediated by the ventral and dorsal striatum (Everitt and Robbins, 2005). Thus, continued or escalated drug use can be viewed as a consequence of strengthening of associative automatic processes, overwhelming or weakening regulatory control processes.
1.1 Implicit Associations and Drug Use
Drug use habits may be considered a strong form of reinforced associative learning (Yin and Knowlton, 2006) and may strengthen motivationally relevant associative memories (see Stacy, 1997). Dopaminergic activity in the striatum reinforces the repetition of certain behaviors and the encoding and processing of proximal stimuli associated with rewarding experiences (e.g, Everitt and Robbins, 2005; Cardinal and Everitt, 2004). Marijuana indirectly activates dopamine systems by stimulating neurons that modulate the release of dopamine through direct effects on cannabinoid receptors (Baler and Volkow, 2006; Koob, 2002). Recent animal models have shown endocannabinoid signaling (through CB1-R) to play a key role in habit development with the striatum implicated as a critical neural substrate for CB1 signaling (Hilario et al., 2007). Further, endocannabinoids have been implicated in drug use habits and rewarding experiences (e.g., Caille, et al., 2007; Corbille, et al. 2007; Giuffrida et al., 1999).
Through repetitive experience, stimuli associated with rewards may come to represent and subsequently cue the behavior. Research on the neurobiology of drug use has shown this behavior to be highly sensitive to prior learning experiences and predictive cues, which become encoded into patterns of association. Cues can then trigger a pattern of activation in memory that is a relatively automatic process, described in connectionist and neural network models (e.g., Hopfield and Tank, 1986; Queller and Smith, 2002). As learned associations in memory are strengthened, patterns of associations signal and drive behavior without the necessary involvement of deliberative or control processes (cf., Stacy et al., 2004; White, 1996; Wiers et al., 2007).
Various brain regions associated with neural systems of reward overlap with associative memory and habit systems (see Stacy et al., 2004). These regions are different from those supporting aspects of controlled cognitive processes and explicit memory (e.g., Squire, 1992; White, 1996). For instance, neural systems of the dorsal striatum have been implicated in habit-based learning and associative memory processes (Knowlton et al., 1996; Wagner et al., 2000; White, 1996; Yin and Knowlton, 2006; Yin et al., 2005).
In humans, validated associative memory procedures of drug use behavior have been shown to tap into and activate pre-existing associations in memory (see Stacy et al., 2004; Rooke et al., 2008; Wiers and Stacy, 2006). The Implicit Association Test (IAT) is a commonly used indirect test of associations in memory (for review, Greenwald et al., 2009). The IAT is a concept categorization task that evaluates the relative strength of associations of contrasted target categories with contrasted attribute categories through rate of processing. The assumption is that individuals react faster when they categorize strongly associated concepts that share a response key (based on past learning experience) and slower when they categorize concepts less likely to be associated that share a response key (Greenwald et al., 1998). In behavioral research, the IAT has been found to effectively differentiate non-substance users from substance users in several studies (e.g., tobacco, Swanson et al. 2001; alcohol, De Houwer et al., 2004; Houben and Wiers, 2007; 2008; Jajodia and Earleywine, 2003, Lindgren et al., 2012; McCarthy and Thompsen, 2006; Thush et al., 2008; Wiers et al., 2002, Wiers et al., 2005; cocaine, Wiers et al., 2007; marijuana, Ames et al., 2007; Field et al., 2004). Ames et al. (2007) found positive outcome associations to be predictive of marijuana use among at-risk adolescents with a marijuana IAT controlling for gender, ethnicity, explicit cognitions, sensation seeking and working memory capacity.
In addition, the neural correlates of non-substance-related IATs have been observed in several imaging studies (e.g; racial preference, Beer et al., 2008; flowers/insect pleasantness, Chee et al., 2000; politics, Knutson et al., 2006; gender and race-related, Knutson et al., 2007; morality, Luo et al., 2006). The IAT elicits neural populations involved in implicit or automatic processing during the task’s compatible trials and effortful processing on incompatible trials. Incompatible trials have been found to elicit greater frontal activity relative to compatible trials in imaging studies involving non-substance-related IATs (e.g., increased activity in the ventrolateral, dorsolateral prefrontal cortex and anterior cingulate: Chee et al., 2000; Luo et al., 2006; left inferior frontal gyrus: Knutson et al., 2006; middle frontal gyrus: Knutson et al., 2007). In addition, on a racial preference IAT, Beer et al. (2008) found significant activity in the caudate, insular cortex, and lateral orbital frontal cortex during compatible association trials. Although these imaging studies used the IAT to evaluate behavioral processes involving implicit associations, this study is the first to elicit neural correlates of a marijuana-specific IAT.
1.1.2 Overview
This study observed differences in hemodynamic response between heavy marijuana using individuals and age-matched non-using controls during performance on compatible and incompatible trials of a marijuana-specific IAT. Among marijuana users (compared to non-users), we predicted that we would observe more neural activity in regions critical for implicit associative memory processes underlying habit during trials in which strength of positive implicit associations toward marijuana use should be detected (i.e., compatible association trials). The regions implicated were the dorsal striatum (caudate/putamen) and ventral striatum.
In addition, we predicted that incompatible trials would elicit more neural activity in regions implicated in executive and inhibitory control processes among all participants, when compared to compatible trials, since during incompatible trials, participants categorize concepts not generally related (requiring more effortful processing). Further, the degree of increased activity in these regions was expected to be less (reflecting poorer control processes) in the marijuana users. Based on earlier IAT studies (e.g., Chee et al., 2000; Luo et al., 2006), the regions implicated were lateral regions of the orbitofrontal cortex, dorsolateral and adjacent ventrolateral region, and inferior frontal gyrus, all regions associated with inhibitory control processes (e.g. Aron et al., 2004).
2. Materials and Methods
Behavioral assessments and neuroimaging took place at the Dornsife Cognitive Neuroscience Imaging Center at the University of Southern California. All study protocol was approved by the Claremont Graduate University and University of Southern California Internal Review Boards and was consistent with the Code of Ethics of the World Medical Association. Recruited participants initially performed a practice IAT task (flower/insect and pleasant/unpleasant categories) to get acquainted with the protocol. The practice task was designed to prevent priming of the concept of focus. Next, participants were situated in the scanner where they underwent a structural scan and then performed a marijuana-specific IAT optimized for the scanner, which took about 30 minutes. The fMRI assessment utilized a mixed design. Participants completed computerized questionnaires consisting of behavioral measures following scanning and were given $95 for participation. The entire assessment took about 1½ hours.
2.1. Participants
Participants were 28 neurologically normal emerging adults ranging in age from 18–25. Written consent was obtained from all study participants. Thirteen participants were heavy marijuana users and 15 were non-users. The mean age of the heavy marijuana users was 21.15 (1.9) and the mean age of the controls was 20.27(2.3). Eighteen percent of heavy marijuana users were female and 67% of non-users were female. All participants were right-handed and native English speakers. Participants were recruited through flyers in stores, coffee shops, sport recreation centers, and medical marijuana dispensaries in the metropolitan Los Angeles area. To be in the study, participants had to be light alcohol or non-drinkers with minimal lifetime use of drugs other than marijuana. Individuals with a history of psychiatric or neurological disorders or of use of medications that affect the central nervous system were excluded from the study.
