Abstract
This review highlights the current status and control of liver fluke infections in the Mekong Basin countries where Opisthorchis and Clonorchis are highly endemic. Updated data on prevalence and distribution have been summarized from presentations in the “96 Years of Opisthorchiasis. International Congress of Liver Flukes”. It is disturbing that despite treatment and control programs have been in place for decades, all countries of the Lower Mekong Basin are still highly endemic with O. viverrini and/or C. sinensis as well as alarmingly high levels of CCA incidence. A common pattern that is emerging in each country is the difference in transmission of O. viverrini between lowlands which have high prevalence versus highlands which have low prevalence. This seems to be associated with wetlands, flooding patterns and human movement and settlement. A more concerted effort from all community, educational, public health and government sectors is necessary to successfully combat this fatal liver disease of the poor.
1. Introduction
The human liver flukes, Opisthorchis viverrini, O. felineus and Clonorchis sinensis remain important public health problems in many parts of the world, particularly in Asia. Clonorchis sinensis is endemic in southern China, Korea and northern Vietnam, whereas O. viverrini is endemic in the Lower Mekong Basin, including Thailand, Lao People’s Democratic Republic (Lao PDR), Cambodia and central Vietnam [1]. Opisthorchis felineus is found in the former USSR and in Central-Eastern Europe [2-4]. Recent reports suggest that up to 15 million people are infected with C. sinensis in China alone [3,5] and another 10 million people are infected with O. viverrini in Thailand and Lao PDR [1,6-8]. Throughout the world 700 million people are at risk of infection with these two liver flukes [3]. The infections are associated with hepatobiliary diseases including hepatomegaly, cholangitis, fibrosis of the periportal system, cholecystitis, and gallstones. They are the major aetiological agents of bile duct cancer, cholangiocarcinoma (CCA), and as such, O. viverrini and C. sinensis have been classified as Type 1 carcinogens (metazoan parasites that are carcinogenic to humans) by the International Agency for Research on Cancer, World Health Organization (WHO) [9,10]. The highest incidence of the liver fluke-associated liver cancer in the world is in Khon Kaen, the Northeast Thailand [11,12]. This report highlights the current status and control of liver fluke infections in Mekong Basin countries where Opisthorchis and Clonorchis are highly endemic (Figure 1). Data on current epidemiology have been summarized from presentations of the member countries in the “96 Years of Opisthorchiasis. International Congress of Liver Flukes” held in Khon Kaen, Thailand, 7-8 March 2011. Additionally, updates in research relevant to opisthorchiasis and clonorchiasis are highlighted.
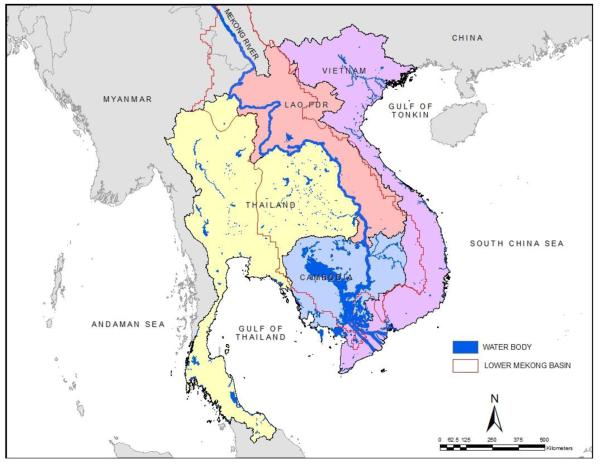
Figure 1.
Geographical areas of the Lower Mekong Basin.
2. Life cycle and transmission
The adult worms of O. viverrini and C. sinensis inhabit the intra- and extrahepatic biliary system. The embryonated eggs containing a miracidium released from the gravid worm is discharged with the bile and eventually passed into environment in the fecal stream. After reaching freshwater, the egg is ingested by snails of the genus Bithynia. The snails are abundant in water reservoirs with shallow water, rice fields and wetlands. Infected snails are frequently found in water bodies close to villages where high fecal contamination occurs (Kaewkes et al., this issue). Within the snail, eggs hatch to release miracidia, which transform to sporocysts. Then sporocysts undergo asexual reproduction to give rise to rediae and finally cercariae. After the cercariae are released from snails, they attach to and penetrate the skin of freshwater fish - belonging to one of ~20 susceptible species of cyprinids - to form infective stage, the metacercaria. In a recent survey, 70% of cyprinid fish, caught from Lawa Lake, Khon Kaen province, Thailand, were found to be infected with O. viverrini (Kaewkes, unpublished). However, the prevalence of C. sinensis infection in fish in Vietnam was quite low, 1.9-5.1% [13].
