Abstract
This study evaluated the effects of bisphenol A (BPA) on human endometrial stromal fibroblast (ESF) differentiation and expression of genes involved in oestrogen metabolism. Human ESF from eight hysterectomy specimens were cultured and treated with 5–100 µmol/l of BPA ± oestradiol or 8-br-cAMP for 48 h. mRNA expression was analysed by real-time reverse-transcription PCR. 8-br-cAMP-induced human ESF decidualization was confirmed by expression of insulin-like growth factor binding protein-1 (IGFBP1) and prolactin secretion. Short-term exposure (48 h) decreased human ESF proliferation (P < 0.04) not due to apoptosis. High doses of BPA significantly induced IGFBP1 mRNA and protein, decreased P450scc mRNA, reversed the 8-br-cAMP-induced increase in HSD17B2 (oestradiol to oestrone conversion) in a dose-dependent manner and down-regulated HSD17B1 expression (oestrone to oestradiol conversion; P ≤ 0.03). 8-br-cAMP significantly potentiated this effect (P = 0.028). BPA had no significant effect on aromatase and PPAR γ expression. The oestrogen-receptor antagonist ICI had no effect on gene expression in BPA-treated cells, and oestrogen receptor α, but not oestrogen receptor β, was significantly down-regulated by high doses of BPA (P = 0.028). BPA has an endocrine-disrupting effect on human ESF function and gene expression but the underlying mechanisms appear not to involve oestrogen-mediated pathways.
Keywords: bisphenol A, decidualization, endometrial stromal cells, eutopic endometrium
Introduction
There are increasing data to support adverse effects on human reproductive health by environmental contaminants and especially endocrine-disrupting chemicals (Diamanti-Kandarakis et al., 2009; Giudice, 2006). Bisphenol A (BPA) is an industrial chemical, a xenobiotic oestrogen and a known endocrine-disrupting chemical. It is widely distributed and is found in water pipes, polycarbonate plastics, metal cans, plastic bottles and baby toys, computers, cell phones and dental sealants (NTP, 2008; vom Saal and Hughes, 2005). Recent data show that nearly every person in the USA is exposed to BPA (Calafat et al., 2005, 2008; Lang et al., 2008; vom Saal and Hughes, 2005) and that it can be passed transplacentally (Ikezuki et al., 2002; Schonfelder et al., 2002). Serum concentrations of BPA in the general population have been reported to be 0.2–20 ng/ml (0.1–10 µmol/l), with the highest concentrations (100 ng/g) found in the placenta (Schonfelder et al., 2002). Thus, there is nearly ubiquitous exposure to this xenoestrogen that has the potential for action on oestrogen-responsive tissues, including the endometrium.
BPA can exert some of its effects by binding to the nuclear steroid oestrogen receptors α and β, acting as an agonist as well as a selective oestrogen-receptor modulator (Chapin et al., 2008; Lee et al., 2007; Welshons et al., 2006). BPA selectively binds to oestrogen receptors α and β and has a higher affinity for oestrogen receptor β in select target cells (Han et al., 2002; Lapensee et al., 2009). BPA may also affect oestrogen receptor β-mediated transcription of target genes by differential degradation of nuclear receptors (Masuyama and Hiramatsu, 2004). It has also been suggested that endocrine-disrupting chemicals may act with endogenous steroid hormones to induce additive effects in target tissues (Buterin et al., 2006).
Several groups have reported the effects of BPA on cells and tissues of the reproductive tract in different species, resulting in recurrent miscarriage, decreased semen quality, altered gonadotrophin hormone secretion, early pubertal onset and altered ovarian granulosa steroidogenesis (Kwintkiewicz and Giudice, 2009; Kwintkiewicz et al., 2010; Oehlmann et al., 2009; Talsness et al., 2009). Adverse reproductive consequences of low-dose BPA exposure have also been reported on oocyte chromosomal constitution (Hunt et al., 2003; Richter et al., 2007; vom Saal and Hughes, 2005). The study centre has recently shown that BPA inhibits FSH-stimulated insulin-like growth factor-I, aromatase and oestradiol synthesis in human primary and immortalized granulosa cells (Kwintkiewicz et al., 2010). While the BPA effect on human spermatozoa in in-vitro studies was less evident (Luconi et al., 2001), male offspring of rats exposed perinatally to BPA had decreased sperm count and motility, resulting in reduced fertility (Salian et al., 2009). Exposure of mice to low BPA doses (0.5–5.0 mg/kg) increased HOXA10 protein expression in the uterus, indicating that HOX genes are common targets for this xenobiotic oestrogen (Smith and Taylor, 2007). A preliminary communication reported that urinary BPA concentrations are inversely associated with the number of retrieved oocytes and peak oestradiol concentrations in women undergoing IVF and embryo transfer (Mok-Lin et al., 2010) and higher serum BPA concentrations have been found in infertile women who did not become pregnant after IVF treatment (Lamb et al., 2008). The latter may be due to a detrimental effect of BPA on ovarian function (decreased production of oestrogen by granulosa cells; Kwintkiewicz et al., 2010) or it may reflect a direct effect of BPA compromising endometrial development and preparation for blastocyst nidation. Since the human endometrial stromal fibroblast (ESF) plays a major role in endometrial receptivity, embryonic implantation and sustained pregnancy, the current study has evaluated the effects of BPA on the expression of several genes involved in oestrogen metabolism, as well as on human ESF differentiation.
