Abstract
Originally described over three hundred years ago, endometriosis is classically defined by the presence of endometrial glands and stroma in extrauterine locations. Endometriosis is an inflammatory, estrogen dependent condition associated with pelvic pain and infertility. This work reviews the disease process from theories regarding origin to the molecular basis for disease sequelae. A thorough understanding of the histopathogenesis and pathophysiology of endometriosis is essential toward the development of novel diagnostic and treatment approaches for this debilitating condition.
Keywords: endometriosis, pathogenesis, pathophysiology, lesion biology
Endometriosis is classically defined as the presence of endometrial glands and stroma in ectopic locations, primarily the pelvic peritoneum, ovaries, and rectovaginal septum. Affecting 6-10% of women of reproductive age, the stigmata of endometriosis include dysmenorrhea, dyspareunia, chronic pelvic pain, irregular uterine bleeding and/or infertility (1). The prevalence of this condition in women experiencing pain, infertility, or both is as high as 35-50% (2). Yet, endometriosis is under-diagnosed and associated with a 6.7 year mean latency from onset of symptoms to definitive diagnosis (3), in part due to the requirement for surgical diagnosis. Endometriosis is a debilitating condition, posing significant quality of life issues for the individual patient (4). The disorder represents a major cause of hysterectomy and hospitalization in the United States, with total annual healthcare costs estimated at $69.4 billion in 2009 (5). The significant individual and public health concerns associated with endometriosis underscore the importance of understanding its pathogenesis and pathophysiology toward prevention and the development of sensitive non-surgical diagnostic assays and effective treatments. The past several decades have witnessed substantial progress toward unraveling the enigma associated with this disorder. Herein, we outline the current understanding of the pathogenesis and pathophysiology of endometriosis.
Histopathogenesis
A unifying theory regarding the origin of endometriosis has remained mystifyingly elusive. Instead, several theories (Fig. 1) have arisen to account for the disparate observations regarding pathogenesis, and these can generally be categorized as those proposing that implants originate from uterine endometrium and those proposing that implants arise from tissues other than the uterus. Intrinsic to these theories are inciting factors and genetic susceptibilities whose roles are beginning to be delineated, though insufficiently established to confirm cause and effect and subsequent development of endometriosis. For example, reports linking endocrine disrupting chemicals (EDCs) with endometriosis (6) suggest these, and endogenous/exogenous estrogens, as potential transforming/inductive/ stimulant candidates in theories of endometriosis pathogenesis. The developmental timing of action of such agents and their roles in influencing other systems that predispose to endometriosis (endocrine, immune, stem/progenitor cells, epigenetic modifications) must be considered in the context of genetic background as well as stimulus-driven reprogramming of the female reproductive tract (7).
Figure 1.
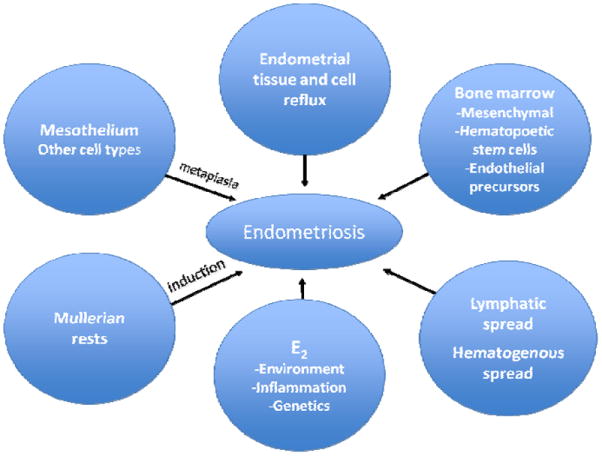
Theories regarding endometriosis pathogenesis. E2=estradiol
Among theories proposing a non-uterine origin of disease, coelomic metaplasia involves the transformation of normal peritoneal tissue to ectopic endometrial tissue (8). Agents responsible for such transformation remain poorly defined, although EDCs may be candidates. The closely related induction theory holds that an endogenous inductive stimulus, such as a hormonal or immunologic factor, promotes the differentiation of cells in the peritoneal lining to endometrial cells (9-10). Finally, the theory of embryonic Mullerian rests, or mullerianosis, purports cells residual from embryologic Mullerian duct migration maintain the capacity to develop into endometriotic lesions under the influence of estrogen beginning at puberty (11) or perhaps in response to estrogen mimetics. These theories find support in epidemiologic studies reporting a twofold increased risk of endometriosis in women exposed to diethylstilbestrol in utero(12).
A more recent proposal suggests extra-uterine stem/progenitor cells originating from bone marrow may differentiate into endometriotic tissue (13). Candidate cell lineages include bone marrow mesenchymal stem progenitors and endothelial progenitors, and this represents an active area of investigation. Support for theories advocating a non-endometrial origin for endometriosis is derived from clinical accounts of histologically confirmed endometriotic tissue in patients without menstrual endometrium, such as individuals with Rokitansky-Kuster-Hauser syndrome (14) and men with prostate cancer undergoing high dose estrogen treatment (15).
The theory of benign metastasis holds that ectopic endometrial implants are the result of lymphatic or hematogenous dissemination of endometrial cells (16-17). Microvascular studies demonstrated flow of lymph from the uterine body into the ovary, rendering possible a role for the lymphatic system in the etiology of ovarian endometriosis. Endometriosis within lymph nodes has been documented in a baboon model of induced endometriosis (18), and in 6-7% of women at lymphadenectomy (19). The strongest evidence for the theory of benign metastasis is derived from reports of histologically proven endometriotic lesions occurring in sites distant from the uterus to include bone, lung and brain (20).
Initially proposed by Sampson in the 1920s, the theory of retrograde menstruation is both intuitively attractive and supported by multiple lines of scientific evidence (21). According to this theory, eutopic endometrium is sloughed via patent fallopian tubes into the peritoneal cavity during menstruation. Indeed, the universality of this phenomenon is supported by the finding of menstrual blood in the peritoneal fluid of up to 90% of healthy women with patent fallopian tubes undergoing laparoscopy during the peri-menstrual time of the cycle (22).
Further support for this etiology is derived from studies of obstructed or compromised outflow tracts. In adolescent girls with congenital outflow obstruction, the prevalence of endometriosis is high (23). Likewise, iatrogenic obstruction of the outflow tract in a non-human primate model results in endometriotic lesions within the peritoneal cavity (24). Even subtle compromise of antegrade menstruation may predispose to endometriosis, as evidenced by the higher prevalence of endometriosis in women with a uterine septum (25) and cervical stenosis (26). The anatomic distribution of endometriotic lesions also favors the retrograde menstruation theory. Superficial implants are more often located in the posterior compartment of the pelvis (27) and in the left hemipelvis (28). The propensity for lesions to implant in the posterior cul de sac is explained by the accumulation of regurgitated menstrual effluent in this most dependent portion of the peritoneal cavity under the influence of gravity. In allowing flow from the anterior to posterior compartment in the upright or supine position, a retroverted uterine position is correlated with the finding of endometriosis (29). By acting as an obstacle to the diffusion of menstrual effluent from the left fallopian tube, the sigmoid colon promotes stasis of this effluent, thereby extending the interval for refluxed endometrial fragments to implant in the left hemipelvis.