Until recently it has been assumed that cats and dogs act as reservoir hosts of O. viverrini in Thailand and Lao PDR. Currently, it has been shown that more than 35% of cats from endemic areas of Thailand are infected with O. viverrini [14] (Aunpromma et al., this issue). Similarly, the infection rate of O. viverrini in cats ranges from 33-60% in southern Vietnam (De, unpublished). Humans are the major definitive host; people become infected when they ingest raw or inadequately cooked fish, such as koi pla (fish salad) and pla som (short fermented fish) particularly in northeast Thailand and Lao PDR and pra hok in Cambodia (Figure 2). After ingestion fish flesh, the encysted metacercariae are digested by gastric and intestinal juices, whereupon excysted juvenile flukes migrate through the ampulla of Vater into the common bile duct and into the intrahepatic bile ducts, where they mature and sexually reproduce. Some flukes can be found in the common bile duct, cystic duct and even in the gallbladder [15].
Figure 2.
Consumption of „Koi pla” a popular raw fish dish in northeast Thailand and Lao PDR.
3. Current prevalence and geographic distribution
The first human cases of O. viverrini infection were reported in Thailand, nearly 100 years ago [16]. To date O. viverrini infection has been reported from other countries including Lao PDR, Cambodia and Vietnam with sporadic case reports from Malaysia, Singapore, and the Philippines [1]. In contrast, C. sinensis was first reported in 1874 from the bile duct contents of a Chinese carpenter who died in Calcutta, India [5,17]. However, it is important to note that human clonorchiasis has been a public health problem in China for more than 2,000 years: C. sinensis eggs have been detected in the fecal remains from an individual who lived during the middle stage of the Warring States Period (475-221 BC) in Hubei, China [18]. No reports of endemicity of clonorchiasis in Mekong Basin countries other than Vietnam have been published, except a report of clonorchiasis cases detected by PCR in a recent survey in a community in the central region of Thailand [19].
In like fashion to other trematodes, the presence and geographical range of suitable snail intermediate host dictates the geographical distributions of these liver flukes. However, because of growing international trading importation of freshwater aquaculture products from endemic countries of the liver flukes as well as other fish borne zoonotic helminthes, it is now increasingly common to detect infected people in nonendemic areas. For example, cases of opisthorchiasis in nonendemic areas such as the United States and Europe have been reported in immigrants from Asia and the cause of infection in some cases was due to the consumption of imported raw or undercooked freshwater fish containing metacercariae [20,21].
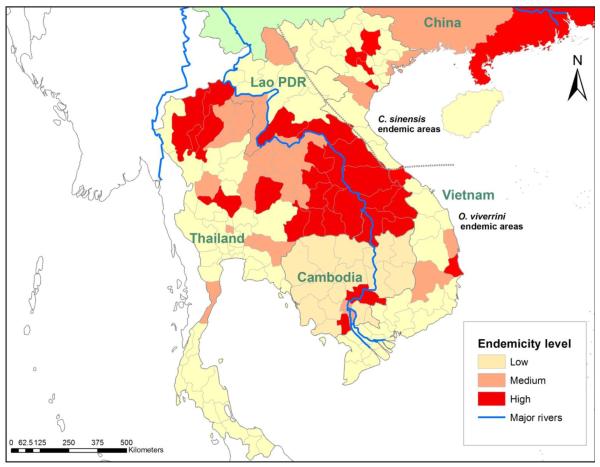
Human liver fluke control programs have been implemented in endemic areas in the Mekong Basin over several decades; disappointingly however, high infection rates still remain in many areas. Current data on the geographical distribution of human liver fluke infections in Thailand, Lao PDR, Cambodia and Vietnam are shown in Figure 3.
Figure 3.
The prevalence of O.viverrini and C.sinensis in Asian countries.
Endemicity level is defined based on prevalence of infections: low – 0 – 5%; medium- 5.1 – 15%; high – greater than 15%.