The majority of the non-using controls had never used marijuana (13) and the other 2 had used marijuana only a few times in their lifetimes. The heavy marijuana users reported using marijuana more than 500 times in the past 3 years and current daily use of marijuana (see Table 1). Users and non-using controls were matched on age, ethnicity, and past 3 year alcohol and tobacco use. Participants were asked to not use the day of testing and this was verbally confirmed the day of the imaging session.
Table 1.
Population Descriptives
| Non-Using Controls N=15 |
Heavy Marijuana Users N=13 |
||||
|---|---|---|---|---|---|
| Mean (sd) | Range | Mean (sd) | Range | P | |
| Age | 20.27 (2.3) | 18–25 | 21.15 (1.9) | 19–25 | .283 |
| Female/Male | 10/5 | 2/11 | <.01 | ||
| Marijuana Use past 3 years 1=none to 7=>500 times |
1.2 (.4) | 1–2 | 6.85 (.56) | 5–7 | <.0001 |
| Last Use of Marijuana 1=>5 years ago to 12=24 hours ago |
1.73 (1.48) | 1–6 | 11.46 (.52) | 11–12 | <.0001 |
| OSPAN score (working memory capacity) |
33.6 (7.78) | 18–42 | 37.25 (7.42) | 21–42 | .228 |
2.2 Questionnaires
Past 3-year substance use (i.e. marijuana, ecstasy, hallucinogens, methamphetamine, etc.) was assessed with a 7-item rating scale (1=“never used” to 7=“>500 times”). Time since last marijuana use was assessed with a 12-item rating scale, ranging from “1= never used” to “12=within the last 24 hours”.
Working memory capacity was assessed as a proxy measure for general fluid intelligence with the OSPAN (see Engle, 2002; Engle et al., 1999; Kane and Engle, 2002), a validated automated operation span task (Unsworth et al., 2005; alpha=.78; test-retest reliability r=.83). The task measures capacity to learn and maintain information in an active state during interference and demands controlled attention (Kane and Engle, 2002). Participants are instructed to remember a series of 3 to 7 letters while solving simple math problems and indicating whether an answer to a problem is true or false (e.g., 8/2+6=10). Math problems serve as distracters requiring control of attention while maintaining letter sequences in short-term memory. A larger number of letters recalled in proper sequence is indicative of higher working memory capacity.
Marijuana Implicit Association Test (IAT)
The marijuana IAT consisted of words and images (cf. Ames et al., 2007). The IAT has shown stable test-retest reliability (median r=.56) and good internal consistency (range .7 to .9; Hofmann et al., 2005; Nosek et al., 2007). Participants observed 5 different words and images. Practice and test trials consisted of the same stimuli (see trials described below). The stimuli categorized were randomly presented. In the scanner, participants were instructed: “Press the #1 key for items that fit into a category on the top left. Press the #4 key for items that fit into a category on the top right. The categories change from time to time. You will not receive any instructions during the task. Go as fast as you can without making mistakes. Please wait for the task to start automatically.”
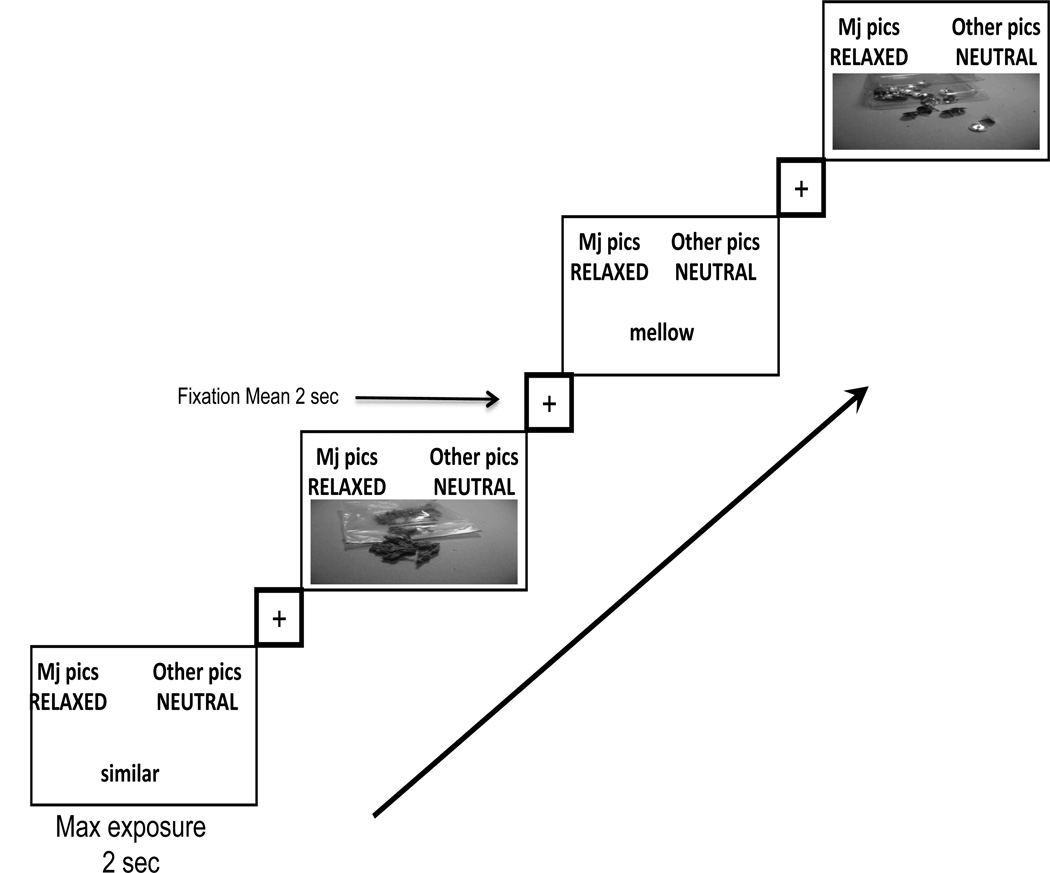
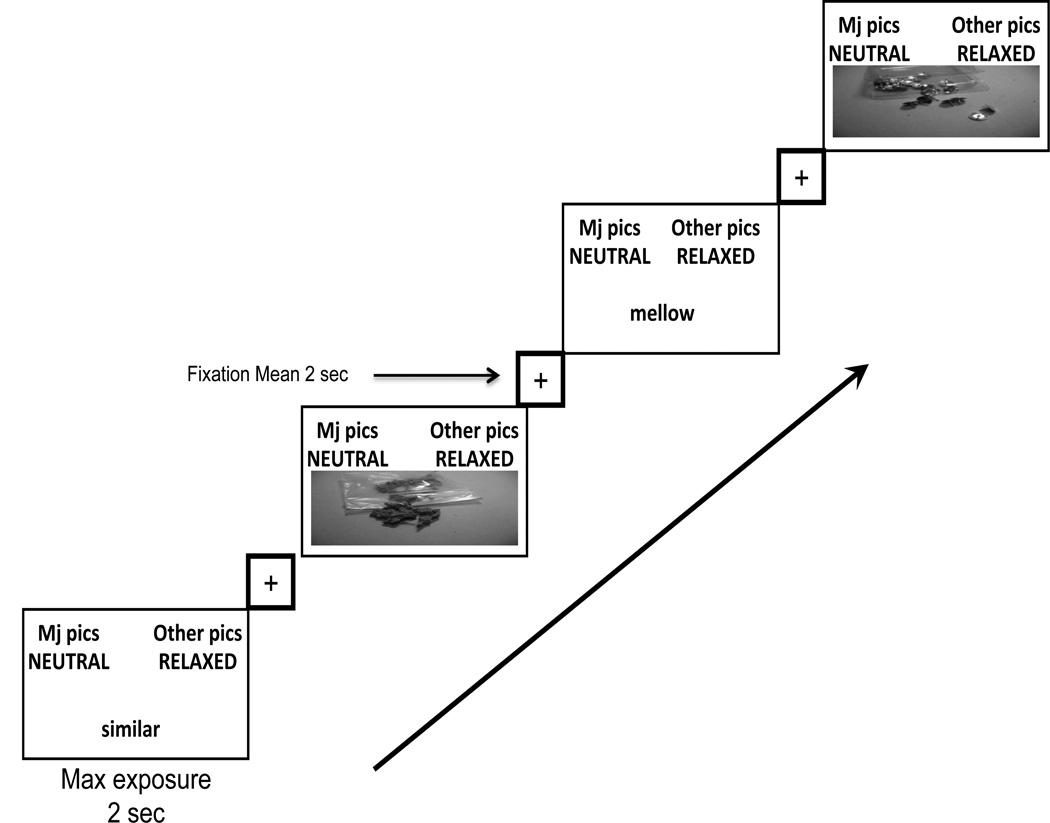
The IAT included as target categories ‘Marijuana Pictures’ and ‘Other Pictures’. Pictures were closely matched in size and shape. ‘Marijuana Pictures’ were images of a marijuana cigarette (joint), rolling papers, pipe, dried marijuana buds, and dried marijuana in a plastic bag. The matched 'Other Pictures’ were images of a ballpoint pen, small memo pad, small flashlight, loose thumbtacks, and thumbtacks in a clear plastic box. Affective categories consisted of a 'Relaxed’ category for positive reinforcement and a 'Neutral’ category. Relaxed category words were chill, calm, cool, mellow and comfortable. The matched Neutral category words were count, square, common, similar, and historical. A standard version of the IAT was used (Nosek et al., 2005) and included 7 blocks of trials: (1) 20 practice trials with target categories only, (2) 20 practice trials with affective categories only, (3) 20 compatible block practice trials with both target and affective categories, (4) 40 compatible block test trials with both target and affective categories, (5) 20 practice trials with target categories in reversed positions, (6) 20 incompatible block practice trials with reversed target categories and affective categories, and (7) 40 incompatible block test trials with reversed target categories and affective categories. The order of IAT incompatible and compatible blocks of trials was counterbalanced across participants. The headers for compatible trials were “marijuana pictures + relaxed words” and “other pictures + neutral words”. The headers for incompatible trials were “marijuana pictures + neutral words” and “other pictures + relaxed words” (see Figure 1 and Figure 2).