A murine model of endometriosis has provided insight into the pathogenesis of peritoneal endometriosis (30). The conditional activation of the K-ras oncogene in endometrial cells deposited into the peritoneum resulted in histologically confirmed peritoneal endometriotic implants in nearly 50% of mice within 8 months. On the other hand, similar activation of the K-ras oncogene in peritoneal cells showed no progression to endometriosis. These preclinical observations favor an endometrial origin to the development of peritoneal lesions.
Though retrograde menstruation explains the physical displacement of endometrial fragments into the peritoneal cavity, additional steps are necessary for the development of endometriotic implants. Escape from immune clearance, attachment to peritoneal epithelium, invasion of the epithelium, establishment of local neurovascularity and continued growth and survival are necessary if endometriosis is to develop from retrograde passage of endometrium. Collectively, investigations involving the pathophysiology of endometriosis have revealed several well supported molecular hallmarks of this disease:
Genetic predisposition
Estrogen dependence
Progesterone resistance
Inflammation
It is the propensity for implantation that best accounts for the discrepancy between the 90% prevalence of retrograde menstruation and the nearly 10% prevalence of the disease. Hereditary or acquired properties of the endometrium, hereditary or acquired defects of the peritoneal epithelium, and/or defective immune clearance of sloughed endometrium are areas of active investigation in the search for the factor or factors that influence predisposition toward implantation of the displaced endometrial cells - a necessary correlate to theories proposing an endometrial origin to disease pathogenesis.
Endometrial cell survival
The evidence for an innate or acquired condition of the endometrial cells as the predisposing factor toward implantation is compelling. The eutopic endometrium from women with endometriosis shares certain alterations with ectopic lesions that are not observed in the endometrium from healthy women. Up-regulation of the anti-apoptotic gene BCL-2 has been shown in both eutopic and ectopic endometrium from affected women (31). In addition to decreased apoptosis, enhanced proliferation may confer a selective survival advantage to endometrium of women predisposed to endometriosis (32).
A genetic alteration of the endometrial cells influencing their tendency to implant may be hereditary, as a heritable component to the disease has been established. The risk for first degree relatives of women with severe endometriosis is six times higher than that for relatives of unaffected women (33). Studies of monozygotic twins demonstrate high concordance rates for histologically confirmed endometriosis (34). Linkage analysis has elucidated candidate genes with biological plausibility. The largest of these involved over 1100 families with two or more affected sib-pairs, and established significance for a susceptibility loci in the regions of chromosome 10q26 and 7p15 (35-36).
Acquired genomic alterations represent a potential source for a conferred survival advantage to sloughed endometrial cells in the establishment of endometriotic implants. The endometrium is a setting of extraordinary cell turnover and consequently, vulnerable to errors of genetic recombination. The occurrence of genomic alteration in eutopic endometrium is well documented, and may be consequent to epigenetic factors or oxidative stress (37). Loss of heterozygosity and somatic mutation of the tumor suppressor gene, PTEN, has been documented in 56% and 21% of solitary endometrial cysts of the ovary, respectively (38). Genomic alterations within endometriotic implants have been described using comparative genomic hybridization (CGH) microarrays (39). Interestingly, the CGH profiles (chromosome loss or gain) clustered by anatomic location of the implant as peritoneal or ovarian.
Finally, increasing evidence supports epigenetic regulation of steroid hormone action in the endometrium (40) and dysregulation in women with endometriosis (41). In particular, aberrant DNA methylation of promoters of genes whose products are critical for normal endometrial progesterone response have been reported in endometriosis and animal models of the disease, with resulting progesterone resistance (42). MicroRNAs (miRNAs) are short non-coding RNAs which generally repress gene expression through mRNA degradation. Differential and ovarian steroid dependent expression of miRNAs in eutopic endometrium from women with and without endometriosis has been demonstrated (43).
The search for an innate or acquired survival advantage of eutopic endometrium toward ectopic implantation has fueled a number of studies comparing eutopic endometrium from women with and without endometriosis. Collectively, these studies reveal striking differences in gene and protein expression that may predispose to disease development, and these have been nicely synopsized recently (44). A partial list of promising candidates is provided in Table 1. Validation of these genes/proteins requires temporally controlled experiments that can only be conducted using preclinical models such as the non-human primate, the only other species documented to spontaneously develop endometriosis (45).
Table 1.
Candidate factors implicated in the pathophysiology of endometriosis
| Gene | Function | Reference(s) |
|---|---|---|
| 17βHSD-2 | Hydroxysteroid dehydrogenase | Zeitoun et al 1998 (47) |
| BCL-2 | Anti-apoptosis | Jones et al 1998 (31) |
| CYP19 | Aromatase enzyme | Noble et al 1996 (101) |
| HOXA10 | Transcription factor | Taylor et al 1999 (102) |
| IL-6 | Cytokine | Harada et al 1997 (81) |
| KRAS | Oncogene | Dinulescu et al 2005 (30) |
| MMP 3,7 | Matrix metalloproteinases | Bruner-Tran et al 2002 (65) |
| NF-KB | Transcription factor | Gonzalez-Ramos et al 2010 (103) |
| PGE2 | Prostaglandin | Badawy et al 1984 (104) |
| PTEN | Tumor suppressor gene | Dinulescu et al 2005 (30) |
| TGF-B | Cytokine | Oosterlynck et al 1994 (61) |
| TNF-α | Cytokine | Eisermann et al 1988 (80) |
Altered hormonal milieu: estrogen dependence and progesterone resistance
Hormonal alterations may influence the ability of endometrial cells to proliferate, attach to the mesothelium and/or evade immune mediated clearance. Long appreciated clinically, the concept of endometriosis as an estrogen dependent disorder is well supported by molecular evidence (46). A striking finding in endometriotic tissue relative to eutopic endometrium is the increased expression of the aromatase enzyme and decreased expression of 17β-hydroxysteroid dehydrogenase (17β-HSD) type 2 (47). The sum consequence of this differential expression profile is a marked increase in the locally bioavailable estradiol concentration. Estradiol stimulates the production of prostaglandin E2 which further stimulates aromatase activity (48). These findings support the capacity of endometriotic lesions for estradiol biosynthesis, and substantiate treatments aimed at promoting a hypoestrogenic peritoneal microenvironment.
In addition to estrogen dependence, there is increasing evidence to support a profile of progesterone resistance in the pathophysiology of endometriosis (49). Endometriotic lesions exhibit an overall reduction in progesterone receptor expression relative to eutopic endometrium, and an absence of progesterone receptor-B (50). Additionally, endometrial expression profiling has documented dysregulation of progesterone responsive genes in the luteal phase (51). An incomplete transition of endometrium from the proliferative to secretory phase has significant molecular implications toward enhancing the survival and implantation of refluxed endometrium.
Evasion from immune clearance
Normally, refluxed endometrial tissue is cleared from the peritoneum by the immune system, and the dysregulation of this clearance mechanism has been implicated in the predisposition to implantation and growth of endometrial cells. Interestingly, larger tissue fragments as opposed to individual cells demonstrate an increased capacity to implant, presumably due to the protection from immune clearance afforded the cells residing on the inner aspects of such fragments (52). Additionally, the eutopic endometrium from women with endometriosis was found to be more resistant to lysis by natural killer (NK) cells than the eutopic endometrium from women without disease (53). Subsequent studies identified the constitutive shedding of intercellular adhesion molecule-1 (ICAM-1) by endometrial stromal cells from women with endometriosis as the potential mechanism by which these cells escape NK cell mediated clearance (54-55). Impaired NK cell function may confer an immune-priveleged status to the refluxed endometrial cells, thereby predisposing to disease. Compromised macrophage function in women with endometriosis (below) may further contribute to decreased clearance of lesions by this cell type.