3.1 Thailand
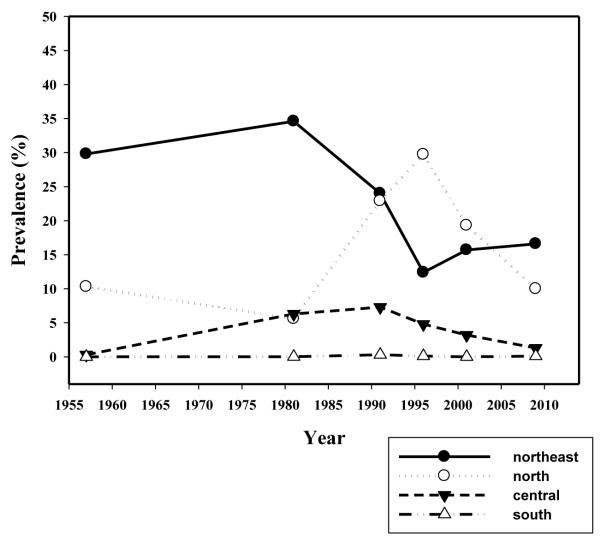
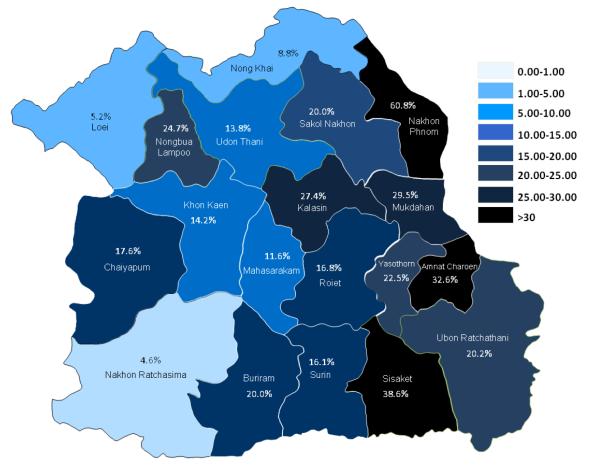
In Thailand, the first report of high prevalence of O. viverrini infection, reaching 100% in certain villages of northeast Thailand was by Sadun (1955) [22]. Almost 30 year later, a near 100% in prevalence and high intensity of O. viverrini was similarly reported in the Chonnabot district of Khon Kaen Province, confirming Khon Kaen to be one of the hot spots of the liver fluke infection in northeast Thailand [23-25]. The first nationwide survey of the four Regions of Thailand during 1980-1981 revealed an overall prevalence of O. viverrini infection of 14%; the Northeast (34.6%), the Central (6.3%), the North (5.6%) and the South (0.01%) regions. As a result of intensive and continuous control programs and public health service activities, the average national prevalence of infection has declined to 9.4% in the year 2000 [7] and went down further to 8.7% in the year 2009. Again, high prevalence of the infection was found in the Northeast (16.6%) followed by the North (10.0%), the Central (1.3%) and the South (0.01%) region of Thailand. Indeed, in the Northeast Region the prevalence in 2009 was similar to that of the previous survey 10 years ago in the year 2000 (15.7%) (Figure 4). Alarmingly, up to 60% prevalence was still found in Nakhon Panom Province in the Northeast Region. Other Provinces in the Northeast Region also showed disturbingly high prevalence of the infection (Figure 5). Based on the data in 2009, O. viverrini infection in Thailand, particularly in the North and Northeast Regions, is still prevalent and the total number of opisthorchiasis cases is estimated to be more than 6 million, the highest in the world. But this estimate is highly likely to be a gross underestimate [15] now that more sensitive diagnostic techniques are available [26].
Figure 4.
Annual prevalences of O.viverrini infection in each region in Thailand from 1957 to 2009. In the Northest, the prevalence tends to decline until 1996 but remains stable thereafter. A peaked prevalence in the north in 1996 may not entirely due to O.viverrini but mixed infection with the minute intestinal flukes which have similar egg morphology. (Data from the Ministry of Public Health).
Figure 5.
Distribution and prevalence of O.viverrini in the Northeast Thailand (surveyed in 2009).
