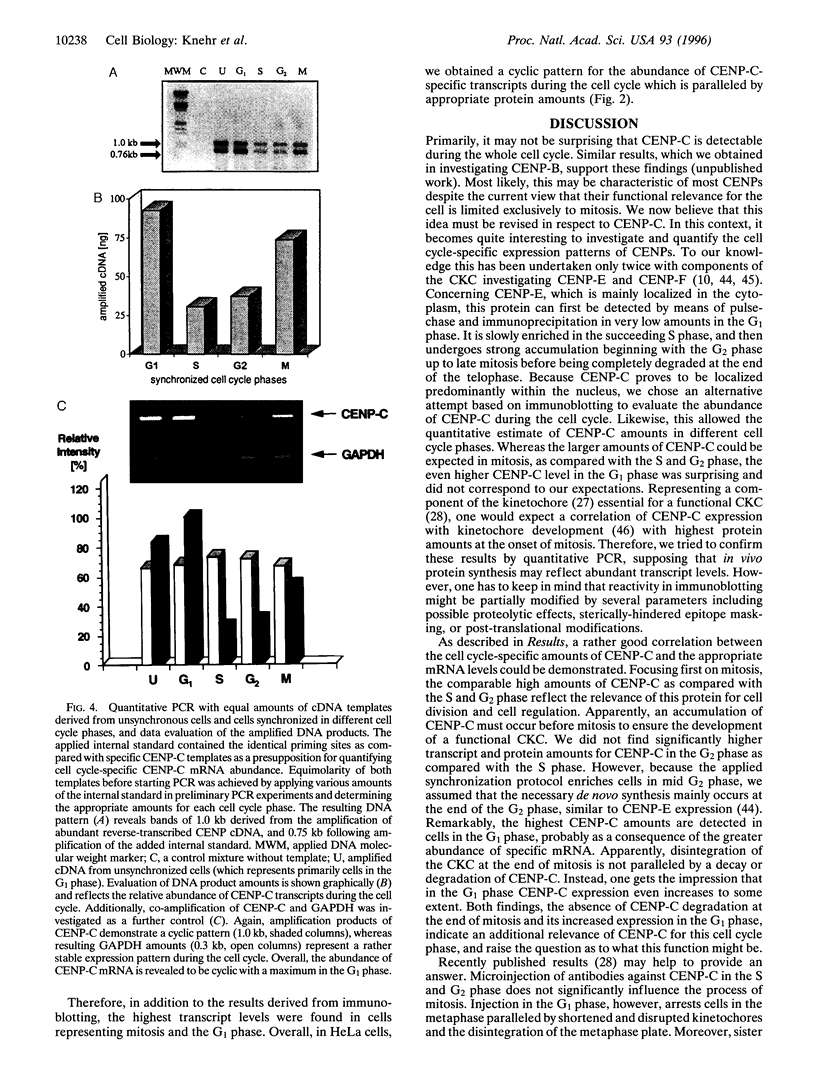
Abstract
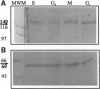
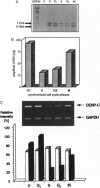
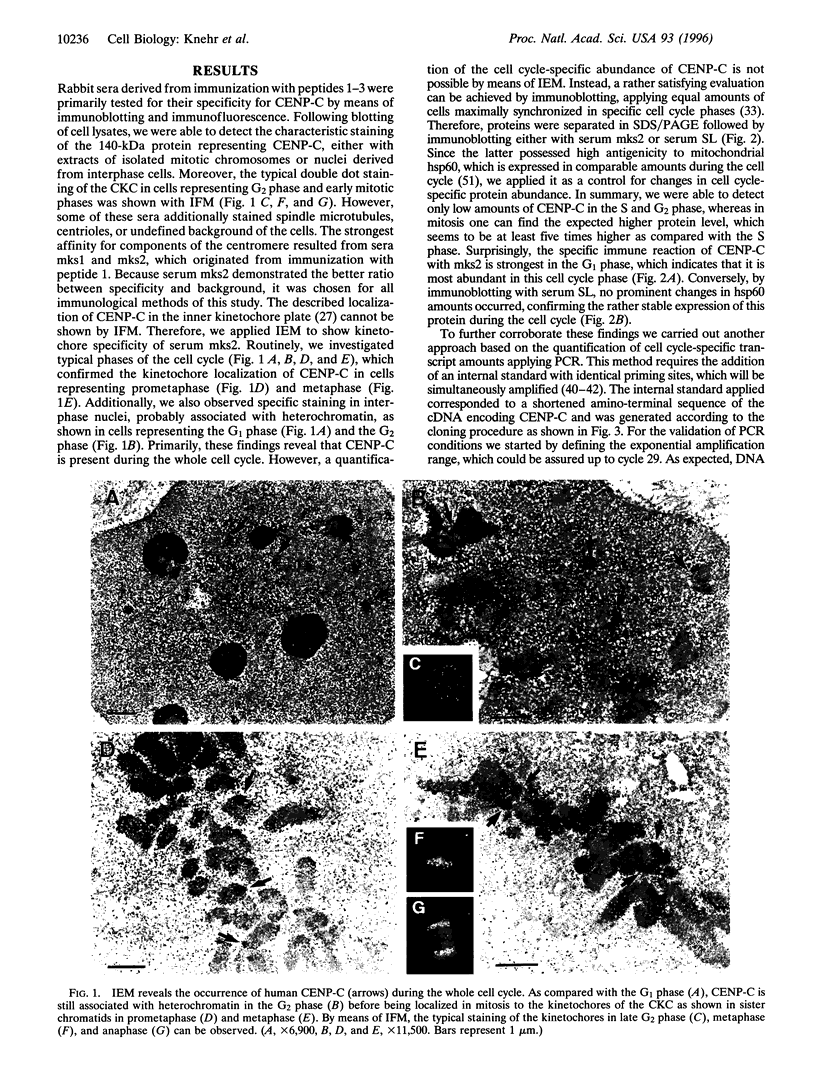
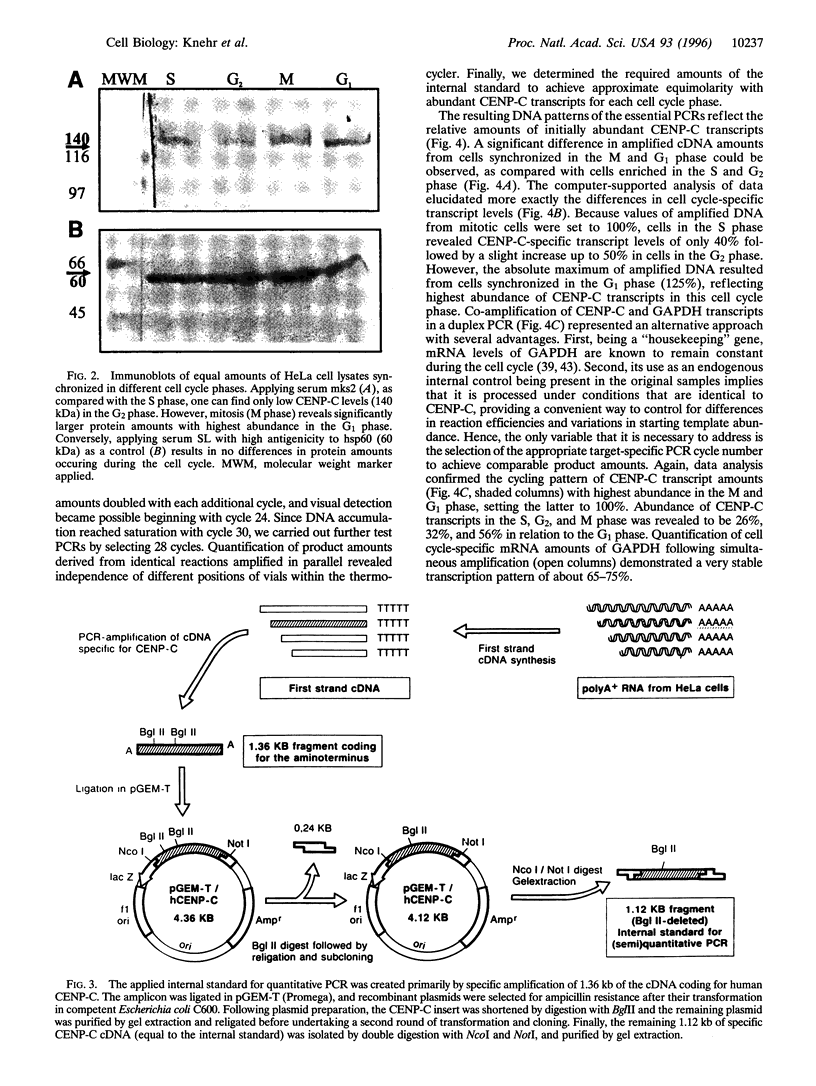
Centromere proteins are localized within the centromere-kinetochore complex, which can be proven by means of immunofluorescence microscopy and immunoelectron microscopy. In consequence, their putative functions seem to be related exclusively to mitosis, namely to the interaction of the chromosomal kinetochores with spindle microtubules. However, electron microscopy using immune sera enriched with specific antibodies against human centromere protein C (CENP-C) showed that it occurs not only in mitosis but during the whole cell cycle. Therefore, we investigated the cell cycle-specific expression of CENP-C systematically on protein and mRNA levels applying HeLa cells synchronized in all cell cycle phases. Immunoblotting confirmed protein expression during the whole cell cycle and revealed an increase of CENP-C from the S phase through the G2 phase and mitosis to highest abundance in the G1 phase. Since this was rather surprising, we verified it by