Further support for a fundamentally altered immune system in the predisposition to endometriosis is derived from studies demonstrating a high concordance of autoimmune (systemic lupus erythematosus, rheumatoid arthritis, Sjogren’s syndrome, autoimmune thyroid disease) and atopic disease (allergies, asthma and eczema) in affected women (56). A number of non-organ specific antibodies have been found in association with endometriosis (57). Several studies have demonstrated clustering of autoimmune thyroid disease with endometriosis associated infertility, as evidenced by a high prevalence of positive anti-TPO titer in this cohort of women (58-59).
Endometrial cell attachment and invasion
Though endometriosis is a benign disorder, the process by which endometrial cells attach and invade surfaces shares features of malignancy. The endometrial stromal cell (ESC) fraction is primarily involved in the interaction of endometrial tissue with the mesothelial cell lining of the peritoneum. A study using ESCs and peritoneal mesothelial cells (PMCs) from a variety of sources in an in vitro binding assay demonstrated that the source of the endometrial stromal cells rather than the source of the peritoneal cells had the greatest impact on the rate of implantation (60).
A heritable or acquired condition of the peritoneum may predispose to the attachment and trans-mesothelial invasion by refluxed endometrial cells. An intact mesothelium is likely to act as a protective barrier against the implantation of regurgitated endometrial tissue. Indeed, in vitro studies showed that endometrial fragments adhered to the peritoneum only at locations where the basement membrane or extracellular matrix was exposed due to mesothelial layer damage (58). Menstrual effluent has a harmful effect on the mesothelium, and may autologously induce the local injury that promotes the implantation of endometrial cells (59). However, the exact factors involved in mediating mesothelial damage are unknown. Gene expression profiling of the peritoneum from subjects with and without endometriosis demonstrated upregulation of MMP-3 during the luteal phase and upregulation of ICAM-1, transforming growth factor-beta (TGF-β) and IL-6 during the menstrual phase (60). The differential expression of these cytokines and growth factors may create a microenvironment that encourages implantation of endometrial cells or protects them from immune mediated clearance. Among the cytokines that are elevated in the peritoneal fluid of women with endometriosis (61), TGF-β was observed to induce endometrial cell invasion in an in vitro model of the peritoneum (62).
Matrix metalloproteinases (MMPs) and their inhibitors (tissue inhibitors of metalloproteinases, TIMPs) are involved in extracellular matrix remodeling and have been implicated in cyclic endometrial turnover and menstruation (63-64). Menstrual cycle phase specific expression of MMPs suggests ovarian steroid regulation. The balance between MMPs and TIMPs is critical in maintaining the appropriate level of MMP activity, and failure to maintain this balance may contribute to matrix breakdown and cellular invasion. Endometrial MMP-7 expression is normally suppressed by progesterone during the secretory phase, yet endometriotic lesions exhibit persistent expression of MMP-7 during this phase (64). In a compelling illustration of intrinsic progesterone resistance in the pathophysiology of endometriosis, the in vitro treatment of eutopic endometrium acquired from affected women with progesterone failed to fully suppress pro-MMP-7 secretion and failed to prevent the ability of the transplanted endometrium to establish experimental disease in mice (65).
Lesional neuroangiogenesis and growth
A rich vascular supply is necessary for the development and sustenance of endometriotic lesions, particularly in the peritoneal microenvironment which is relatively avascular compared to the eutopic endometrium. Neoangiogenesis and capillary recruitment are visibly associated with endometriotic lesions at laparoscopy, most notably in the context of the red vesicular phenotype (Figure 2). In addition, nerves frequently accompany angiogenesis (neuroangiogensis), likely contributing to pain associated with this disorder (66). Gene expression profiling of menstrual phase endometrium in women with endometriosis demonstrated upregulation of tumor necrosis factor-α (TNF-α), interleukin-8 (IL-8) and MMP-3 (67). As IL-8 and TNF-α promote proliferation and adhesion of endometrial cells and angiogenesis, an overabundance of these cytokines may facilitate growth and local neovascularization. Vascular endothelial growth factor (VEGF) has been consistently detected in high concentration in peritoneal fluid from women with endometriosis, and the level appears to correlate with stage of disease (68). VEGF is abundantly expressed in the glandular compartment of peritoneal implants, in endometriomas, and is secreted by activated peritoneal macrophages (69). The expression of VEGF exhibits a cycle phase dependence consistent with ovarian steroid regulation. Evidence for VEGF as the prominent angiogenic factor is compelling. Other angiogenic factors implicated in disease pathophysiology include angiogenin (70), platelet-derived endothelial growth factor (71), and macrophage migration inhibitory factor (72).
Figure 2.
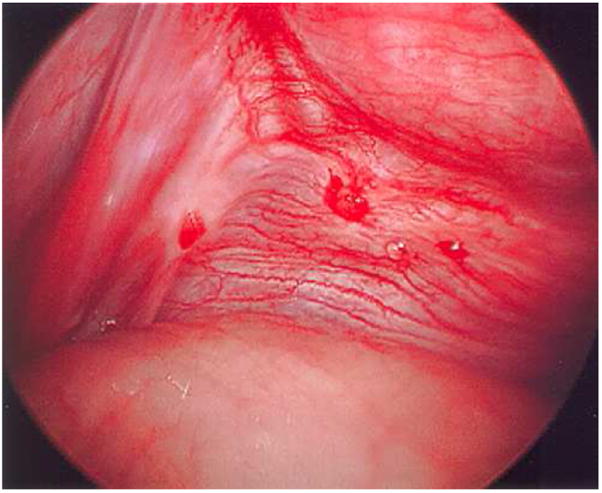
Red vesicular lesions with focal hemorrhage observed on the posterior aspect of the broad ligament. Note prominent focal vascularity in immediate vicinity of lesions.
Growth factors may play a fundamental role in stimulating ectopic endometrial growth and differentiation. Hepatocyte growth factor is a mitogen and morphogen for endometrial epithelial cells when co-cultured with stromal cells and may play a role in the regeneration of endometrial glands in ectopic locations (73). Epidermal growth factor (EGF) (74), insulin-like growth factors (IGF) (75), platelet derived growth factor (76), and basic fibroblast growth factor (74) are potent mitogens for endometrial stromal cells in vitro. IGF-1 is an anti-apoptotic growth factor and may enhance cell survival. EGF and IGF mediate estrogen actions in many tissues and, thus, are potential participants in the pathophysiology of endometriosis.
Inflammation
Increasing evidence supports conceptualization of endometriosis as a pelvic inflammatory condition. In women with endometriosis, the peritoneal fluid is remarkable for an increased number of activated macrophages and important differences in the cytokine/chemokine profile (77). A proteomics approach identified a unique protein structurally similar to haptoglobin in the peritoneal fluid of patients with endometriosis (78). This protein was subsequently found to bind to macrophages, reduce their phagocytic capacity and increase their production of interleukin 6. Other cytokines or chemokines found to be increased in the peritoneal fluid of patients with endometriosis include macrophage migration inhibitory factor (79), TNF-α (80), IL-1β, IL-6 (81), IL-8, regulated on activation, normal T expressed and secreted (RANTES), and monocyte chemoattractant protein-1 (MCP-1) (82). The latter three are chemoattractants, which facilitate the recruitment of macrophages. Whether observed cytokine profiles are a cause or a consequence of endometriosis remains to be definitively determined. The non-human primate model of endometriosis may allow the dissection of the temporal relationship between lesional development and cytokine profiles.