Materials and methods
Subjects
Endometrial biopsies were obtained from eight subjects undergoing hysterectomy for benign conditions such as fibroids and pelvic organ prolapse (Table 1). None had endometriosis or adenomyosis by direct visualization at surgery or on pathology reports of hysterectomy specimens. The participating subjects were 36–49 years old (mean 43.6 ± 1.85), not pregnant and had not been on any hormonal medication within 3 months before surgery. The study was approved by the UCSF Committee on Human Research. Written informed consent was obtained from all participating subjects. Samples were also obtained through the UCSF NIH Human Endometrial Tissue and DNA Bank with appropriate institutional review, approvals and written informed consent from all participating subjects.
Table 1.
Characteristics of patients who donated endometrial biopsy samples for the study and investigations on human endometrial stromal fibroblasts.
| Patient | Cycle phase | Diagnosis | Age (years) | Ethnicity | Experimental protocol |
|---|---|---|---|---|---|
| 229 | LSE | Pelvic pain | 47 | Caucasian | BrdU |
| 236 | PE | Symptomatic pelvic prolapse | 47 | Caucasian | BrdU |
| 237 | ESE | Intramural myoma, left paratubal cyst | 39 | Caucasian | Cell cultures |
| 275 | PE | Intramural myoma | 36 | Black | Cell cultures |
| 293 | PE | Intramural myoma | 49 | Caucasian | Cell cultures, BrdU, TUNEL |
| 310 | ESE | Intramural myoma | 44 | Caucasian | Cell cultures |
| 316 | ESE | Intramural myoma | 38 | Caucasian | Cell cultures |
| 326 | PE | Intramural myoma | 49 | Asian | Cell cultures |
None of the patients had endometriosis.
BrdU = bromodeoxyuridine incorporation assay; ESE = early secretory endometrium; LSE = late secretory endometrium; PE = proliferative endometrium; TUNEL = terminal deoxynucleotidyl transferase dUTP nick end labelling apoptosis assay.
Isolation and culture of human ESF
Human ESF were isolated from eight hysterectomy specimens, as described elsewhere (Aghajanova et al., 2009, 2010). Cells were cultured and propagated in DMEM/MCDB-105 medium containing 10% charcoal-stripped fetal bovine serum (FBS), insulin (5 µg/ml), gentamicin, penicillin and streptomycin (all reagents from Sigma–Aldrich, St. Louis, USA, unless stated otherwise). At passage 2, cells were plated in 60-mm plates according to the study design (see below), and after cells became near confluent, media were changed to low-serum medium (DMEM/MCDB-105 medium containing ascorbic acid, transferrin and gentamicin with 2% charcoal-stripped FBS) and cultured for 24 h prior to the onset of treatment. Human ESF in duplicate were treated with 5, 25, 50 and 100 µmol/l of BPA with or without 10 nmol/l oestradiol or 0.5 mmol/l 8-br-cAMP or 1 µmol/l oestrogen-receptor antagonist (ICI) for 48 h, with appropriate controls. In order to investigate the potential effect of BPA on the decidualization process, treatment of human ESF with cAMP was started 48 h prior to BPA treatment. BPA doses were selected to cover the ‘physiological’ serum concentrations detected in the general population (0.1–10 µmol/l; Schonfelder et al., 2002) and concentrations above those. Conditioned medium and cell lysates in RLT lysis buffer containing β-mercaptoethanol (Qiagen, Valencia, USA) were collected for protein concentration and mRNA expression analyses by enzyme-linked immunosorbent assay (ELISA) and real-time reverse-transcription (RT-PCR), respectively.
ELISA
Conditioned media from cultured human ESF were subjected to ELISA to quantify the decidualization markers, insulin-line growth factor binding protein-1 (IGFBP1) and prolactin (PRL), according to the manufacturer’s instructions (Alpha Diagnostic, San Antonio, USA and Diagnostic 250 Systems Labs, Webster, USA, respectively). All samples were assayed in duplicate and a standard curve was run in each experiment. Concentrations of IGFBP1 and PRL for each sample were normalized to total RNA. Inter- and intra-assay coefficients of variation for the IGFBP1 ELISA were 5.0–7.4% and 2.4–3.4%, respectively, and for PRL were 6.7–10.4% and 7.8–8.2%, respectively.
RNA isolation and real-time RT-PCR
Total RNA was purified using Qiagen RNeasy Plus Mini kit (Qiagen) according to the manufacturer’s instructions. Samples were stored in RNase-free H2O and quantified by spectroscopy. The purity was analysed by the 260/280 absorbance ratio and RNA integrity was assessed using an Agilent Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA) with RNA integrity number ranging from 9.7–10. For quantitative RT-PCR analysis, 1 µg of RNA was converted to cDNA using the iScript cDNA Synthesis kit (Bio-Rad Laboratories, Hercules, USA). The real-time RT-PCR reaction was carried out for 40 cycles with primers listed in Table 2.
Table 2.