3.2 Lao PDR
It has been estimated that over 2 million people are infected with O. viverrini in Lao PDR [8]. High infection rate occurs in the central and southern lowlands (Figure 3). A nationwide survey of 29,846 primary school children from 17 Provinces, including the Vientiane municipality, showed an average prevalence of O. viverrini infection of 10.9% with high prevalence in Khammuane, Saravane and Savannakhet Provinces of 32.2%, 21.5% and 25.9%, respectively [27]. Additionally, there are several reports of O. viverrini infection in children and adults from 1996 to present with high prevalence ranging from 37-86% [28-34]. A very recent survey in Champasack Province, southern Lao PDR has shown the prevalence of 64% for O. viverrini infection among 669 persons from 3 Districts [31]. Furthermore, the prevalence of the infection was strikingly high in Khong (92.0%) and Mounlapamok Districts (90.9%) which are located in the lowlands, whereas the prevalence was low in Paksong (5.7%) which is in the highlands. Hence, opisthorchiasis is highly endemic in Lao PDR, particularly in lowland areas.
3.3 Cambodia
Few reports are available on prevalence of O. viverrini infection in Cambodia. During 1981-1982, two of 102 Cambodian refugees in the United States were found to be positive for C. sinensis eggs (Parish, 1985), but we consider that it is likely they were eggs of O. viverrini by current knowledge. Subsequently, egg-positive cases have been reported in a number of Provinces [35]. High infection rates have been detected among communities located along the Mekong River flood plain (Figure 3). A survey of 251 primary schoolchildren from Kampong Cham Province revealed a 4.0% prevalence of O. viverrini infection [36]. A recent study revealed a high infection rate of O. viverrini of 32% in Takeo Province [37], whereas a separate survey conducted 2010 in Kampong Cham Province found an 18% prevalence of O. viverrini infection (Sinuon, unpublished). It has also been found in 2006 by Japanese parasitologists [38] that there were highly prevalent in villages in Takeo Province (26.9%) and a village in Kandal Province (10.7%). These workers also surveyed Kampong Cham Province and found four positive villages with the highest prevalence of 44.8% [38].
A high infection rate of 11% was recorded in school children in Kandal Province, south of Phnom Penh. This correlates with a high prevalence of O. viverrini metacercariae found in 10 species of freshwater cyprinid fish with the infection rate ranging from 2.1% to 66.7% of the fish captured from Kandal Province [39].
In contrast, a relatively low (0.7%) human infection rate has been recorded in northwestern Cambodia, including the highlands of Oddar Meanchey Province [40]. Although limited, data currently available suggest that O. viverrini prevalence levels in Cambodia are similar to those seen in the neighboring Lower Mekong Basin countries. Recently it has been estimated that 600,000 people are infected by O. viverrini in Cambodia [10]. However, this is a potentially marked underestimate of numbers of people infected because data are limited and incomplete and there is no accurate nationwide public health process of diagnosis and reporting of O. viverrini infections. Comprehensive studies on opisthorchiasis should be conducted throughout Cambodia.
3.4 Vietnam
In Vietnam, both C. sinensis and O. viverrini infections have been reported with C. sinensis distributed in 21 provinces in the north and O. viverrini distributed in 11 provinces in the south (Figure 3). An epidemiological survey in 12 of 61 provinces showed that C. sinensis was highly prevalent in nine northern provinces with the prevalence ranging from 0.2% to 26.0%, specifically the Red River delta region [41,42]. For O. viverrini, the prevalence has been found to range from 15.2% to 36.9% in three southern provinces where it is endemic [41]. The cumulative prevalence of O. viverrini in 11 provinces in southern Vietnam has been found to be 36.9 % (Phu Yen), 32% (Quang Tri), 11.9 % (Binh Dinh), 7.6 % (Dac Lac), 4.6% (Quang Nam), 1.4% (Khanh Hoa), 0.3% (Da Nang) and Thua Thien Hue (sporadic case reports). Six endemic districts in central Vietnam - Nui Thanh, Mo Duc, Mhu My, Song Cau, Tuy An and Buon Don -showed a high prevalence up to 40% of O. viverrini [42].
A study in 1992 in Phu Yen province has shown that infection rate was 43.5% in men and 29.4% in women. The highest prevalence O. viverrini infection has been recorded in people aged between 40-59 years old. Consumption of raw fish is common in opisthorchiasis endemic areas, where it was found that the proportion of people who ate raw or undercooked fish was 46% in Phu Yen Province, 61.3% in Binh Dinh Province, and 74% in Dac Lac Province (De, unpublished).