The peritoneal microenvironment in the setting of endometriosis is notably rich in prostaglandins, and these mediators likely play a central role in disease pathophysiology as well as clinical sequelae of pain and infertility. Peritoneal macrophages from women with endometriosis express higher levels of cyclo-oxygenase-2 (COX-2) and release significantly higher amounts of prostaglandins than macrophages from healthy women (83). At the lesional level, TNF-α promotes endometrial cell production of prostaglandin F2αand prostaglandin E2 (84). IL-1β activation of COX-2 increases production of PGE2 which activates steroidogenic acute regulatory (StAR) and aromatase (85). By upregulating PGE2 synthesis, estrogen completes a positive feedback loop that promotes the increased local bioavailability of estradiol (86). This pathway highlights the interplay of estrogen dependence and inflammation in endometriosis.
Inflammation is not only present in the peritoneal microenvironment, but also in the eutopic endometrium of women with endometriosis. As progesterone has well described anti-inflammatory properties, these changes may reflect attenuated progesterone action at the level of the endometrium. An increase in macrophage numbers is present in women with endometriosis throughout the menstrual cycle (87). Compared to disease-free controls, eutopic endometrium from women with endometriosis showed an increased basal production of interleukin-6 (88). IL-6 plays a prominent role in many chronic inflammatory conditions and is secreted by macrophages as well as epithelial endometrial cells. Interestingly, IL-6 was shown to significantly stimulate aromatase expression in cultured endometriotic stromal cells (89).
The inflammatory environment within the pelvis may contribute to the pathophysiology of pain perception in symptomatic women with endometriosis. Figure 3 summarizes the roles of estradiol (local and systemic), growth factors, prostaglandins, and other inflammatory stimuli in the pathogenesis of pain. It is believed that nerve fibers in endometriosis implants influence dorsal root neurons within the central nervous system, increasing pain perception in patients (66). The pathophysiology of pain has recently been extensively reviewed (90).
Figure 3.
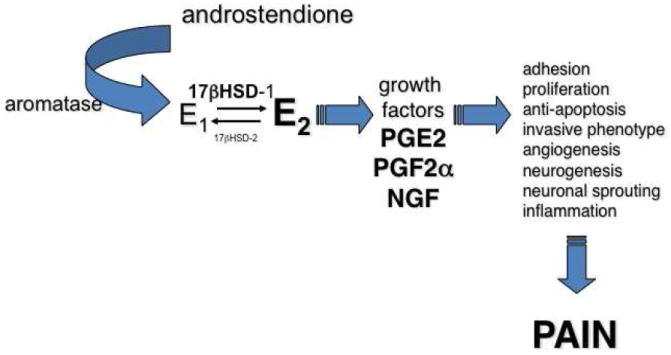
Local estradiol production in endometriotic lesions and eutopic endometrium, inflammation, and pain. 17βHSD = 17β hydroxysteroid dehydrogenase; E1=estrone; E2=estradiol; PGE2=prostaglandin E2; PGF2α=prostaglandin F2α; NGF= nerve growth factor.
Lesional progression and sequelae
Clinical and molecular lines of evidence converge to support a stagewise phenotypic progression associated with peritoneal endometriotic lesions. These stages include red vesicular, black powder-burn, and fibrotic lesional phenotypes. Longituginal placebo-controlled trials with second look laparoscopy have demonstrated that 71-83% of untreated lesions will progress or remain stable over a 12-month period (91-92). The earliest lesion is the red vesicular subtype (Figure 4A). Red vesicular lesions have been cytoarchitecturally defined as a cluster of communicating glands (93), are more biochemically active than black powder-burn lesions (94), and may be more responsive to cyclic sex hormones than other lesion subtypes (95). Laparoscopy timed to menstruation has observed these lesions to be focally hemorrhagic in response to progesterone withdrawal (96). MMP-1 is expressed focally in red peritoneal lesions regardless of the menstrual phase, but not in black peritoneal lesions (97). Foci of MMP-1 expression closely correlate with matrix breakdown and with the absence of progesterone receptors in adjacent epithelial cells, suggesting MMP-1 expression may be involved in tissue remodeling and bleeding of these early endometriotic lesions. Lesional bleeding could be the precursor to development of fibrin mediated adhesions (98). Most lesions evolve toward cicatrization (Fig 4c). A temporal progression of peritoneal lesions from red vesicular to fibrotic stages is supported by a large prospective surgical study finding red vesicular lesions predominantly in younger (20-25 year old) women and white plaques predominantly in older (41-45 year old) women (99). The cyclic inflammatory reaction to the peritoneal endometriotic lesion may result in a peritoneal defect referred to as an Allen-Masters window, a finding more frequently encountered in women with endometriosis (100).
Figure 4.
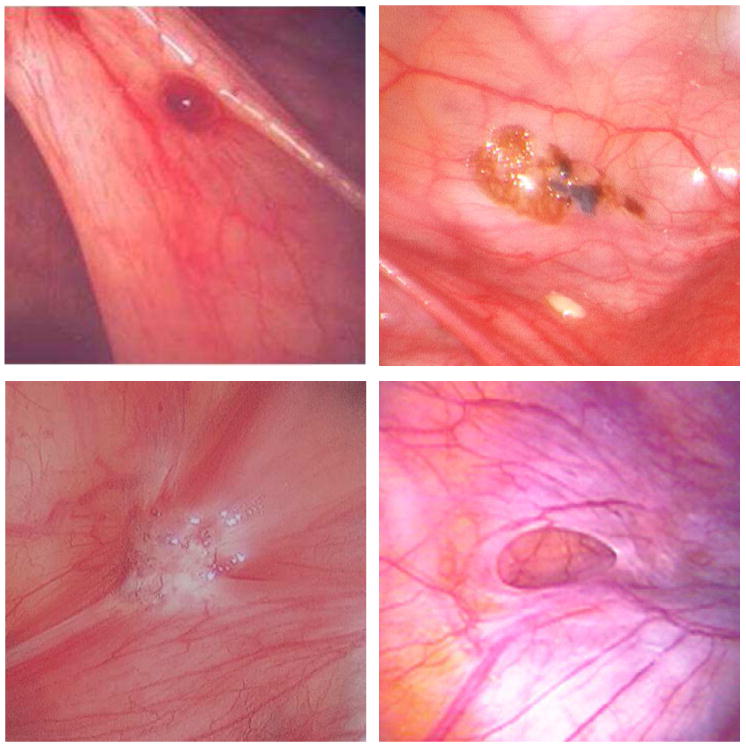
Phenotypic subtypes of peritoneal endometriosis. A. Red vesicular lesion B. Powder burn lesion C. Fibrotic lesion D. Allen-Masters peritoneal defect.