Figure 1.
Temporal layout of 40 Compatible trials or implicit associations toward marijuana use. Individuals react faster when categorizing strongly associated concepts that share a response key. This should be fairly easy to do for someone with past experience with marijuana use. Temporal jitter was used in the presentation of the fixation with onset timing ranging from 1–4.5 seconds, with a mean exposure of 2 seconds, followed by stimuli presentation. Maximum exposure of test stimuli was for 2 seconds. After a participant pressed a response key, the screen would go blank for the remainder of the 2 seconds. Total trial time ranged from 3 to 6.5 seconds.
Figure 2.
Temporal layout of 40 Incompatible trials. In this figure the attribute categories are switched. Individuals react slower when categorizing concepts not typically associated that share a response key. These trials require more effortful processing across all subjects.
Trials on blocks 3 and 4 were included in the analyses for compatible association trials and blocks 6 and 7 were included for incompatible association trials (or the reverse when counterbalanced). It is the standard in behavioral studies to include practice blocks 3 and 6 in analyses of the IAT effect (Nosek et al, 2005), and that protocol was followed in the fMRI analyses.
For the IAT run, blocks of compatible trials and incompatible trials were randomly ordered and counterbalanced. Within each block, trials and fixation were presented in an order specified by OPTSEQ (Dale, 1999) to enhance design efficiency. Total trial time ranged from 3 to 6.5 seconds. Temporal jitter was used in fixation presentation with onset timing ranging from 1–4.5 seconds, and mean exposure of 2 seconds, followed by stimuli presentation. Maximum exposure for the test stimuli was 2 seconds. The visual screen would go blank for the remainder of the 2 seconds following a participant’s key press response.
Response latencies were collected along with the scans and scored according to the scoring algorithm described by Greenwald et al. (2003) to obtain a D-600 measure. Higher D-600 scores reflect a greater difference between compatible and incompatible categorization scores, whereas lower D-600 scores reflect a smaller difference between compatible and incompatible scores.
2.3 Imaging parameters and data pre-processing
A Siemens 3T Magnetom Tim/Trio MR scanner fitted with head coil arrays for parallel imaging to minimize signal loss and image distortion was used for imaging. An MRI-compatible response box with a fiber-optics response pad and 4 buttons that accepts a trigger from the scanner was used to collect participants’ responses. Participants lay supine on the scanner bed while visual stimuli were back-projected onto a screen and viewed through a mirror attached onto the head coil. Functional images were acquired using a z-shim gradient, single-shot T2*-weighted echo EPI sequence with prospective acquisition correction. This sequence is dedicated to reduce signal loss in the prefrontal and orbitofrontal regions. The scanning parameters were as follows: TR = 2000 ms (whole brain); TE = 25 ms; flip angle =90°; 64 × 64 matrix size with resolution 3×3 mm2; Bandwidth:1906 Hz/pixel. Thirty-five 3 mm axial slices were acquired to cover the cerebrum and most of the cerebellum with no gap. The anatomical T1-weighted structural scan was acquired using an MPRAGE sequence (TI =800 ms; TR = 2530 ms; TE = 3.1 ms; flip angle 10°; 208 sagittal slices; 256 × 256 matrix size with spatial resolution as 1×1×1mm3).
The fMRI data underwent preprocessing to improve signal detection and sensitivity of statistical analyses. The functional data were slice acquisition timing corrected, motion corrected, co-registered to the anatomical image, normalized to the standardized template and spatially smoothed with a Gaussian kernel (4 mm full width at half maximum). A high pass filter was applied to the functional data to minimize low frequency noise such as cardiac cycles and respiration (Friston et al., 2000; Wang et al., 2005). No participants’ data were excluded from the analyses since all movement parameters were less than 2 mm along all axes.