Primer sequences.
| Gene | Primer sequence (5′ to 3′) | |
|---|---|---|
| Sense | Antisense | |
| PRL | CATCAACAGCTGCCACACTT | CGTTTGGTTTGCTCCTCAAT |
| IGFBP1 | CTATGATGGCTCGAAGGCTC | TTCTTGTTGCAGTTTGGCAG |
| Oestreogen receptor α | TGGAGATCTTCGACATGCTG | GATGTGGGAGAGGATGAGGA |
| Oestreogen receptor β | GATGAGGGGAAATGCGTAGA | ATCACCCAAACCAAAGCATC |
| P450scc | TCACCCCATCTCCGTGAC | GGCTTTTCAGGCATCAGAAT |
| Cyp19A1 | TGCATGGCAAGCTCCTCA | TTTGCGCATGACCAAGTCCA |
| HSD17B1 | CTGGACGTAAGGGACTCAAAA | CTTCTCCATGAAGGCGGTGT |
| HSD17B2 | GAAAGGCTGGCATCTTATGG | TCCAGCTTTTCCCACTTGTC |
| PPAR γ | GAGGGCCAAGGCTTCATGA | AGGCTTTCGCAGGCTCTTTAG |
| PRA | CCTCGGACACCTTGCCTGAA | CGCCAACAGAGTGTCCAAGAC |
| PRAB | TAGTGAGGGGGCAGTGGAAC | AGGAGGGGGTTTCGGGAATA |
| RPL19 | GCAGATAATGGGAGGAGCC | GCCCATCTTTGATGAGCTTC |
Bromodeoxyuridine incorporation assay
In order to assess if BPA exposure affects the proliferative capacity of human ESF, cells cultured with and without BPA for 48 h were subjected to a BrdU assay (Roche Diagnostics, Mannheim, Germany). Human ESF from three subjects (Table 1) were plated in triplicate at 5 × 104 cells/well in 96-well plates and serum starved for 24 h before being incubated with or without BPA for 48 h and, in the last 18 h, incubated with bromodeoxyuridine (BrdU). Cell proliferation was measured by colourimetric absorbance of BrdU, according to the manufacturer’s instructions, using a 96-well plate reader at 450 nm wavelength (Bio-Rad Laboratories).
TUNEL apoptosis assay
For this assay, human ESF from three subjects (Table 1) were cultured in duplicate on eight-well chamber slides and treated with or without different BPA concentrations for 48 h. Thereafter media were removed and cells were washed with phosphate-buffered saline (PBS). Cells were fixed with 3% paraformaldehyde for 20 min at room temperature, washed with PBS, permeabilized with methanol for 20 min at room temperature and subsequently washed with PBS. Slides were incubated with TdT (terminal deoxynucleotidyl transferase)-mediated dUDP nick-end labelling (TUNEL) solution (TdT and digoxigenin-dUTP; Roche Diagnostics) in a humidified chamber at 37°C for 1 h. Positive control cells were incubated with 6 µmol/l camptothecin for 12 h before fixation. Duplicate negative controls were run in each set of experiments. Colour development for localization of cells containing labelled DNA strand breaks was performed by incubating the chamber slides with Fast Red substrate solution for 10 min. After rinsing with PBS, slides were mounted with 4,6-diamidino-2-phenylindole (nuclear staining) and analysed under a fluorescent microscope (Leica Microsystems, Wetzlar, Germany). TUNEL staining was quantitated by counting the number of positively stained cells (green colour) per 100 cells within three different high-power (×20) fields for each subject and each treatment group. Values represent the mean percentage of positively stained cells ± SEM.
Statistical evaluation
Statistical analysis for the ELISA, BrdU proliferation assay and TUNEL assay data was performed using a two-tailed type-3 Student’s t-test and for the quantitative RT-PCR the non-parametric Mann Whitney U-test was used. Significance was determined at P ≤ 0.05.