4. Prevention and control
Prevention and control programs against human liver fluke infection have been implemented in the Mekong Basin over several decades, specifically in Thailand. Unfortunately, high infection rates still persist in endemic areas as described above. The main reason of the failure of liver fluke control programs is likely to be the lack of culturally sensitive and educationally informed information concerning “raw attitudes” in eating behavior of the people in the Lower Mekong Basin [43]. Governmental control programs may be partially successful during active campaigns but this is not sustainable. Control strategies, therefore, should be designed to accommodate a more community-oriented approach and not a top down policy. Ecosystem health or EcoHealth is one of the approaches that may be ideal for liver fluke control, since the disease is a complex problem involving not only humans but several intermediate and reservoir hosts, different environments, government and non-government sectors including communities and individual villages.
The “Lawa model” is an opisthorchiasis control program using an EcoHealth approach, which is currently being implemented in the opisthorchiasis endemic area at Lawa Lake, Khon Kaen province, Thailand. This model involves equitable trans-disciplinary and stakeholder participation at every level. Many researchers from various disciplines, i.e. medicine, veterinary medicine, public health, environmental sciences, agriculture, biology and biotechnology have been recruited to collaborate in research, public health and environmentally related components. Multi-level stakeholders including villagers, community leaders, health volunteers, local health officers and governors, teachers/headmasters, and religious leaders/monks actively participate. Consensus agreements on implementation strategies are finalized after several rounds of small group meetings and public hearings under the supervision of the researchers. The control strategies include treatment of humans and mammalian reservoir hosts (to reduce the excretion of eggs), improved sanitation (to prevent eggs from reaching water sources) and health promotion including information, education and communication (IEC) to discourage consumption of raw fish and to improve sanitary practices.
Control of snail intermediate hosts by molluscicides is not considered feasible because of the widespread distribution of snails for example in the Lawa complex [44] (Petney et al 2011in this issue), and low prevalence of fluke infection of snails even in endemic communities where high prevalence occurred in humans, and because molluscicides destroy non-target mollusks and other invertebrates as well. To be most effective, IEC strategies should be designed and delivered in a culturally-sensitive manner with the aim of stimulating behavioural change as well as by providing easily understandable information and therefore raising awareness of the problem. Targetting young people, for example school-aged children, may be a feasible approach for successful long-term control [45] (Figure 6). Village health volunteers are key players for health education since each health volunteer takes care of about ten households in Thailand. Their activities during the delivery of health education to villagers are impressive (Figure 7). Large-scale efforts in endemic areas by public health agencies have certainly had a major impact on reducing the intensity of infections, although the efficacy and cost-effectiveness remains to be determined. Multi-sectoral participation among government agencies, private organizations including non-governmental organizations (NGOs) and the residents of the endemic areas can facilitate sustainable parasite disease control. A Pilot EcoHealth approach for human liver fluke control is aimed to implement in endemic areas of Lao PDR, Cambodia, Vietnam and southern China.
Figure 6.
School-based campaign for prevention and control of opisthorchiasis in the community in the Lawa lake complex, Khon Kaen, Thailand.
Figure 7.
Community-based health education program run by village health volunteer workers in Khon Kaen, Thailand.
Because of sustained, elevated CCA incidence in northeast Thailand and the recognition that O. viverrini is a carcinogenic parasite and the fundamental risk factor for CCA [10], the liver fluke surveillance and control has received more attention from governmental and public sectors. In northeast Thailand, where more concerted action from academic researchers and public health workers has led to comprehensive investigations ranging from the prevalence of liver fluke infection to treatment and surgical therapy of CCA, it is anticipated that the hidden dangers of this underestimated problem that affects millions of poor and less privileged people can soon be ameliorated, improving the quality of life and leading to other socioeconomic advances.
Highlights.
This review highlights the current status and control of Opisthorchis and Clonorchis infections in the Lower Mekong Basin countries.
Data were summarized from presentations in the “96 Years of Opisthorchiasis. International Congress of Liver Flukes” in Khon Kaen Thailand.
A more concerted effort from all community, educational, public health and government sectors is necessary to successfully combat this fatal liver disease of the poor.