2.4 Data analysis
SAS® software Version 9.3 (SAS Institute Inc., 2011) was used for analyses of demographics and behavioral measures. Differences between groups on demographics, marijuana and other drug use, and the IAT D-600 measure were evaluated using t and F tests.
The fMRI whole brain analyses were performed using SPM8 (www.fil.ion.ucl.ac.uk/spm). To visually plot the direction of interaction effects, Marsbar (http://marsbar.sourceforge.net/) implemented in MATLAB (Mathworks Inc.) was used to extract the percent signal change in regions where interaction effects were observed. The analyses used a standard two-level GLM model for fMRI analysis. In the first level, the GLM included two regressors representing two types of stimuli (compatible and incompatible trials). Fixation served as baseline. Separate analyses with random effects were carried out in the second level (Friston et al., 1995). First, to identify within-group activation for compatible and incompatible trials, one sample t-tests were performed for the marijuana users and controls separately. Voxels were considered significant at p < .005 (uncorrected) with clusters larger than 30 voxels. This threshold is equivalent to p corrected < 0.05 based on Monte Carlo simulations (Cox, 1996).
To compare group differences on compatible and incompatible trials, a 2 (group: marijuana users or non-users) X 2 (IAT blocks of trials: compatible or incompatible) full-factor random effects (Friston et al., 1995) model was used in the second level analysis. F tests were used to detect the main effects of condition and group and their interaction. The significance threshold was set at an exploratory uncorrected p < .005 with clusters larger than 30 voxels. Percent signal change was calculated for each participant under each condition separately.
3. Results
3.1. Behavioral data
The IAT effect (D-600) was calculated as the mean reaction time in incompatible blocks minus the mean reaction time in compatible blocks divided by the pooled standard deviation for the two blocks (see Greenwald et al., 1998). A 2×2×2×2 multivariate ANOVA (2 within block types: compatible vs. incompatible by 2 within practice blocks: practice vs. test by 2 between groups: users vs. non-users by 2 between block orders: compatible first vs. incompatible first) showed a main univariate effect for block type F(1, 25) = 14.42, p<.001 and practice block F(1, 25)=14.62, p<.001. The mean response time for the compatible trials (blocks 3 and 4, M=798ms, SD=207) was less than the mean for the incompatible trials (blocks 6 and 7, M=864ms, SD=216) indicating that participants overall had positive associations for marijuana. The mean response time for the practice blocks (blocks 3 and 6, M=855ms, SD=206) was larger than the mean for the test blocks (blocks 4 and 7, M=807ms, SD=211) indicating that participants responded quicker after some practice. Practice effects are common in the IAT (Nosek et al., 2005). No other main effects and no 2-way interactions were significant. The IAT effect (D-600 score) for both groups was small. The mean D-600 measure for the heavy marijuana users was .268 (SD=.36; range −.5 to .8 seconds), and the mean D-600 measure for the non-using controls was .299 (SD=.29.; range −.09 to .9 seconds).
A second multivariate ANOVA with the same factors was run for the proportion of errors in each block. There was a main univariate effect for block type, F(1, 25) = 14.42, p<.001, such that the mean proportion of errors for compatible trials (M=.044, SD=.034) was less than the mean for incompatible trials (M=.065, SD=.045), indicating that participants overall made fewer errors in the compatible blocks. No other main effects or interactions were significant. The mean proportion of errors for marijuana users was .051 (SD=.035) and for non-using controls, .057 (SD=.035).
There was a significant difference between groups on time since last drug use, with non-using controls reporting last drug use as being approximately 5 years ago and marijuana users reporting a mean of last use 24 hours ago (p<.0001, see Table 1). There were no significant differences between groups with respect to cigarette, alcohol, methamphetamine, tranquilizer, opiate or hallucinogen use, with self-reported use of these drugs minimal. Self-reported cocaine use was also minimal but marijuana users reported slightly more use (mean=1.3, SD=.48) than non users (mean=1, SD=0, p=.039). There were no significant differences between groups in working memory capacity (p=.228).
3.1.1. fMRI data
Table 2 provides whole brain analysis peak activity t statistics for the non-using controls and marijuana users during the incompatible and compatible trials (relative to fixation). During compatible trials, the marijuana users showed significant bilateral activity in predicted regions of the dorsal striatum (caudate and putamen). In conjunction with this intensified striatal and implicit activity in marijuana users, increased frontal activity was also observed, perhaps reflecting an increased effort to exert inhibitory control over more automatic tendencies. Increased activity during compatible trials was also observed in the hippocampus. During compatible trials, we observed no significant regional activity among non-users, and during incompatible trials, we observed no significant regional activity among the heavy users. Significant activity was observed across several sub-regions of the lateral prefrontal cortex among non-using controls during the more effortful incompatible trials. These sub-regions include the left middle frontal gyrus, the operculum region of the inferior frontal gyrus, as well as the more anterior region of the left inferior frontal gyrus, and in the right superior frontal gyrus. Other activation in non-users included the left hippocampus and right caudate (see Table 2).
Table 2.
Significant Regions of Activity within Groups during Compatible and Incompatible Trials
| Hemisphere | Region | X | Y | Z | Voxels in Cluster |
T statistic |
|
|---|---|---|---|---|---|---|---|
| Compatible Association Trials | |||||||
| Non Using Controls | |||||||
| Marijuana Users | Left | Inferior Frontal Gyrus | −46 | 28 | 24 | 2223 | 6.16 |
| Right | Posterior Cingulate Cortex | 6 | −40 | 6 | 85 | 4.85 | |
| Left | Inferior Frontal Gyrus Operculum | −54 | 12 | 0 | 85 | 4.24 | |
| Right | Putamen | 30 | −16 | 0 | 63 | 4.18 | |
| Right | Caudate | 20 | 24 | 8 | 223 | 3.99 | |
| Left | Putamen | −24 | 6 | 12 | 100 | 3.74 | |
| Left | Caudate | −18 | −4 | 20 | 49 | 3.73 | |
| Right | Hippocampus | 22 | −28 | −6 | 30 | 3.65 | |
| Incompatible Association Trials | |||||||
| Non Using Controls | Left | Frontal Inferior Gyrus Operculum | −58 | 12 | 2 | 165 | 5.70 |
| Right | Superior Frontal Gyrus | 30 | 24 | 50 | 2179 | 5.70 | |
| Left | Hippocampus | −28 | −30 | −4 | 95 | 4.85 | |
| Left | Middle Frontal Gyrus | −42 | 54 | 10 | 750 | 4.59 | |
| Left | Inferior Frontal Gyrus | −48 | 30 | 24 | 261 | 4.30 | |
| Right | Caudate | 18 | 10 | 22 | 252 | 4.02 | |
| Marijuana Users | |||||||
Note: All peak activity based on whole brain analyses. Results are for single group/condition results relative to fixation. Significance set at p<.005, uncorrected, voxels>30. Bolded regions represent predicted regions.