Results
Bisphenol A decreases proliferation in human ESF with no effect on apoptosis
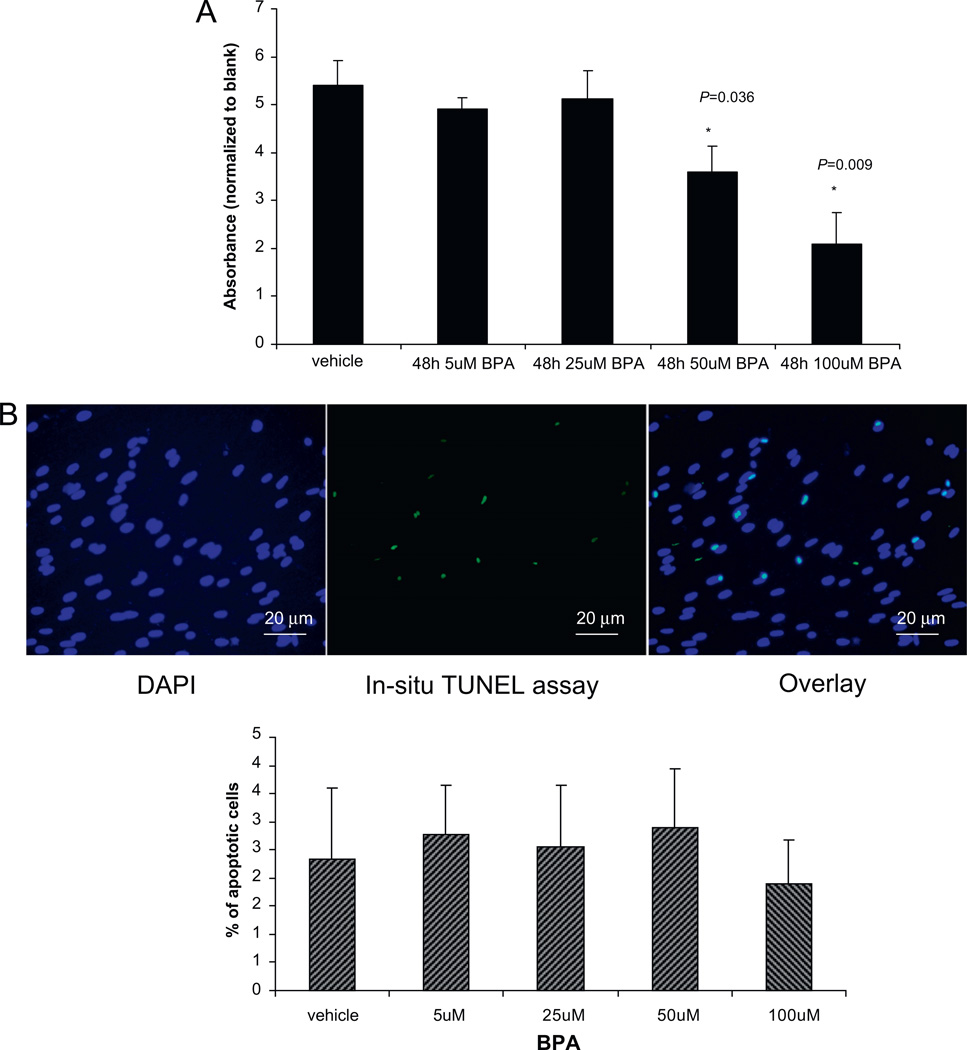
To assess if human ESF proliferation potential and apoptosis are affected by exposure to BPA, this study performed BrdU incorporation and TUNEL apoptosis assays (Figure 1A and B). The data demonstrated that BPA treatment significantly decreased proliferation of human ESF at higher doses (50 and 100 µmol/l; P = 0.036 and P = 0.009, respectively; Figure 1A). In order to assess if the observed changes in proliferation were associated with cell death (apoptosis), the TUNEL assay was performed in human ESF treated with or without BPA for 48 h (Figure 1B), with positive and negative controls. Interestingly, there was no difference in human ESF cell death upon treatment with any BPA concentration used.
Figure 1.
(A) Endometrial stromal fibroblasts (n = 3, in triplicate) treated with 5–100 µmol/l bisphenol A (BPA) for 48 h and cell proliferation analysed by BrdU incorporation. Asterisks indicate significant differences at P < 0.05. (B) Endometrial stromal fibroblasts on tissue chamber slides incubated with 5–100 µmol/l BPA for 48 h and analysed by TdT (terminal deoxynucleotidyl transferase)-mediated dUDP nick-end labelling (TUNEL) assay for apoptosis. Blue-stained nuclei/DNA reflect the total number of cells. The number of positively stained cells (green colour) per 100 blue cells was counted within three different high-power magnification (×40) fields for each subject and each treatment group. Values represent the mean percentage of positively stained cells ± SEM. No significant differences were observed. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Bisphenol A effect on decidualization of human ESF and expression of steroidogenic enzyme and oestrogen- and progesterone-receptor mRNA
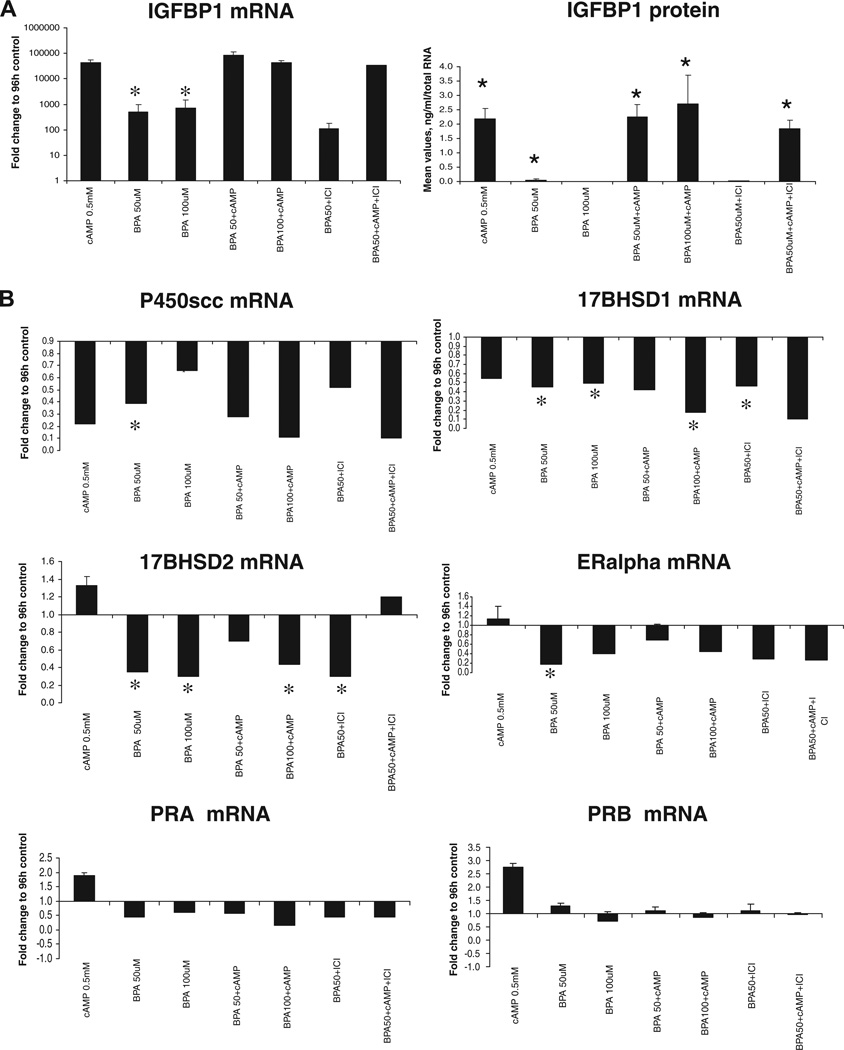
BPA at low doses (5 and 25 µmol/l) had no effect on the expression of oestrogen receptors α and β, P450scc, CYP19A1, HSD17B1, HSD17B2, PPAR γ, IGFBP1, PRL, PRA and PRAB (data not shown). However, BPA significantly induced IGFBP1 mRNA and protein expression at a concentration of 50 µmol/l (P = 0.03 and P = 0.028, respectively); however, it was not able to potentiate the effect of cAMP on IGFBP1 expression (Figure 2A). BPA alone had no effect on PRL mRNA and protein expression (data not shown). High doses of BPA significantly decreased mRNA expression for P450scc (Cyp11A, the steroidogenic enzyme that converts cholesterol to pregnenolone and initiates the cascade; P = 0.02; Figure 2B). BPA reversed the cAMP-induced increase in 17β-hydroxysteroid dehydrogenase type 2 (HSD17B2, converting oestradiol to oestrone) and decreased HSD17B2 mRNA expression in a dose-dependent manner at high concentrations (50 and 100 µmol/l). These high doses of BPA also down-regulated 17β-hydroxysteroid dehydrogenase type 1 (HSD17B1) expression (oestrone to oestradiol conversion) to the same extent as cAMP and even potentiated its effect (P = 0.028; Figure 2B). Surprisingly, BPA had no significant effect on aromatase or PPAR γ mRNA expression (data not shown), in contrast to recent data on human granulosa cells (Kwintkiewicz et al. 2010).