Acknowledgements
This work was supported by the International Development and Research Centre (IDRC), the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, through the Health Cluster (SHeP-GMS), Khon Kaen University, and National Institutes of Health, USA award numbers UO1-AI065871 and R01CA155297. We acknowledge the support of the Faculty of Medicine, Resident International Professor Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–50. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- [2].Armignacco O, Caterini L, Marucci G, Ferri F, Bernardini G, Natalini Raponi G, et al. Human illnesses caused by Opisthorchis felineus flukes, Italy. Emerg Infect Dis. 2008;14:1902–5. doi: 10.3201/eid1412.080782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Keiser J, Utzinger J. Emerging foodborne trematodiasis. Emerg Infect Dis. 2005;11:1507–14. doi: 10.3201/eid1110.050614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Sithithaworn P, Haswell-Elkins M. Epidemiology of Opisthorchis viverrini. Acta Trop. 2003;88:187–94. doi: 10.1016/j.actatropica.2003.02.001. [DOI] [PubMed] [Google Scholar]
- [5].Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- [6].Andrews RH, Sithithaworn P, Petney TN. Opisthorchis viverrini: an underestimated parasite in world health. Trends Parasitol. 2008;24:497–501. doi: 10.1016/j.pt.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Jongsuksuntigul P, Imsomboon T. Opisthorchiasis control in Thailand. Acta Trop. 2003;88:229–32. doi: 10.1016/j.actatropica.2003.01.002. [DOI] [PubMed] [Google Scholar]
- [8].WHO Control of foodborne trematode infections. Report of a WHO Study Group. World Health Organ Tech Rep Ser. 1995;849:1–157. [PubMed] [Google Scholar]
- [9].Bouvard V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F, et al. A review of human carcinogens--Part B: biological agents. Lancet Oncol. 2009;10:321–2. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- [10].IARC A review of human carcinogens part B: Biological Agents. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2011;100B:457. [PMC free article] [PubMed] [Google Scholar]
- [11].Shin HR, Oh JK, Masuyer E, Curado MP, Bouvard V, Fang YY, et al. Epidemiology of cholangiocarcinoma: an update focusing on risk factors. Cancer Sci. 2010;101:579–85. doi: 10.1111/j.1349-7006.2009.01458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Vatanasapt V, Uttaravichien T, Mairiang EO, Pairojkul C, Chartbanchachai W, Haswell-Elkins M. Cholangiocarcinoma in north-east Thailand. Lancet. 1990;335:116–7. doi: 10.1016/0140-6736(90)90591-r. [DOI] [PubMed] [Google Scholar]
- [13].Thu ND, Dalsgaard A, Loan LT, Murrell KD. Survey for zoonotic liver and intestinal trematode metacercariae in cultured and wild fish in An Giang Province, Vietnam. Korean J Parasitol. 2007;45:45–54. doi: 10.3347/kjp.2007.45.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Enes JE, Wages AJ, Malone JB, Tesana S. Prevalence of Opisthorchis viverrini infection in the canine and feline hosts in three villages, Khon Kaen Province, northeastern Thailand. Southeast Asian J Trop Med Public Health. 2010;41:36–42. [PMC free article] [PubMed] [Google Scholar]
- [15].Sithithaworn P, Tesana S, Pipitgool V, Kaewkes S, Thaiklar K, Pairojkul C, et al. Quantitative post-mortem study of Opisthorchis viverrini in man in north-east Thailand. Trans R Soc Trop Med Hyg. 1991;85:765–8. doi: 10.1016/0035-9203(91)90449-9. [DOI] [PubMed] [Google Scholar]
- [16].Leiper RT. Notes of the occurence of parasites presumably rare in man in man. J R Army Med Corps. 1915;24:569–75. [Google Scholar]
- [17].Grove D. A history of human helminthology. CAB International; UK: 1990. pp. 141–57. [Google Scholar]