Findings for the 2 by 2 full-factor analyses were as follows: 1) no region showed a significant main effect for group or condition (ps>.005, uncorrected); and 2) a significant group by condition interaction was found in both subcortical and cortical hypothesized regions (all ps<.005, uncorrected). That is, the effect of group on BOLD response varied depending on the compatible and incompatible conditions, described below (see Table 3).
Table 3.
Significant Regions of Activity Associated with the Group by Condition Interaction
| Hemisphere | Region | X | Y | Z | Voxels in Cluster |
F statistic |
|---|---|---|---|---|---|---|
| Left | Caudate | −8 | 12 | 12 | 322 | 15.34 |
| Right | Caudate | 10 | 8 | 16 | 283 | 14.79 |
| Right | Putamen | 30 | −14 | 0 | 46 | 14.07 |
| Left | Putamen | −28 | 0 | 12 | 64 | 12.20 |
| Right | Inferior Frontal Cortex | 44 | 16 | 4 | 48 | 10.74 |
Note: All Fs, p<.005, uncorrected, voxels>30
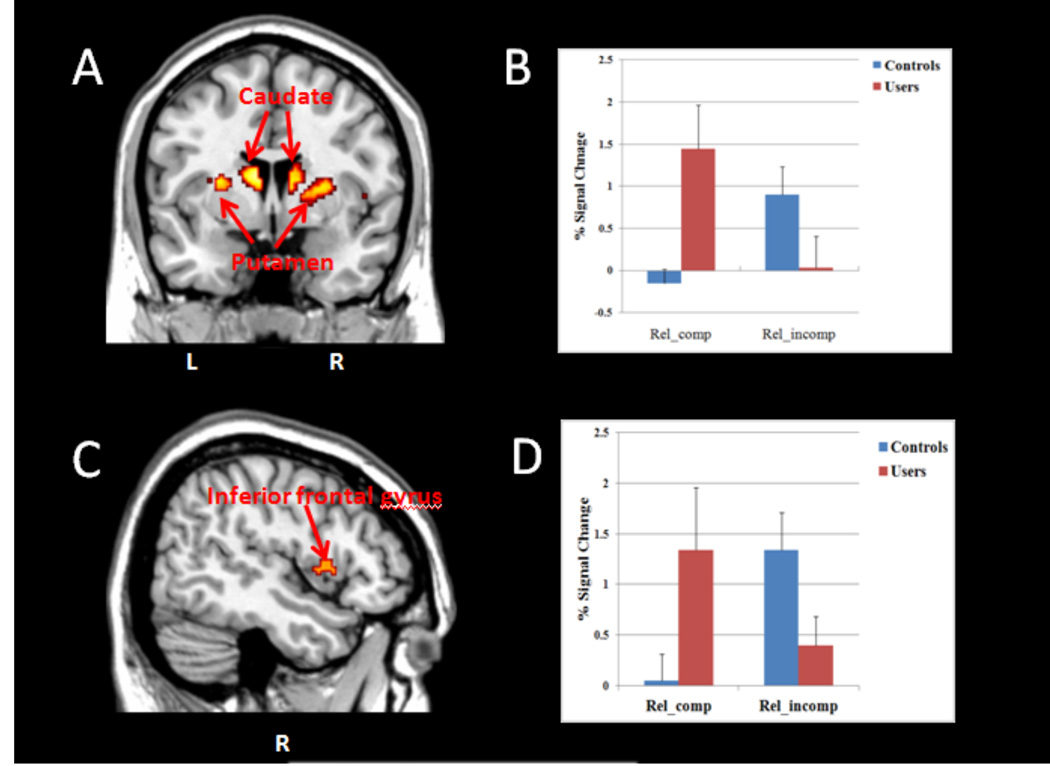
The marijuana users showed significantly greater bilateral activity in the caudate and putamen during performance on the compatible trials of the IAT relative to non-using controls (p<.005; see Figure 3).
Figure 3.
Interaction effect during compatible & incompatible trials by user and control groups in subcortical and cortical regions. A. Coronal view of activity in the caudate and putamen. B. Percent (%) signal change for both caudate and putamen. Error bars denote within-subject error. C. Sagittal view of the activity in the right inferior frontal gyrus. D. % signal change for the right inferior frontal gyrus. Error bars denote within-subject error. Significance set at p<.005, uncorrected, voxels>30.
In addition, the marijuana users showed greater activity in the right inferior frontal gyrus region relative to non-users during compatible association trials. Alternatively, during the incompatible trials, the non-using controls showed greater activity in the right inferior frontal gyrus region of the prefrontal cortex relative to the users.
4. Discussion
The present study extends behavioral research on implicit associative processes underlying habit by observing the neural correlates of marijuana-relevant memory associations during IAT performance. This is the first study to observe differences in hemodynamic response between marijuana users and non-using controls on a marijuana-specific IAT, providing preliminary evidence for the neural substrates recruited by the task. The imaging data showed an interaction between groups and task conditions. Findings revealed a difference among marijuana users and non-users in regions of the brain implicated in habit-based (associative) learning (see Robbins et al., 2008). Heavy marijuana users showed increased bilateral activity in the caudate and putamen, during compatible trials (trials of implicit associations for positive outcomes of marijuana use). This finding is consistent with our prediction that these trials would elicit greater activity in these regions among heavy users. This pattern of activation in heavy users is also consistent with recent findings in animal models regarding CB1 activity in the dorsal striatum and the role of endocannibinoids in affecting habit formation and habitual responding (see Hilario et al., 2007). Individuals with repetitive experiences with marijuana use should be able to perform trials comprised of concepts highly associated with marijuana fairly easily, requiring little need for engagement of more deliberative or control processes because they likely have stronger associations compared to a non-user who may not have these associations.
Additionally, some activity in the right inferior frontal gyrus (RIFG) was observed during compatible trials among the heavy marijuana users relative to the non-users. It is possible that this result was observed due to the need for inhibitory control over intensified striatal activity in the marijuana users. That is, the heavy marijuana users were working harder to inhibit habit-based (automatic or implicit) tendencies or suppressing well-learned associations requiring the engagement of frontal regions. The RIFG has been implicated in inhibitory control (Aron et al., 2003; Aron & Poldrack, 2004) and more recently has been implicated in a more general role of executive processing including detection of salient cues during task performance (Hampshire et al., 2010).
Further, although the ventral striatum (accumbens) has traditionally been implicated in motivation and reward and was predicted to show activity on compatible trials among our users, there was little activity observed during task performance. This finding is consistent with some theories that argue that what may originate as motivational (supported by the nucleus accumbens) in essence transfers control to the dorsal striatum, which supports habitual responding, following repetition of rewarding behavior, and without need for involvement of the accumbens (e.g., see Porrino et al., 2004; Robbins et al., 2008; cf. Yin & Knowlton, 2006). It is also possible that simply viewing stimuli related to marijuana was not in and of itself rewarding.