Figure 2.
(A) Insulin-like growth factor binding protein-1 (IGFBP1) mRNA expression and protein secretion in conditioned medium from human endometrial stromal fibroblasts treated with 0.5 mmol/l cAMP, 50 and 100 µmol/l bisphenol A (BPA) and 1 µmol/l oestrogen receptor antagonist (ICI) for 48 h (n = 6). Asterisks indicate significant differences at P ≤ 0.05. Error bars represent SEM. (B) Real-time reverse-transcription PCR analysis of P450scc, HSD17B1, HSD17B2, oestrogen receptor α, PRA and PRB mRNA in human endometrial stromal fibroblasts treated with 0.5 mmol/l cAMP, 50 and 100 µmol/l BPA and 1 µmol/l ICI for 48 h (n = 6). Asterisks indicate significant differences at P ≤ 0.05 (Mann Whitney U-test). Error bars represent SEM.
The present study did not observe effects of oestradiol on BPA action, when cells were treated with both agents (data not shown). The oestrogen-receptor antagonist ICI had no effect on gene expression in BPA-treated cells (Figure 2), suggesting that the BPA effect on the investigated genes in human ESF is not mediated by an oestrogen receptor. However, oestrogen receptor α was significantly down-regulated by BPA at 50 µmol/l (P = 0.028; Figure 2B). Of note, there was no significant effect of BPA on oestrogen receptor β or on progesterone receptors PRA and PRAB expression (Figure 2B).
Discussion
Herein, as far as is known, this study demonstrates for the first time the effect of bisphenol A on human ESF in vitro. It shows that even short-term exposure (48 h) of endometrial stromal cells to BPA significantly decreases cell proliferation and this is not due to increased cell death (no toxic effect of BPA at the concentrations studied). Indeed, its promotion of the decidual phenotype suggests that it acts more like a progestin.
Steroidogenic pathway
This study shows that BPA effects on the steroidogenic pathway in endometrial stromal cells (decreased P450scc) could lead to decreased conversion of cholesterol in the cells towards its first metabolite, pregnenolone, which can lead to decreased production of the hormone that supports implantation and pregnancy – progesterone. Furthermore, even though BPA had no significant effect on aromatase mRNA expression, it decreased the expression of HSD17B1 and HSD17B2, which are involved in the inter-conversion of oestrone and oestradiol. Whether such effects are observed at the endometrial tissue level in vivo is unknown and is worthy of further investigation. Interaction of BPA with oestrogen metabolism may play a role in development or support of already existing oestrogen-dependent disorders, such as leiomyomas or endometriosis. Interestingly, Cobellis et al. (2009) did not detect any bisphenols in the sera from healthy women by HPLC; whereas BPA and its derivative BPB were detected in 63.8% of women with endometriosis, suggesting a relationship between BPA serum 252 L Aghajanova, LC Giudice concentrations and endometriosis. Additional studies are needed to understand the observed relationship between BPA and endometriosis.