Conclusion
Since the original clinical description of endometriosis, much has been accomplished in furthering our understanding of this debilitating disease. Though no theory of pathogenesis can account for all of the described manifestations of endometriosis, the retrograde menstruation theory has gained widespread acceptance as an explanation for the dissemination of endometrial cells. The exact factor or factors that orchestrate the survival and subsequent implantation of the displaced endometrium remain unknown. Innate or acquired properties of the endometrium and defective immune clearance are systems of interest in elucidating the establishment of endometriotic implants. Disease heterogeneity, particularly in lesional phenotype, requires adherence to histopathologic confirmation of implants in clinical and molecular research. The underpinnings for the observed hallmarks of inflammation, estrogen dependence, and progesterone resistance in the pathophysiology of endometriosis associated pain and infertility are areas of active research. With further advances in our understanding of endometriosis, preventive strategies, novel non-surgical diagnostic modalities and targeted therapeutics hold great promise of becoming realities.
Footnotes
Financial support: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. 1997;24:235–58. doi: 10.1016/s0889-8545(05)70302-8. [DOI] [PubMed] [Google Scholar]
- 2.Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D’Hooghe T. High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertil Steril. 2009;92:68–74. doi: 10.1016/j.fertnstert.2008.04.056. [DOI] [PubMed] [Google Scholar]
- 3.Nnoaham KE, Hummelshoj L, Webster P, d’Hooghe T, de Cicco Nardone F, de Cicco Nardone C, et al. Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries. Fertil Steril. 2011;96:366–73 e8. doi: 10.1016/j.fertnstert.2011.05.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308:1587–9. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- 5.Simoens S, Dunselman G, Dirksen C, Hummelshoj L, Bokor A, Brandes I, et al. The burden of endometriosis: costs and quality of life of women with endometriosis and treated in referral centres. Hum Reprod. 2012;27:1292–9. doi: 10.1093/humrep/des073. [DOI] [PubMed] [Google Scholar]
- 6.Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, et al. Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–40. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulun SE. Endometriosis. N Engl J Med. 2009;360:268–79. doi: 10.1056/NEJMra0804690. [DOI] [PubMed] [Google Scholar]
- 8.Iwanoff N. Dusiges cystenhaltiges uterusfibromyom compliciert durch sarcom und carcinom. (Adenofibromyoma cysticum sarcomatodes carcinomatosum) Monatsch Geburtshilfe Gynakol. 1898;7:295–300. [Google Scholar]
- 9.Levander G, Normann P. The pathogenesis of endometriosis; an experimental study. Acta Obstet Gynecol Scand. 1955;34:366–98. doi: 10.3109/00016345509158287. [DOI] [PubMed] [Google Scholar]
- 10.Merrill JA. Endometrial induction of endometriosis across Millipore filters. Am J Obstet Gynecol. 1966;94:780–90. [PubMed] [Google Scholar]
- 11.Russell W. Aberrant portions of the mullerian duct found in an ovary. Ovarian cysts of mullerian origin. Bull Johns Hopkins Hosp. 1899;10:8. [Google Scholar]
- 12.Missmer SA, Hankinson SE, Spiegelman D, Barbieri RL, Michels KB, Hunter DJ. In utero exposures and the incidence of endometriosis. Fertil Steril. 2004;82:1501–8. doi: 10.1016/j.fertnstert.2004.04.065. [DOI] [PubMed] [Google Scholar]
- 13.Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann N Y Acad Sci. 2008;1127:106–15. doi: 10.1196/annals.1434.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rosenfeld DL, Lecher BD. Endometriosis in a patient with Rokitansky-Kuster-Hauser syndrome. Am J Obstet Gynecol. 1981;139:105. doi: 10.1016/0002-9378(81)90418-x. [DOI] [PubMed] [Google Scholar]
- 15.Schrodt GR, Alcorn MO, Ibanez J. Endometriosis of the male urinary system: a case report. J Urol. 1980;124:722–3. doi: 10.1016/s0022-5347(17)55627-x. [DOI] [PubMed] [Google Scholar]
- 16.Halban J. Metastatic hysteroadenosis. Wien klin Wochenschr. 1924;37:1205–6. [Google Scholar]
- 17.Sampson JA. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am J Pathol. 1927;3:93–110 43. [PMC free article] [PubMed] [Google Scholar]
- 18.Hey-Cunningham AJ, Fazleabas AT, Braundmeier AG, Markham R, Fraser IS, Berbic M. Endometrial stromal cells and immune cell populations within lymph nodes in a nonhuman primate model of endometriosis. Reprod Sci. 18:747–54. doi: 10.1177/1933719110397210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Javert CT. The spread of benign and malignant endometrium in the lymphatic system with a note on coexisting vascular involvement. Am J Obstet Gynecol. 1952;64:780–806. doi: 10.1016/s0002-9378(16)38796-8. [DOI] [PubMed] [Google Scholar]
- 20.Jubanyik KJ, Comite F. Extrapelvic endometriosis. Obstet Gynecol Clin North Am. 1997;24:411–40. doi: 10.1016/s0889-8545(05)70311-9. [DOI] [PubMed] [Google Scholar]
- 21.Sampson JA. Peritoneal endometriosis due to menstrual dissemination of endometrial tissue into the peritoneal cavity. Am J Obstet Gynecol. 1927;14:442–69. [Google Scholar]
- 22.Halme J, Hammond MG, Hulka JF, Raj SG, Talbert LM. Retrograde menstruation in healthy women and in patients with endometriosis. Obstet Gynecol. 1984;64:151–4. [PubMed] [Google Scholar]
- 23.Sanfilippo JS, Wakim NG, Schikler KN, Yussman MA. Endometriosis in association with uterine anomaly. Am J Obstet Gynecol. 1986;154:39–43. doi: 10.1016/0002-9378(86)90389-3. [DOI] [PubMed] [Google Scholar]
- 24.D’Hooghe TM, Bambra CS, Suleman MA, Dunselman GA, Evers HL, Koninckx PR. Development of a model of retrograde menstruation in baboons (Papio anubis) Fertil Steril. 1994;62:635–8. [PubMed] [Google Scholar]
- 25.Nawroth F, Rahimi G, Nawroth C, Foth D, Ludwig M, Schmidt T. Is there an association between septate uterus and endometriosis? Hum Reprod. 2006;21:542–4. doi: 10.1093/humrep/dei344. [DOI] [PubMed] [Google Scholar]
- 26.Barbieri RL. Stenosis of the external cervical os: an association with endometriosis in women with chronic pelvic pain. Fertil Steril. 1998;70:571–3. doi: 10.1016/s0015-0282(98)00189-7. [DOI] [PubMed] [Google Scholar]