Finally, during the incompatible trials, which require more effortful processing of information since participants categorize concepts not generally related, the non-using group showed greater activity in some frontal regions (although not the specific regions predicted) relative to fixation. Alternatively, heavy marijuana users showed no significant activity in this study. This result only partially supported our prediction that these trials would elicit more neural activity in frontal regions among all participants, but less activity was expected among the heavy marijuana users perhaps as a result of chronic effects from use (e.g., poorer executive and controlled processing). This finding is similar to some other research that has shown diminished control ability and reduced frontal activity in populations with substance problems (e.g., see Dom et al., 2005; Everitt et al., 2008; Koob and Volkow, 2010).
In sum, the marijuana users showed greater activity in regions of the brain implicated in more habit-based associative learning during compatible trials relative to fixation and relative to non-users, while the non-using controls showed greater activity in regions of the brain requiring more deliberative/controlled processing during incompatible trials relative to fixation. These findings are consistent with practice-related changes that occur in neural systems and the transition from predominantly effortful or control-related systems to more automatic (or implicit) processing with experience (e.g., Chein and Schneider, 2005; Schneider and Chein, 2003).
Behaviorally, although we did not find a significant difference between the marijuana users and non-using control group on the D-600 measure of the IAT, we found significant effects for block type. The mean response latencies for the compatible trials were less than the mean for the incompatible trials across all participants, and there were fewer errors during compatible trials than incompatible trials. It is possible that we found no group effect because the stimuli presented are culturally common and limit the ability to behaviorally differentiate users from non-users when categorizing the presented stimuli. The categories and stimuli used are important determinants of behavioral IAT effects and should clearly represent the concepts being evaluated (see Nosek et al., 2007). Future drug-related imaging research with the IAT might benefit by using more personalized stimuli generated by the participants being studied to improve the utility of these tasks in assessing individual differences in automatic associations.
While the behavioral measures were not sensitive in detecting differences among groups, measurement of task-related neural response was sensitive in revealing group differences. Such findings provide support for the importance and unique advantage of using fMRI in detecting differences in functioning of specific brain regions that otherwise might not be detected by conventional behavioral measures. Neural comparisons were sensitive in detecting associative processes underlying habit, reflected by neural response to more or less effortful processing of concepts related to marijuana.
Responses to indirect assessments of drug-relevant associations, like the IAT, are likely rooted in associative learning, with associative strength being a key determinant of information processing expressed as accessibility or activation differences that influence behavior. By eliciting activation of associative marijuana-relevant memories through performance on this indirect test, we were able to increase our understanding of group differences in associative structures influenced by marijuana use and observe neural correlates of associative effects of a repeated behavior.
4.1. Limitations
Although functional imaging provides insight into neural activation of cognitive processes associated with tasks, findings are correlational and other variables may have an effect. fMRI has been criticized for lacking precision in localizing task activation areas and distributed substrates involved in information processing (Huettel et al., 2004). Additionally, it is not possible to rule out that differences in brain activity found may not be specific to the marijuana-related IAT. It is possible that we observed differences in neural response resulting from some executive functioning (e.g., working memory/attention) and/or motor deficits among marijuana dependent individuals as a result of residual effects from long-term chronic use. Further, it is not possible to rule out effects of recent use or being under the influence during the task despite asking participants to not use the day of the scans and obtaining verbal reports of non-use (e.g., see Chang and Chronicle, 2007; Eldreth et al., 2004; Iversen, 2003; Lundqvist, 2005).
Additionally, heavy marijuana using participants were recruited to compare to non-users; however, the level of use scale was not sensitive enough to differentiate heaviness of the users and therefore, we were unable to evaluate correlations between heaviness (no variance in the variable) and brain activity in regions of interest. Further, we did not have explicit measures of attitudes toward marijuana and were therefore unable to evaluate brain activity with respect to these measures. Future drug-related imaging studies of the IAT might want to incorporate more sensitive measures of use as well as key explicit measures.
Finally, the groups in the study were not equal with respect to gender. However, based on findings from our behavioral research, we did not expect gender differences on indirect tests of associations involving drugs (e.g., Ames et al., 2007; Ames et al., 2005). In sum, future studies might consider evaluating potential gender differences on this task.
5. Conclusions
The findings from this study contribute insight into the neural correlates of marijuana-related associative memory processes assessed with the IAT and are consistent with a theoretical framework of appetitive behaviors proposing that (1) implicit associations underlying habit are mediated through neural circuitry dependent on the striatum, and (2) deliberative/controlled behaviors are mediated through circuitry more dependent on the prefrontal cortex (see Bechara et al., 2006). This work is a step toward understanding the biology of implicit associative drug-related processes underlying habit. Understanding neural mediators of drug use such as associative memory processes can help in the development of prevention and treatment interventions.
Highlights.
Marijuana users showed activity in the caudate/putamen on compatible IAT trials.
Non-users showed activity in the inferior frontal gyrus on incompatible IAT trials.
Findings are consistent with a dual process framework of appetitive behaviors.
Implicit associations underlying habit are mediated through the striatum.
Reflective/controlled behaviors are mediated through the prefrontal cortex.
Acknowledgments
This research was supported by grants from the National Institute on Drug Abuse (DA024772, DA023368, DA024659) and the National Institute on Alcohol Abuse and Alcoholism (AA017996) and the National Cancer Institute (CA152062). We thank Marcia McGuire for her support on this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure/Conflict of Interest Statement
The authors declare that no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional services and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest.