Decidualization
Development of a receptive endometrium to blastocyst nidation and the invasive phase of implantation into the maternal stromal compartment of the endometrium are regulated primarily by oestradiol and progesterone. The present study demonstrates that BPA may trigger differentiation of endometrial stroma even in the absence of decidualization stimuli from the surrounding environment. This can advance the development of endometrial stroma, which will not match the developmental stage of an embryo when it arrives in the uterine cavity, potentially compromising implantation and pregnancy success.
High doses of BPA
The above-mentioned effects on human ESF gene expression were evident upon exposure to higher doses of BPA, 50 and 100 µmol/l, coinciding with the report on the Ishikawa cell line (Naciff et al., 2010). The latter presented significant changes in global gene expression of Ishikawa cells after 48 h of exposure to 1 nmol/l−100 µmol/l BPA. Interestingly, after 48 h, BPA up-regulated KLF4, inhibin E, PTEN, HOXA10, osteopontin (secreted phosphoprotein 1), cadherins 6 and 8 (cell adhesion molecules), activated leukocyte cell adhesion molecule (ALCAM) and IGFBP6 and down-regulated EGR1, MMP10, stanniocalcin 1 (STC1), ALDH1A1, cadherin 18 and IGFBP3 genes, which are known to be expressed in endometrial stromal cells and involved in endometrial physiology (Aghajanova et al., 2010; Talbi et al., 2006). Moreover, up-regulation in Ishikawa epithelial cells of HOXA10 and osteopontin (Naciff et al., 2010; Smith and Taylor, 2007), which are important for endometrial receptivity in vivo (Daftary and Taylor, 2001; Lessey, 2002), is in line with the current finding of IGFBP1 up-regulation by BPA in human endometrial stromal cells at the same concentration. These data support potential effects of high-dose BPA exposure on endometrial maturation and function, with possible consequences on endometrium– embryo communication.
Apoptosis
Consistent with the present observations on the lack of effect of BPA on human ESF survival and viability, Naciff et al. (2010) also did not observe an effect of BPA on Ishikawa epithelial cell apoptosis. Bredhult et al. (2009) showed that primary endometrial endothelial cells upon 24 h of 50 µmol/l BPA exposure had a slight decrease in proliferation, similar to the present findings at 48 h of exposure in human ESF.
The mechanism of BPA action is not known with certainty, as its relationship with oestrogen receptors may differ between different species and cell/tissue types. In Ishikawa cells, BPA-mediated HOXA10 induction was blocked by the oestrogen-receptor antagonist ICI (Smith and Taylor, 2007); whereas herein, ICI had no effect on BPA-mediated gene expression, suggestive of a non-oestrogen receptor-mediated mechanism of action in this cell type.
Serum BPA and tissue proliferative potential
Based on its common pro-oestrogenic properties, it was anticipated that women with endometrial hyperplasia would have higher serum BPA concentrations compared with healthy controls (Hiroi et al., 2004). However, BPA serum concentrations in women with simple hyperplasia did not differ from women without hyperplasia (2.9 ± 2.0 versus 2.5 ± 1.5 ng/ml, respectively). Moreover, unexpectedly, BPA serum concentrations in women with complex endometrial hyperplasia with malignant potential and endometrial carcinoma were significantly lower compared with the other two groups (1.4 ± 0.5 ng/ml). These observations are consistent with the present observation of a decreased proliferation potential of BPA-treated human ESF, which, together with the enhanced decidualized phenotype observed herein, suggests a progestational effect of BPA in the endometrium. In neuroblastoma and breast cancer cell lines, BPA promotes cell proliferation (Fernandez and Russo, 2010; Zhu et al., 2009, 2010), indicating a complex and tissue specific effect of BPA.
In a human granulosa cell line and in primary human granulosa cells, BPA inhibited FSH-induced aromatase expression and hence oestradiol synthesis (Kwintkiewicz et al., 2010). The BPA effect on granulosa cells was mediated by increased expression of PPAR γ (Kwintkiewicz et al., 2010), an effect not observed herein with human ESF. Impaired function of granulosa cells could affect oocyte development and quality and result in dysregulation of steroid hormone-dependent tissues throughout the body, including endometrium. Thus, while the in-vitro evidence in human granulosa and endometrial stromal cells demonstrate impaired steroidogenesis and likely impaired endometrial development, respectively, with a potential influence on oocyte and endometrial quality, recent preliminary clinical data demonstrate a negative correlation between BPA and oestradiol concentrations in human follicular fluid and lower oocyte fertilization rates during IVF treatment (Lamb et al., 2008). This can reflect the combined effect of BPA on the ovary/oocyte, and potentially on endometrium/endometrial receptivity, contributing to compromised female fertility.