- 27.Dmowski WP, Radwanska E. Current concepts on pathology, histogenesis and etiology of endometriosis. Acta Obstet Gynecol Scand Suppl. 1984;123:29–33. doi: 10.3109/00016348409156978. [DOI] [PubMed] [Google Scholar]
- 28.Al-Fozan H, Tulandi T. Left lateral predisposition of endometriosis and endometrioma. Obstet Gynecol. 2003;101:164–6. doi: 10.1016/s0029-7844(02)02446-8. [DOI] [PubMed] [Google Scholar]
- 29.Jenkins S, Olive DL, Haney AF. Endometriosis: pathogenetic implications of the anatomic distribution. Obstet Gynecol. 1986;67:335–8. [PubMed] [Google Scholar]
- 30.Dinulescu DM, Ince TA, Quade BJ, Shafer SA, Crowley D, Jacks T. Role of K-ras and Pten in the development of mouse models of endometriosis and endometrioid ovarian cancer. Nat Med. 2005;11:63–70. doi: 10.1038/nm1173. [DOI] [PubMed] [Google Scholar]
- 31.Jones RK, Searle RF, Bulmer JN. Apoptosis and bcl-2 expression in normal human endometrium, endometriosis and adenomyosis. Hum Reprod. 1998;13:3496–502. doi: 10.1093/humrep/13.12.3496. [DOI] [PubMed] [Google Scholar]
- 32.Wingfield M, Macpherson A, Healy DL, Rogers PA. Cell proliferation is increased in the endometrium of women with endometriosis. Fertil Steril. 1995;64:340–6. doi: 10.1016/s0015-0282(16)57733-4. [DOI] [PubMed] [Google Scholar]
- 33.Simpson JL, Elias S, Malinak LR, Buttram VC., Jr Heritable aspects of endometriosis. I. Genetic studies. Am J Obstet Gynecol. 1980;137:327–31. doi: 10.1016/0002-9378(80)90917-5. [DOI] [PubMed] [Google Scholar]
- 34.Hadfield RM, Mardon HJ, Barlow DH, Kennedy SH. Endometriosis in monozygotic twins. Fertil Steril. 1997;68:941–2. doi: 10.1016/s0015-0282(97)00359-2. [DOI] [PubMed] [Google Scholar]
- 35.Treloar SA, Wicks J, Nyholt DR, Montgomery GW, Bahlo M, Smith V, et al. Genomewide linkage study in 1,176 affected sister pair families identifies a significant susceptibility locus for endometriosis on chromosome 10q26. Am J Hum Genet. 2005;77:365–76. doi: 10.1086/432960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, et al. Genome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nat Genet. 43:51–4. doi: 10.1038/ng.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo SW, Wu Y, Strawn E, Basir Z, Wang Y, Halverson G, et al. Genomic alterations in the endometrium may be a proximate cause for endometriosis. Eur J Obstet Gynecol Reprod Biol. 2004;116:89–99. doi: 10.1016/j.ejogrb.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Sato N, Tsunoda H, Nishida M, Morishita Y, Takimoto Y, Kubo T, et al. Loss of heterozygosity on 10q23.3 and mutation of the tumor suppressor gene PTEN in benign endometrial cyst of the ovary: possible sequence progression from benign endometrial cyst to endometrioid carcinoma and clear cell carcinoma of the ovary. Cancer Res. 2000;60:7052–6. [PubMed] [Google Scholar]
- 39.Wu Y, Strawn E, Basir Z, Wang Y, Halverson G, Jailwala P, et al. Genomic alterations in ectopic and eutopic endometria of women with endometriosis. Gynecol Obstet Invest. 2006;62:148–59. doi: 10.1159/000093130. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Ho SM. Epigenetics meets endocrinology. J Mol Endocrinol. 46:R11–32. doi: 10.1677/jme-10-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Houshdaran S, Zelenko Z, Tamaresis JS, Irwin JC, Giudice LC. Abnormal epigenetic signature in eutopic endometrium of subjects with severe endometriosis. Reprod Sci. 2011;18:191A. [Google Scholar]
- 42.Guo SW. Epigenetics of endometriosis. Mol Hum Reprod. 2009;15:587–607. doi: 10.1093/molehr/gap064. [DOI] [PubMed] [Google Scholar]
- 43.Pan Q, Luo X, Toloubeydokhti T, Chegini N. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod. 2007;13:797–806. doi: 10.1093/molehr/gam063. [DOI] [PubMed] [Google Scholar]
- 44.May KE, Villar J, Kirtley S, Kennedy SH, Becker CM. Endometrial alterations in endometriosis: a systematic review of putative biomarkers. Hum Reprod Update. 2011;17:637–53. doi: 10.1093/humupd/dmr013. [DOI] [PubMed] [Google Scholar]
- 45.D’Hooghe TM, Kyama CM, Chai D, Fassbender A, Vodolazkaia A, Bokor A, et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16:152–61. doi: 10.1177/1933719108322430. [DOI] [PubMed] [Google Scholar]
- 46.Kitawaki J, Kado N, Ishihara H, Koshiba H, Kitaoka Y, Honjo H. Endometriosis: the pathophysiology as an estrogen-dependent disease. J Steroid Biochem Mol Biol. 2002;83:149–55. doi: 10.1016/s0960-0760(02)00260-1. [DOI] [PubMed] [Google Scholar]
- 47.Zeitoun K, Takayama K, Sasano H, Suzuki T, Moghrabi N, Andersson S, et al. Deficient 17beta-hydroxysteroid dehydrogenase type 2 expression in endometriosis: failure to metabolize 17beta-estradiol. J Clin Endocrinol Metab. 1998;83:4474–80. doi: 10.1210/jcem.83.12.5301. [DOI] [PubMed] [Google Scholar]
- 48.Noble LS, Takayama K, Zeitoun KM, Putman JM, Johns DA, Hinshelwood MM, et al. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J Clin Endocrinol Metab. 1997;82:600–6. doi: 10.1210/jcem.82.2.3783. [DOI] [PubMed] [Google Scholar]
- 49.Bulun SE, Cheng YH, Yin P, Imir G, Utsunomiya H, Attar E, et al. Progesterone resistance in endometriosis: link to failure to metabolize estradiol. Mol Cell Endocrinol. 2006;248:94–103. doi: 10.1016/j.mce.2005.11.041. [DOI] [PubMed] [Google Scholar]
- 50.Attia GR, Zeitoun K, Edwards D, Johns A, Carr BR, Bulun SE. Progesterone receptor isoform A but not B is expressed in endometriosis. J Clin Endocrinol Metab. 2000;85:2897–902. doi: 10.1210/jcem.85.8.6739. [DOI] [PubMed] [Google Scholar]
- 51.Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, et al. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology. 2007;148:3814–26. doi: 10.1210/en.2006-1692. [DOI] [PubMed] [Google Scholar]
- 52.Nap AW, Groothuis PG, Demir AY, Maas JW, Dunselman GA, de Goeij AF, et al. Tissue integrity is essential for ectopic implantation of human endometrium in the chicken chorioallantoic membrane. Hum Reprod. 2003;18:30–4. doi: 10.1093/humrep/deg033. [DOI] [PubMed] [Google Scholar]
- 53.Oosterlynck DJ, Cornillie FJ, Waer M, Vandeputte M, Koninckx PR. Women with endometriosis show a defect in natural killer activity resulting in a decreased cytotoxicity to autologous endometrium. Fertil Steril. 1991;56:45–51. doi: 10.1016/s0015-0282(16)54414-8. [DOI] [PubMed] [Google Scholar]