- [18].Wu Z, Guan Y, Zhou Z. [Study of an ancient corpse of the Warring States period unearthed from Tomb No. 1 at Guo-Jia Gang in Jingmen City (A comprehensive study)] J Tongji Med Univ. 1996;16:1–5. 10. doi: 10.1007/BF02889033. [DOI] [PubMed] [Google Scholar]
- [19].Traub RJ, Macaranas J, Mungthin M, Leelayoova S, Cribb T, Murrell KD, et al. A new PCR-based approach indicates the range of Clonorchis sinensis now extends to Central Thailand. PLoS Negl Trop Dis. 2009;3:e367. doi: 10.1371/journal.pntd.0000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Stauffer WM, Sellman JS, Walker PF. Biliary liver flukes (Opisthorchiasis and Clonorchiasis) in immigrants in the United States: often subtle and diagnosed years after arrival. J Travel Med. 2004;11:157–9. [PubMed] [Google Scholar]
- [21].Yossepowitch O, Gotesman T, Assous M, Marva E, Zimlichman R, Dan M. Opisthorchiasis from imported raw fish. Emerg Infect Dis. 2004;10:2122–6. doi: 10.3201/eid1012.040410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sadun EH. Studies on Opisthorchis viverrini in Thailand. Am J Hyg. 1955;62:81–115. doi: 10.1093/oxfordjournals.aje.a119772. [DOI] [PubMed] [Google Scholar]
- [23].Upatham ES, Viyanant V, Kurathong S, Rojborwonwitaya J, Brockelman WY, Ardsungnoen S, et al. Relationship between prevalence and intensity of Opisthorchis viverrini infection, and clinical symptoms and signs in a rural community in north-east Thailand. Bull World Health Organ. 1984;62:451–61. [PMC free article] [PubMed] [Google Scholar]
- [24].Upatham ES, Viyanant V, Kurathong S, Brockelman WY, Menaruchi A, Saowakontha S, et al. Morbidity in relation to intensity of infection in Opisthorchiasis viverrini: study of a community in Khon Kaen, Thailand. Am J Trop Med Hyg. 1982;31:1156–63. doi: 10.4269/ajtmh.1982.31.1156. [DOI] [PubMed] [Google Scholar]
- [25].Upatham ES, Brockelman WY, Viyanant V, Lee P, Kaengraeng R, Prayoonwiwat B. Incidence of endemic Opisthorchis viverrini infection in a village in northeast Thailand. Am J Trop Med Hyg. 1985;34:903–6. doi: 10.4269/ajtmh.1985.34.903. [DOI] [PubMed] [Google Scholar]
- [26].Johansen MVSP, Bergquist R, Utzinger J. Towards improved diagnosis of zoonotic trematode infections in Southeast Asia. Advanced Parasitology. 2010;73:171–95. doi: 10.1016/S0065-308X(10)73007-4. [DOI] [PubMed] [Google Scholar]
- [27].Rim HJ, Chai JY, Min DY, Cho SY, Eom KS, Hong SJ, et al. Prevalence of intestinal parasite infections on a national scale among primary schoolchildren in Laos. Parasitol Res. 2003;91:267–72. doi: 10.1007/s00436-003-0963-x. [DOI] [PubMed] [Google Scholar]
- [28].Chai JY, Han ET, Shin EH, Sohn WM, Yong TS, Eom KS, et al. High prevalence of Haplorchis taichui, Phaneropsolus molenkampi, and other helminth infections among people in Khammouane province, Lao PDR. Korean J Parasitol. 2009;47:243–7. doi: 10.3347/kjp.2009.47.3.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kobayashi J, Vannachone B, Sato Y, Manivong K, Nambanya S, Inthakone S. An epidemiological study on Opisthorchis viverrini infection in Lao villages. Southeast Asian J Trop Med Public Health. 2000;31:128–32. [PubMed] [Google Scholar]
- [30].Kobayashi J, Vannachone B, Xeutvongsa A, Manivang K, Ogawa S, Sato Y, et al. Prevalence of intestinal parasitic infection among children in two villages in Lao PDR. Southeast Asian J Trop Med Public Health. 1996;27:562–5. [PubMed] [Google Scholar]