References
- Adinoff B. Neurobiologic processes in drug reward and addiction. Harvard Review of Psychiatry. 2004;12:305–320. doi: 10.1080/10673220490910844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames SL, Franken IHA, Coronges K. Implicit cognition and drugs of abuse. In: Wiers RW, Stacy AW, editors. Handbook of Implicit Cognition and Addiction. Thousand Oaks, CA, US: SAGE Publications; 2006. pp. 363–378. [Google Scholar]
- Ames SL, Grenard JL, Thush C, Sussman S, Wiers RW, Stacy AW. Comparison of indirect assessments of association as predictors of marijuana use among at-risk adolescents. Experimental and Clinical Psychopharmacology. 2007;15:204–218. doi: 10.1037/1064-1297.15.2.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames SL, Sussman S, Dent CW, Stacy AW. Implicit cognition and dissociative experiences as predictors of adolescent substance use. American Journal of Drug & Alcohol Abuse. 2005;31:129–162. [PubMed] [Google Scholar]
- Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature Neuroscience. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Sciences. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Baler RD, Volkow ND. Drug addiction: The neurobiology of disrupted self-control. Trends in Molecular Medicine. 2006;12:559–566. doi: 10.1016/j.molmed.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision making, impulse control and loss of willpower to resist drugs: a neurocognitive perspective. Nature Neuroscience. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (Part I): Impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (Part II): Myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Bechara A, Noel X, Crone EA. Loss of willpower: Abnormal neural mechanisms of impulse control and decision making in addiction. In: Wiers RW, Stacy AW, editors. Handbook of Implicit Cognition and Addiction. Thousand Oaks: Sage Publications, Inc; 2006. pp. 215–233. [Google Scholar]
- Beer JS, Stallen M, Lombardo MW, Gonsalkorale K, Cunningham WA, Sherman JW. The quadruple process model approach to examining the neural underpinnings of prejudice. Neuroimage. 2008;43:775–783. doi: 10.1016/j.neuroimage.2008.08.033. [DOI] [PubMed] [Google Scholar]
- Corbille AG, Valjent E, Marsicano G, Ledent C, Lutz B, Herve D, Girault JA. Role of cannabinoid type 1 receptors in locomotor activity and striatal signaling in response to psychostimulants. The Journal of Neuroscience. 2007;27:6937–6947. doi: 10.1523/JNEUROSCI.3936-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit LH, Muir JL, Balleine BW. Lesions of mediodorsal thalamus and anterior thalamic nuclei produce dissociable effects on instrumental conditioning in rats. European Journal of Neuroscience. 2003;18:1286–1294. doi: 10.1046/j.1460-9568.2003.02833.x. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Everitt BJ. Neural and psychological mechanisms underlying appetitive learning: Links to drug addiction. Current Opinion in Neurobiology. 2004;14:156–162. doi: 10.1016/j.conb.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Chang L, Chronical EP. Functional imaging studies in cannabis users. The Neurosocientist. 2007;13:422–432. doi: 10.1177/1073858406296601. [DOI] [PubMed] [Google Scholar]
- Chee MWL, Sriram N, Soon CS, Lee KM. Dorsolateral prefrontal cortex and the implicit association of concepts and attributes. NeuroReport. 2000;11:135–140. doi: 10.1097/00001756-200001170-00027. [DOI] [PubMed] [Google Scholar]
- Chein JM, Schneider W. Neuroimaging studies of practice-related change: fMRI and meta analytic evidence of a domain-general control network for learning. Cognitive Brain Research. 2005;25:607–623. doi: 10.1016/j.cogbrainres.2005.08.013. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computational Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8:109–114. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Houwer J, Crombez G, Koster EHW, De Beul N. Implicit alcohol-related cognitions in a clinical sample of heavy drinkers. Journal of Behavior Therapy & Experimental Psychiatry. 2004;35:275–286. doi: 10.1016/j.jbtep.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Dom G, Sabbe B, Hulstijn W, van den Brink W. Substance use disorders and the orbitofrontal cortex: Systematic review of behavioural decision-making and neuroimaging studies. British Journal of Psychiatry. 2005;187:209–220. doi: 10.1192/bjp.187.3.209. [DOI] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, et al. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. NeuroImage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Engle RW, Tuholski SW, Laughlin JE, Conway ARA. Working memory, short- term memory and general fluid intelligence: A latent variable approach. Journal of Experimental Psychology: General. 1999;128:309–331. doi: 10.1037//0096-3445.128.3.309. [DOI] [PubMed] [Google Scholar]
- Engle RW. Working memory capacity as executive attention. Current Directions in Psychological Science. 2002:19–23. [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical Transactions of the Royal Society B: Biological Sciences. 2008;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Dickinson A, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Research. 2001;36:129–138. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: From actions to habits to compulsion. Nature Neuroscience. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Field M, Mogg K, Bradley BP. Cognitive bias and drug craving in recreational cannabis users. Drug & Alcohol Dependence. 2004;74:105–111. doi: 10.1016/j.drugalcdep.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JB, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Friston KJ, Josephs O, Zarahn E, Holmes AP, Rouquette S, Poline JB. To smooth or not to smooth?: Bias and efficiency in fMRI time-series analysis. NeuroImage. 2000;12:196–208. doi: 10.1006/nimg.2000.0609. [DOI] [PubMed] [Google Scholar]
- Giuffrida A, Parsons LH, Kerr TM, Rodriguez de Fonseca F, Navarro M, Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nature Neuroscience. 1999;2:358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, McGhee DE, Schwartz JLK. Measuring individual differences in implicit cognition: The implicit association test. Journal of Personality & Social Psychology. 1998;74:1464–1480. doi: 10.1037//0022-3514.74.6.1464. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Nosek BA, Banaji MR. Understanding and using the Implicit Association Test: I. An improved scoring algorithm. Journal of Personality & Social Psychology. 2003;85:197–216. doi: 10.1037/0022-3514.85.2.197. [DOI] [PubMed] [Google Scholar]
- Greenwald AG, Poehlman TA, Uhlmann EL, Banaji MR. Understanding and using the Implicit Association Test: III. Meta-analysis of predictive validity. Journal of Personality and Social Psychology. 2009;97:17–41. doi: 10.1037/a0015575. [DOI] [PubMed] [Google Scholar]
- Hampshire A, Chamberlain SR, Monti MM, Duncan J, Owen AM. The role of the right inferior frontal gyrus: inhibition ad attentional control. Neuroimage. 2010;50:1313–1319. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilario MRF, Clouse E, Yin HH, Costa RM. Endocannabinoid signaling is critical for habit formation. Frontiers in Integrative Neuroscience. 2007;1:1–12. doi: 10.3389/neuro.07.006.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann W, Gawronski B, Gschwendner T, Le H, Schmitt M. A meta-analysis on the correlation between the Implicit Association Test and explicit self-report measures. Personality and Social Psychological Bulletin. 2005;31:1369–1385. doi: 10.1177/0146167205275613. [DOI] [PubMed] [Google Scholar]
- Hopfield JJ, Tank DW. Computing with neural circuits: A model. Science. 1986;233:625–633. doi: 10.1126/science.3755256. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW. Assessing implicit alcohol associations with the Implicit Association Test: Fact or artifact? Addictive Behaviors. 2006:1346–1362. doi: 10.1016/j.addbeh.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW. Are drinkers implicitly positive about drinking alcohol? Personalizing the Alcohol-IAT to reduce negative extrapersonal contamination. Alcohol and Alcoholism. 2007;42:301–307. doi: 10.1093/alcalc/agm015. [DOI] [PubMed] [Google Scholar]