- 54.Somigliana E, Vigano P, Gaffuri B, Guarneri D, Busacca M, Vignali M. Human endometrial stromal cells as a source of soluble intercellular adhesion molecule (ICAM)-1 molecules. Hum Reprod. 1996;11:1190–4. doi: 10.1093/oxfordjournals.humrep.a019353. [DOI] [PubMed] [Google Scholar]
- 55.Somigliana E, Vigano P, Gaffuri B, Candiani M, Busacca M, Di Blasio AM, et al. Modulation of NK cell lytic function by endometrial secretory factors: potential role in endometriosis. Am J Reprod Immunol. 1996;36:295–300. doi: 10.1111/j.1600-0897.1996.tb00179.x. [DOI] [PubMed] [Google Scholar]
- 56.Sinaii N, Cleary SD, Ballweg ML, Nieman LK, Stratton P. High rates of autoimmune and endocrine disorders, fibromyalgia, chronic fatigue syndrome and atopic diseases among women with endometriosis: a survey analysis. Hum Reprod. 2002;17:2715–24. doi: 10.1093/humrep/17.10.2715. [DOI] [PubMed] [Google Scholar]
- 57.Van Voorhis BJ, Stovall DW. Autoantibodies and infertility: a review of the literature. J Reprod Immunol. 1997;33:239–56. doi: 10.1016/s0165-0378(97)00025-9. [DOI] [PubMed] [Google Scholar]
- 58.Gerhard I, Becker T, Eggert-Kruse W, Klinga K, Runnebaum B. Thyroid and ovarian function in infertile women. Hum Reprod. 1991;6:338–45. doi: 10.1093/oxfordjournals.humrep.a137335. [DOI] [PubMed] [Google Scholar]
- 59.Poppe K, Glinoer D, Van Steirteghem A, Tournaye H, Devroey P, Schiettecatte J, et al. Thyroid dysfunction and autoimmunity in infertile women. Thyroid. 2002;12:997–1001. doi: 10.1089/105072502320908330. [DOI] [PubMed] [Google Scholar]
- 60.Lucidi RS, Witz CA, Chrisco M, Binkley PA, Shain SA, Schenken RS. A novel in vitro model of the early endometriotic lesion demonstrates that attachment of endometrial cells to mesothelial cells is dependent on the source of endometrial cells. Fertil Steril. 2005;84:16–21. doi: 10.1016/j.fertnstert.2004.10.058. [DOI] [PubMed] [Google Scholar]
- 61.Oosterlynck DJ, Meuleman C, Waer M, Koninckx PR. Transforming growth factor-beta activity is increased in peritoneal fluid from women with endometriosis. Obstet Gynecol. 1994;83:287–92. [PubMed] [Google Scholar]
- 62.Liu YG, Tekmal RR, Binkley PA, Nair HB, Schenken RS, Kirma NB. Induction of endometrial epithelial cell invasion and c-fms expression by transforming growth factor beta. Mol Hum Reprod. 2009;15:665–73. doi: 10.1093/molehr/gap043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rodgers WH, Matrisian LM, Giudice LC, Dsupin B, Cannon P, Svitek C, et al. Patterns of matrix metalloproteinase expression in cycling endometrium imply differential functions and regulation by steroid hormones. J Clin Invest. 1994;94:946–53. doi: 10.1172/JCI117461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Osteen KG, Bruner KL, Sharpe-Timms KL. Steroid and growth factor regulation of matrix metalloproteinase expression and endometriosis. Semin Reprod Endocrinol. 1996;14:247–55. doi: 10.1055/s-2007-1016334. [DOI] [PubMed] [Google Scholar]
- 65.Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87:4782–91. doi: 10.1210/jc.2002-020418. [DOI] [PubMed] [Google Scholar]
- 66.Asante A, Taylor RN. Endometriosis: the role of neuroangiogenesis. Annu Rev Physiol. 2011;73:163–82. doi: 10.1146/annurev-physiol-012110-142158. [DOI] [PubMed] [Google Scholar]
- 67.Kyama CM, Overbergh L, Debrock S, Valckx D, Vander Perre S, Meuleman C, et al. Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertil Steril. 2006;85:1667–75. doi: 10.1016/j.fertnstert.2005.11.060. [DOI] [PubMed] [Google Scholar]
- 68.McLaren J, Prentice A, Charnock-Jones DS, Smith SK. Vascular endothelial growth factor (VEGF) concentrations are elevated in peritoneal fluid of women with endometriosis. Hum Reprod. 1996;11:220–3. doi: 10.1093/oxfordjournals.humrep.a019023. [DOI] [PubMed] [Google Scholar]
- 69.Shifren JL, Tseng JF, Zaloudek CJ, Ryan IP, Meng YG, Ferrara N, et al. Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab. 1996;81:3112–8. doi: 10.1210/jcem.81.8.8768883. [DOI] [PubMed] [Google Scholar]
- 70.Suzumori N, Zhao XX, Suzumori K. Elevated angiogenin levels in the peritoneal fluid of women with endometriosis correlate with the extent of the disorder. Fertil Steril. 2004;82:93–6. doi: 10.1016/j.fertnstert.2003.11.043. [DOI] [PubMed] [Google Scholar]
- 71.Fujimoto J, Sakaguchi H, Hirose R, Tamaya T. Expression of platelet-derived endothelial cell growth factor (PD-ECGF) related to angiogenesis in ovarian endometriosis. J Clin Endocrinol Metab. 1999;84:359–62. doi: 10.1210/jcem.84.1.5372. [DOI] [PubMed] [Google Scholar]
- 72.Yang Y, Degranpre P, Kharfi A, Akoum A. Identification of macrophage migration inhibitory factor as a potent endothelial cell growth-promoting agent released by ectopic human endometrial cells. J Clin Endocrinol Metab. 2000;85:4721–7. doi: 10.1210/jcem.85.12.7003. [DOI] [PubMed] [Google Scholar]
- 73.Sugawara J, Fukaya T, Murakami T, Yoshida H, Yajima A. Increased secretion of hepatocyte growth factor by eutopic endometrial stromal cells in women with endometriosis. Fertil Steril. 1997;68:468–72. doi: 10.1016/s0015-0282(97)00226-4. [DOI] [PubMed] [Google Scholar]
- 74.Huang JC, Papasakelariou C, Dawood MY. Epidermal growth factor and basic fibroblast growth factor in peritoneal fluid of women with endometriosis. Fertil Steril. 1996;65:931–4. [PubMed] [Google Scholar]
- 75.Giudice LC, Dsupin BA, Gargosky SE, Rosenfeld RG, Irwin JC. The insulin-like growth factor system in human peritoneal fluid: its effects on endometrial stromal cells and its potential relevance to endometriosis. J Clin Endocrinol Metab. 1994;79:1284–93. doi: 10.1210/jcem.79.5.7525631. [DOI] [PubMed] [Google Scholar]
- 76.Halme J, White C, Kauma S, Estes J, Haskill S. Peritoneal macrophages from patients with endometriosis release growth factor activity in vitro. J Clin Endocrinol Metab. 1988;66:1044–9. doi: 10.1210/jcem-66-5-1044. [DOI] [PubMed] [Google Scholar]
- 77.Rana N, Braun DP, House R, Gebel H, Rotman C, Dmowski WP. Basal and stimulated secretion of cytokines by peritoneal macrophages in women with endometriosis. Fertil Steril. 1996;65:925–30. [PubMed] [Google Scholar]
- 78.Sharpe-Timms KL, Piva M, Ricke EA, Surewicz K, Zhang YL, Zimmer RL. Endometriotic lesions synthesize and secrete a haptoglobin-like protein. Biol Reprod. 1998;58:988–94. doi: 10.1095/biolreprod58.4.988. [DOI] [PubMed] [Google Scholar]