This study has demonstrated effects of BPA at high doses on human ESF proliferation and decidualization. However, before meaningful conclusions could be drawn about whether BPA exposure can alter endometrial function, several issues are worthy of consideration. First, the in-vitro system used herein is a model system without other cell types of the endometrium that communicate in vivo with the human ESF in a changing hormonal milieu. Whether the human ESF would respond similarly with these other cells present and in different hormonal states remains to be determined. Second, most of the effects of BPA on human ESF in vitro were observed at high concentrations – higher than commonly reported in humans. However, repeated exposures to this endocrine-disrupting chemical with a short half-life could increase serum (and perhaps tissue) concentrations to those that have been demonstrated to have similar effects as doses shown herein. Finally, the physiological relevance of effects of BPA on endometrial cells would depend on timing of exposure (in utero, neonatal, adolescent or adult), dose of exposure, duration of exposure and whether other chemicals are concomitantly present. These challenges are best addressed with animal models, although this study’s in-vitro system suggests that adult exposure to high concentrations of BPA could advance endometrial stromal differentiation, which could compromise embryo implantation.
Acknowledgements
This research was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/NIH through co-operative agreement 1U54HD055764–04 as part of the Specialized Co-operative Centres Program in Reproduction and Infertility Research.
Biography

Lusine Aghajanova, MD, PhD, is currently doing her residency in obstetrics and gynaecology at Baylor College of Medicine, Houston, Texas. She obtained her doctoral degree at the Department of Obstetrics and Gynecology at Karolinska Institute, Stockholm and completed her postdoctoral fellowship at the Department of Obstetrics, Gynecology and Reproductive Sciences at University of California, San Francisco. Being a clinician with particular interest in reproduction, she has focused her research on human implantation and several factors affecting that process, particularly environmental, and biomarkers of uterine receptivity, as well as endometrial dysfunction in women with unexplained infertility and endometriosis.
Footnotes
Declaration: The authors report no financial or commercial conflicts of interest.
Contributor Information
L Aghajanova, Email: aghajanoval@obgyn.ucsf.edu, aghajanova@yahoo.com.
LC Giudice, Email: giudice@obgyn.ucsf.edu.
References
- Aghajanova L, Horcajadas JA, Weeks JL, et al. The protein kinase A pathway-regulated transcriptome of endometrial stromal fibroblasts reveals compromised differentiation and persistent proliferative potential in endometriosis. Endocrinology. 2010;151:1341–1355. doi: 10.1210/en.2009-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aghajanova L, Hamilton A, Kwintkiewicz J, Vo KC, Giudice LC. Steroidogenic enzyme and key decidualization marker dysregulation in endometrial stromal cells from women with versus without endometriosis. Biol. Reprod. 2009;80:105–114. doi: 10.1095/biolreprod.108.070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredhult C, Sahlin L, Olovsson M. Gene expression analysis of human endometrial endothelial cells exposed to Bisphenol A. Reprod. Toxicol. 2009;28:18–25. doi: 10.1016/j.reprotox.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Buterin T, Koch C, Naegeli H. Convergent transcriptional profiles induced by endogenous estrogen and distinct xenoestrogens in breast cancer cells. Carcinogenesis. 2006;27:1567–1578. doi: 10.1093/carcin/bgi339. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2005;113:391–395. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ. Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin RE, Adams J, Boekelheide K, et al. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Res. B. Dev. Reprod. Toxicol. 2008;83:157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- Cobellis L, Colacurci N, Trabucco E, Carpentiero C, Grumetto L. Measurement of bisphenol A and bisphenol B levels in human blood sera from healthy and endometriotic women. Biomed. Chromatogr. 2009;23:1186–1190. doi: 10.1002/bmc.1241. [DOI] [PubMed] [Google Scholar]
- Daftary GS, Taylor HS. Molecular markers of implantation: clinical implications. Curr. Opin. Obstet. Gynecol. 2001;13:269–274. doi: 10.1097/00001703-200106000-00004. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez SV, Russo J. Estrogen and xenoestrogens in breast cancer. Toxicol. Pathol. 2010;38:110–122. doi: 10.1177/0192623309354108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC. Infertility and the environment: the medical context. Semin. Reprod. Med. 2006;24:129–133. doi: 10.1055/s-2006-944418. [DOI] [PubMed] [Google Scholar]
- Han DH, Denison MS, Tachibana H, Yamada K. Relationship between estrogen receptor-binding and estrogenic activities of environmental estrogens and suppression by flavonoids. Biosci. Biotechnol. Biochem. 2002;66:1479–1487. doi: 10.1271/bbb.66.1479. [DOI] [PubMed] [Google Scholar]
- Hiroi H, Tsutsumi O, Takeuchi T, et al. Differences in serum bisphenol a concentrations in premenopausal normal women and women with endometrial hyperplasia. Endocr. J. 2004;51:595–600. doi: 10.1507/endocrj.51.595. [DOI] [PubMed] [Google Scholar]