- Houben K, Wiers RW. Implicitly positive about alcohol? Implicit positive associations predict drinking behavior. Addictive Behaviors. 2008;33:979–986. doi: 10.1016/j.addbeh.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. Sunderland, Massachusetts: Sinauer Associates; 2004. [Google Scholar]
- Iversen L. Cannabis and the brain. Brain. 2003;126:1252–1270. doi: 10.1093/brain/awg143. [DOI] [PubMed] [Google Scholar]
- Jajodia A, Earleywine M. Measuring alcohol expectancies with the Implicit Association Test. Psychology of Addictive Behaviors. 2003;17:126–133. doi: 10.1037/0893-164x.17.2.126. [DOI] [PubMed] [Google Scholar]
- Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: An individual-differences perspective. Psychonomic Bulletin Review. 2002;9:637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- Knowlton BJ, Mangels JA, Squire LR. A neostriatal habit learning system in humans. Science. 1996;273:1399–1402. doi: 10.1126/science.273.5280.1399. [DOI] [PubMed] [Google Scholar]
- Knutson KM, Wood JN, Spampinato MV, Grafman J. Politics on the brain: An fMRI investigation. Social. Neuroscience. 2006;1:25–40. doi: 10.1080/17470910600670603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson KM, Mah L, Manly CF, Grafman J. Neural correlates of automatic beliefs about gender and race. Human Brain Mapping. 2007;28:915–930. doi: 10.1002/hbm.20320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neurobiology of drug addiction. In: Kandel DB, editor. Stages and pathways of drug involvement: Examining the gateway hypothesis. New York, NY, US: Cambridge University Press; 2002. pp. 337–361. [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of Addiction. Neuropsychopharmacology Reviews. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindgren KP, Neighbors C, Teachman BA, Wiers RW, Westgate E, Greenwald AG. Drink Therefore I am: Validating Alcohol-Related Implicit Association Tests. Psychology of Addictive Behaviors. 2012 doi: 10.1037/a0027640. Advance online publication. doi: 10.1037/a0027640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo Q, Nakic M, Wheatley T, Richell R, Martin A, Blair RJ. The neural basis of implicit moral attitude--an IAT study using event-related fMRI. NeuroImage. 2006;30:1449–1457. doi: 10.1016/j.neuroimage.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Cognitive consequences of cannabis use: Comparison with abuse of stimulants and heroin with regard to attention, memory and executive functions. Pharmacololgy Biochemistry and Behavior. 2005;81:319–330. doi: 10.1016/j.pbb.2005.02.017. [DOI] [PubMed] [Google Scholar]
- McCarthy DM, Thompsen DM. Implicit and explicit measures of alcohol and smoking cognitions. Psychology of Addictive Behavior. 2006;20:436–444. doi: 10.1037/0893-164X.20.4.436. [DOI] [PubMed] [Google Scholar]
- Nosek BA, Greenwald AG, Banaji MR. The implicit association test at age 7: A methodological and conceptual review. In: Bargh JA, editor. Automatic Processes in Social Thinking and Behavior. Psychology Press; 2007. [Google Scholar]
- Nosek BA, Greenwald AG, Banaji MR. Understanding and using the Implicit Association Test: II. Method variables and construct validity. Personality and Social Psychological Bulletin. 2005;31:166–180. doi: 10.1177/0146167204271418. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. The Journal of Neuroscience. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queller S, Smith ER. Subtyping versus bookkeeping in stereotype learning and change: Connectionist simulations and empirical findings. Journal of Personality & Social Psychology. 2002;82:300–313. [PubMed] [Google Scholar]
- Robbins TW, Ersche KD, Everitt BJ. Drug addiction and the memory systems of the brain. Annals of the New York Academy of Sciences. 2008;1141:1–21. doi: 10.1196/annals.1441.020. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. Mechanisms of action of addictive stimuli. Incentive-sensitization and addiction. Addiction. 2001;96:103–114. doi: 10.1046/j.1360-0443.2001.9611038.x. [DOI] [PubMed] [Google Scholar]
- Rooke SE, Hine DW, Thorsteinsson EB. Implicit cognition and substance use: A meta-analysis. Addictive Behaviors. 2008;33:1314–1328. doi: 10.1016/j.addbeh.2008.06.009. [DOI] [PubMed] [Google Scholar]
- SAS Institute Inc. SAS® 9.3. Cary, NC: SAS Institute Inc; 2011. [Google Scholar]
- Schneider W, Chein JM. Controlled & automatic processing: Behavior, theory, and biological mechanisms. Cognitive Science: A Multidisciplinary Journal. 2003;27:525–559. [Google Scholar]
- Squire LR. Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychological Review. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stacy AW. Memory activation and expectancy as prospective predictors of alcohol and marijuana use. Journal of Abnormal Psychology. 1997;106:61–73. doi: 10.1037//0021-843x.106.1.61. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Ames SL, Knowlton B. Neurologically plausible distinctions in cognition relevant to drug abuse etiology and prevention. Substance Use and Misuse. 2004;39:1571–1623. doi: 10.1081/ja-200033204. [DOI] [PubMed] [Google Scholar]
- Stacy AW, Wiers RW. Implicit cognition and addiction: A tool for explaining paradoxical behavior. Annual Review of Clinical Psychology. 2010;6:551–575. doi: 10.1146/annurev.clinpsy.121208.131444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JE, Rudman LA, Greenwald AG. Using the Implicit Association Test to investigate attitude-behaviour consistency for stigmatised behaviour. Cognition & Emotion. 2001;15:207–230. [Google Scholar]
- Thush C, Wiers RW, Ames SL, Grenard JL, Sussman S, Stacy AW. Apples and oranges? Comparing implicit measures of alcohol-related cognition predicting alcohol use in at-risk adolescents. Psychology of Addictive Behaviors. 2008:587–591. doi: 10.1037/0893-164X.21.4.587. [DOI] [PubMed] [Google Scholar]
- Unsworth N, Heitz RP, Schrock JC, Engle RW. An automated version of the operation span task. Behavior Research Methods Instrumentation and Computers. 2005;37:498–505. doi: 10.3758/bf03192720. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive: Involvement of the orbitofrontal cortex. Cerebral Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Wagner AD, Maril A, Schacter DL. Interactions between forms of memory: When priming hinders new episodic learning. Journal of Cognitive Neuroscience. 2000;12:S52–S60. doi: 10.1162/089892900564064. [DOI] [PubMed] [Google Scholar]
- Wang J, Wang Z, Aguirre GK, Detre JA. To smooth or not to smooth? ROC analysis of perfusion fMRI data. Magnetic Resonance Imaging. 2005;23:75–81. doi: 10.1016/j.mri.2004.11.009. [DOI] [PubMed] [Google Scholar]
- White NM. Addictive drugs as reinforcers: Multiple partial actions on memory systems. Addiction. 1996;91:921–949. [PubMed] [Google Scholar]
- Wiers RW, Bartholow BD, van den Wildenberg E, Thush C, Engels R, Sher K, Grenard J, Ames S, Stacy AW. Automatic and controlled processes and the development of addictive behavior in adolescents: A review and a model. Pharmacology, Biochemistry and Behavior. 2007;86:263–83. doi: 10.1016/j.pbb.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Houben K, de Kraker J. Implicit cocaine associations in active cocaine users and controls. Addictive Behaviors. 2007;32:1284–1289. doi: 10.1016/j.addbeh.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Van de Luitgaarden J, Van den Wildenberg E, Smulders FTY. Challenging implicit and explicit alcohol-related cognitions in young heavy drinkers. Addiction. 2005;100:806–819. doi: 10.1111/j.1360-0443.2005.01064.x. [DOI] [PubMed] [Google Scholar]
- Wiers RW, van Woerden N, Smulders FTY, de Jong PJ. Implicit and explicit alcohol-related cognitions in heavy and light drinkers. Journal of Abnormal Psychology. 2002;111:648–658. doi: 10.1037/0021-843X.111.4.648. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Stacy AW. Handbook of Implicit Cognition and Addiction. Thousand Oaks, CA, US: SAGE Publications; 2006. [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ, Balleine BW. The role of the dorsomedial striatum in instrumental conditioning. European Journal of Neuroscience. 2005;22:513–523. doi: 10.1111/j.1460-9568.2005.04218.x. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]