- 79.Kats R, Collette T, Metz CN, Akoum A. Marked elevation of macrophage migration inhibitory factor in the peritoneal fluid of women with endometriosis. Fertil Steril. 2002;78:69–76. doi: 10.1016/s0015-0282(02)03189-8. [DOI] [PubMed] [Google Scholar]
- 80.Eisermann J, Gast MJ, Pineda J, Odem RR, Collins JL. Tumor necrosis factor in peritoneal fluid of women undergoing laparoscopic surgery. Fertil Steril. 1988;50:573–9. doi: 10.1016/s0015-0282(16)60185-1. [DOI] [PubMed] [Google Scholar]
- 81.Harada T, Yoshioka H, Yoshida S, Iwabe T, Onohara Y, Tanikawa M, et al. Increased interleukin-6 levels in peritoneal fluid of infertile patients with active endometriosis. Am J Obstet Gynecol. 1997;176:593–7. doi: 10.1016/s0002-9378(97)70553-2. [DOI] [PubMed] [Google Scholar]
- 82.Akoum A, Metz CN, Al-Akoum M, Kats R. Macrophage migration inhibitory factor expression in the intrauterine endometrium of women with endometriosis varies with disease stage, infertility status, and pelvic pain. Fertil Steril. 2006;85:1379–85. doi: 10.1016/j.fertnstert.2005.10.073. [DOI] [PubMed] [Google Scholar]
- 83.Wu MH, Sun HS, Lin CC, Hsiao KY, Chuang PC, Pan HA, et al. Distinct mechanisms regulate cyclooxygenase-1 and -2 in peritoneal macrophages of women with and without endometriosis. Mol Hum Reprod. 2002;8:1103–10. doi: 10.1093/molehr/8.12.1103. [DOI] [PubMed] [Google Scholar]
- 84.Chen DB, Yang ZM, Hilsenrath R, Le SP, Harper MJ. Stimulation of prostaglandin (PG) F2 alpha and PGE2 release by tumour necrosis factor-alpha and interleukin-1 alpha in cultured human luteal phase endometrial cells. Hum Reprod. 1995;10:2773–80. doi: 10.1093/oxfordjournals.humrep.a135790. [DOI] [PubMed] [Google Scholar]
- 85.Attar E, Tokunaga H, Imir G, Yilmaz MB, Redwine D, Putman M, et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J Clin Endocrinol Metab. 2009;94:623–31. doi: 10.1210/jc.2008-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bulun SE, Yang S, Fang Z, Gurates B, Tamura M, Zhou J, et al. Role of aromatase in endometrial disease. J Steroid Biochem Mol Biol. 2001;79:19–25. doi: 10.1016/s0960-0760(01)00134-0. [DOI] [PubMed] [Google Scholar]
- 87.Berbic M, Schulke L, Markham R, Tokushige N, Russell P, Fraser IS. Macrophage expression in endometrium of women with and without endometriosis. Hum Reprod. 2009;24:325–32. doi: 10.1093/humrep/den393. [DOI] [PubMed] [Google Scholar]
- 88.Tseng JF, Ryan IP, Milam TD, Murai JT, Schriock ED, Landers DV, et al. Interleukin-6 secretion in vitro is up-regulated in ectopic and eutopic endometrial stromal cells from women with endometriosis. J Clin Endocrinol Metab. 1996;81:1118–22. doi: 10.1210/jcem.81.3.8772585. [DOI] [PubMed] [Google Scholar]
- 89.Velasco I, Rueda J, Acien P. Aromatase expression in endometriotic tissues and cell cultures of patients with endometriosis. Mol Hum Reprod. 2006;12:377–81. doi: 10.1093/molehr/gal041. [DOI] [PubMed] [Google Scholar]
- 90.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17:327–46. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sutton CJ, Ewen SP, Whitelaw N, Haines P. Prospective, randomized, double-blind, controlled trial of laser laparoscopy in the treatment of pelvic pain associated with minimal, mild, and moderate endometriosis. Fertil Steril. 1994;62:696–700. doi: 10.1016/s0015-0282(16)56990-8. [DOI] [PubMed] [Google Scholar]
- 92.Telimaa S, Ronnberg L, Kauppila A. Placebo-controlled comparison of danazol and high-dose medroxyprogesterone acetate in the treatment of endometriosis after conservative surgery. Gynecol Endocrinol. 1987;1:363–71. doi: 10.3109/09513598709082709. [DOI] [PubMed] [Google Scholar]
- 93.Donnez J, Nisolle M, Casanas-Roux F. Three-dimensional architectures of peritoneal endometriosis. Fertil Steril. 1992;57:980–3. [PubMed] [Google Scholar]
- 94.Vernon MW, Beard JS, Graves K, Wilson EA. Classification of endometriotic implants by morphologic appearance and capacity to synthesize prostaglandin F. Fertil Steril. 1986;46:801–6. [PubMed] [Google Scholar]
- 95.Nieminen U. On the Vasculature of Ectopic Endometrium with Decidual Reaction. Acta Obstet Gynecol Scand. 1963;42:151–9. doi: 10.3109/00016346309157877. [DOI] [PubMed] [Google Scholar]
- 96.Burney RO, Lathi RB. Menstrual bleeding from an endometriotic lesion. Fertil Steril. 2009;91:1926–7. doi: 10.1016/j.fertnstert.2008.08.125. [DOI] [PubMed] [Google Scholar]
- 97.Kokorine I, Nisolle M, Donnez J, Eeckhout Y, Courtoy PJ, Marbaix E. Expression of interstitial collagenase (matrix metalloproteinase-1) is related to the activity of human endometriotic lesions. Fertil Steril. 1997;68:246–51. doi: 10.1016/s0015-0282(97)81510-5. [DOI] [PubMed] [Google Scholar]
- 98.Vipond MN, Whawell SA, Thompson JN, Dudley HA. Peritoneal fibrinolytic activity and intra-abdominal adhesions. Lancet. 1990;335:1120–2. doi: 10.1016/0140-6736(90)91125-t. [DOI] [PubMed] [Google Scholar]
- 99.Koninckx PR, Meuleman C, Demeyere S, Lesaffre E, Cornillie FJ. Suggestive evidence that pelvic endometriosis is a progressive disease, whereas deeply infiltrating endometriosis is associated with pelvic pain. Fertil Steril. 1991;55:759–65. doi: 10.1016/s0015-0282(16)54244-7. [DOI] [PubMed] [Google Scholar]
- 100.Chatman DL. Pelvic peritoneal defects and endometriosis: Allen-Masters syndrome revisited. Fertil Steril. 1981;36:751–6. doi: 10.1016/s0015-0282(16)45921-2. [DOI] [PubMed] [Google Scholar]
- 101.Noble LS, Simpson ER, Johns A, Bulun SE. Aromatase expression in endometriosis. J Clin Endocrinol Metab. 1996;81:174–9. doi: 10.1210/jcem.81.1.8550748. [DOI] [PubMed] [Google Scholar]
- 102.Taylor HS, Bagot C, Kardana A, Olive D, Arici A. HOX gene expression is altered in the endometrium of women with endometriosis. Hum Reprod. 1999;14:1328–31. doi: 10.1093/humrep/14.5.1328. [DOI] [PubMed] [Google Scholar]
- 103.Gonzalez-Ramos R, Van Langendonckt A, Defrere S, Lousse JC, Colette S, Devoto L, et al. Involvement of the nuclear factor-kappaB pathway in the pathogenesis of endometriosis. Fertil Steril. 94:1985–94. doi: 10.1016/j.fertnstert.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 104.Badawy SZ, Cuenca V, Marshall L, Munchback R, Rinas AC, Coble DA. Cellular components in peritoneal fluid in infertile patients with and without endometriosis. Fertil Steril. 1984;42:704–8. [PubMed] [Google Scholar]