- Hunt PA, Koehler KE, Susiarjo M, et al. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr. Biol. 2003;13:546–553. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum. Reprod. 2002;17:2839–2841. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Giudice LC. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin. Reprod. Med. 2009;27:43–51. doi: 10.1055/s-0028-1108009. [DOI] [PubMed] [Google Scholar]
- Kwintkiewicz J, Nishi Y, Yanase T, Giudice LC. Peroxisome proliferator-activated receptor-gamma mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ. Health Perspect. 2010;118:400–406. doi: 10.1289/ehp.0901161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb JD, Bloom MS, vom Saal FS, Taylor JA, Sandler JR, Fujimoto VY. Serum Bisphenol A (BPA) and reproductive outcomes in couples undergoing IVF. Fertil. Steril. 2008;90:S186. [Google Scholar]
- Lang IA, Galloway TS, Scarlett A, et al. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. JAMA. 2008;300:1303–1310. doi: 10.1001/jama.300.11.1303. [DOI] [PubMed] [Google Scholar]
- Lapensee EW, Tuttle TR, Fox SR, Ben-Jonathan N. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-alpha-positive and -negative breast cancer cells. Environ. Health Perspect. 2009;117:175–180. doi: 10.1289/ehp.11788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YM, Seong MJ, Lee JW, et al. Estrogen receptor independent neurotoxic mechanism of bisphenol A, an environmental estrogen. J. Vet. Sci. 2007;8:27–38. doi: 10.4142/jvs.2007.8.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessey BA. Implantation defects in infertile women with endometriosis. Ann. NY Acad. Sci. 2002;955:265–280. doi: 10.1111/j.1749-6632.2002.tb02787.x. discussion 293–5, 396–406. [DOI] [PubMed] [Google Scholar]
- Luconi M, Bonaccorsi L, Forti G, Baldi E. Effects of estrogenic compounds on human spermatozoa: evidence for interaction with a nongenomic receptor for estrogen on human sperm membrane. Mol. Cell. Endocrinol. 2001;178:39–45. doi: 10.1016/s0303-7207(01)00416-6. [DOI] [PubMed] [Google Scholar]
- Masuyama H, Hiramatsu Y. Involvement of suppressor for Gal 1 in the ubiquitin/proteasome-mediated degradation of estrogen receptors. J. Biol. Chem. 2004;279:12020–12026. doi: 10.1074/jbc.M312762200. [DOI] [PubMed] [Google Scholar]
- Mok-Lin E, Ehrlich S, Williams PL, et al. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int. J. Androl. 2010;33:385–393. doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naciff JM, Khambatta ZS, Reichling TD, et al. The genomic response of Ishikawa cells to bisphenol A exposure is dose- and time-dependent. Toxicology. 2010;270:137–149. doi: 10.1016/j.tox.2010.02.008. [DOI] [PubMed] [Google Scholar]
- NTP. NTP-CERHR Monograph on the Potential Human Reproductive and Developmental Effects of Bisphenol A. NTP Cerhr. Mon. 2008 i-III1. [PubMed]
- Oehlmann J, Schulte-Oehlmann U, Kloas W, et al. A critical analysis of the biological impacts of plasticizers on wildlife. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364:2047–2062. doi: 10.1098/rstb.2008.0242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter CA, Birnbaum LS, Farabollini F, et al. In vivo effects of bisphenol A in laboratory rodent studies. Reprod. Toxicol. 2007;24:199–224. doi: 10.1016/j.reprotox.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salian S, Doshi T, Vanage G. Perinatal exposure of rats to Bisphenol A affects the fertility of male offspring. Life Sci. 2009;85:742–752. doi: 10.1016/j.lfs.2009.10.004. [DOI] [PubMed] [Google Scholar]
- Schonfelder G, Wittfoht W, Hopp H, Talsness CE, Paul M, Chahoud I. Parent bisphenol A accumulation in the human maternal-fetal-placental unit. Environ. Health Perspect. 2002;110:A703–A707. doi: 10.1289/ehp.110-1241091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CC, Taylor HS. Xenoestrogen exposure imprints expression of genes (Hoxa10) required for normal uterine development. FASEB J. 2007;21:239–246. doi: 10.1096/fj.06-6635com. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Talsness CE, Andrade AJ, Kuriyama SN, Taylor JA, vom Saal FS. Components of plastic: experimental studies in animals and relevance for human health. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2009;364:2079–2096. doi: 10.1098/rstb.2008.0281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS, Hughes C. An extensive new literature concerning low-dose effects of bisphenol A shows the need for a new risk assessment. Environ. Health Perspect. 2005;113:926–933. doi: 10.1289/ehp.7713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welshons WV, Nagel SC, vom Saal FS. Large effects from small exposures. III: Endocrine mechanisms mediating effects of bisphenol A at levels of human exposure. Endocrinology. 2006;147:S56–S69. doi: 10.1210/en.2005-1159. [DOI] [PubMed] [Google Scholar]
- Zhu H, Xiao X, Zheng J, Zheng S, Dong K, Yu Y. Growth-promoting effect of bisphenol A on neuroblastoma in vitro and in vivo. J. Pediatr. Surg. 2009;44:672–680. doi: 10.1016/j.jpedsurg.2008.10.067. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zheng J, Xiao X, et al. Environmental endocrine disruptors promote invasion and metastasis of SK-N-SH human neuroblastoma cells. Oncol. Rep. 2010;23:129–139. [PubMed] [Google Scholar]