- [31].Sayasone S, Mak TK, Vanmany M, Rasphone O, Vounatsou P, Utzinger J, et al. Helminth and intestinal protozoa infections, multiparasitism and risk factors in Champasack province, Lao People’s Democratic Republic. PLoS Negl Trop Dis. 2011;5:e1037. doi: 10.1371/journal.pntd.0001037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sayasone S, Odermatt P, Phoumindr N, Vongsaravane X, Sensombath V, Phetsouvanh R, et al. Epidemiology of Opisthorchis viverrini in a rural district of southern Lao PDR. Trans R Soc Trop Med Hyg. 2007;101:40–7. doi: 10.1016/j.trstmh.2006.02.018. [DOI] [PubMed] [Google Scholar]
- [33].Sayasone S, Vonghajack Y, Vanmany M, Rasphone O, Tesana S, Utzinger J, et al. Diversity of human intestinal helminthiasis in Lao PDR. Trans R Soc Trop Med Hyg. 2009;103:247–54. doi: 10.1016/j.trstmh.2008.10.011. [DOI] [PubMed] [Google Scholar]
- [34].Sithithaworn P, Sukavat K, Vannachone B, Sophonphong K, Ben-Embarek P, Petney T, et al. Epidemiology of food-borne trematodes and other parasite infections in a fishing community on the Nam Ngum reservoir, Lao PDR. Southeast Asian J Trop Med Public Health. 2006;37:1083–90. [PubMed] [Google Scholar]
- [35].Stich AH, Biays S, Odermatt P, Men C, Saem C, Sokha K, et al. Foci of Schistosomiasis mekongi, Northern Cambodia: II. Distribution of infection and morbidity. Trop Med Int Health. 1999;4:674–85. doi: 10.1046/j.1365-3156.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- [36].Lee KJ, Bae YT, Kim DH, Deung YK, Ryang YS, Kim HJ, et al. Status of intestinal parasites infection among primary school children in Kampongcham, Cambodia. Korean J Parasitol. 2002;40:153–5. doi: 10.3347/kjp.2002.40.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sohn WM, Kim HJ, Yong TS, Eom KS, Jeong HG, Kim JK, et al. Echinostoma ilocanum Infection in Oddar Meanchey Province, Cambodia. Korean J Parasitol. 2011;49:187–90. doi: 10.3347/kjp.2011.49.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Miyamoto KMH, Kato-Hayashi N, Chigusa Y. Prevalence of opisthorchiasis in Cambodia. the Proc 80th Annual Meeting of Jpn Soc Parasitol; Tokyo. July 2011.2011. p. 67. [Google Scholar]
- [39].Touch S, Komalamisra C, Radomyos P, Waikagul J. Discovery of Opisthorchis viverrini metacercariae in freshwater fish in southern Cambodia. Acta Trop. 2009;111:108–13. doi: 10.1016/j.actatropica.2009.03.002. [DOI] [PubMed] [Google Scholar]
- [40].Sohn WM, Shin EH, Yong TS, Eom KS, Jeong HG, Sinuon M, et al. Adult Opisthorchis viverrini Flukes in Humans, Takeo, Cambodia. Emerg Infect Dis. 2011;17:1302–4. doi: 10.3201/eid1707.102071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].De NV, Murrell KD, Cong le D, Cam PD, Chau le V, Toan ND, et al. The food-borne trematode zoonoses of Vietnam. Southeast Asian J Trop Med Public Health. 2003;34(Suppl 1):12–34. [PubMed] [Google Scholar]
- [42].MVP/WPRO, The Western Pacific Region. World Health Organization; 2008. WHO Review on the epidemiological profile of helminthiases and their control in the Western Pacific Region, 1997-2008. [Google Scholar]
- [43].Grundy-Warr C, Andrews RH, Sithithaworn P, Petney TN, Sripa B, Laithavewat L, et al. Raw attitudes, wetland cultures, life-cycles: Socio-cultural dynamics relating to Opisthorchis viverrini in the Mekong Basin. Parasitol Int. 2011 doi: 10.1016/j.parint.2011.06.015. (in press) [DOI] [PubMed] [Google Scholar]
- [44].Brockelman WY, Upatham ES, Viyanant V, Ardsungnoen S, Chantanawat R. Field studies on the transmission of the human liver fluke, Opisthorchis viverrini, in northeast Thailand: population changes of the snail intermediate host. Int J Parasitol. 1986;16:545–52. doi: 10.1016/0020-7519(86)90091-3. [DOI] [PubMed] [Google Scholar]
- [45].Ziegler AD, Andrews RH, Grundy-Warr C, Sithithaworn P, Petney TN. Fighting liverflukes with food safety education. Science. 2011;331:282–3. doi: 10.1126/science.331.6015.282-b. [DOI] [PubMed] [Google Scholar]