quantifying phase-specific mRNA levels of CENP-C, paralleled by the amplification of suitable internal standards, using the polymerase chain reaction. The results were in excellent agreement with abundant protein amounts and confirmed the cyclic behavior of CENP-C during the cell cycle. In consequence, we postulate that in addition to its role in mitosis, CENP-C has a further role in the G1 phase that may be related to cell cycle control.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apostolakos M. J., Schuermann W. H., Frampton M. W., Utell M. J., Willey J. C. Measurement of gene expression by multiplex competitive polymerase chain reaction. Anal Biochem. 1993 Sep;213(2):277–284. doi: 10.1006/abio.1993.1421. [DOI] [PubMed] [Google Scholar]
- Bischoff F. R., Maier G., Tilz G., Ponstingl H. A 47-kDa human nuclear protein recognized by antikinetochore autoimmune sera is homologous with the protein encoded by RCC1, a gene implicated in onset of chromosome condensation. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8617–8621. doi: 10.1073/pnas.87.21.8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., Pepper D., Berns M. W., Tan E., Brinkley B. R. Kinetochore structure, duplication, and distribution in mammalian cells: analysis by human autoantibodies from scleroderma patients. J Cell Biol. 1981 Oct;91(1):95–102. doi: 10.1083/jcb.91.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Coulson R. M., Yen T. J., Cleveland D. W. Cyclin-like accumulation and loss of the putative kinetochore motor CENP-E results from coupling continuous synthesis with specific degradation at the end of mitosis. J Cell Biol. 1994 Jun;125(6):1303–1312. doi: 10.1083/jcb.125.6.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai A., Mitchison T. J. A new role for motor proteins as couplers to depolymerizing microtubules. J Cell Biol. 1995 Jan;128(1-2):1–4. doi: 10.1083/jcb.128.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou Q. P., Levin A. H., Zhao S., Pardee A. B. Cyclin E and cyclin A as candidates for the restriction point protein. Cancer Res. 1993 Apr 1;53(7):1493–1497. [PubMed] [Google Scholar]
- Earnshaw W. C., Mackay A. M. Role of nonhistone proteins in the chromosomal events of mitosis. FASEB J. 1994 Sep;8(12):947–956. doi: 10.1096/fasebj.8.12.8088460. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Rothfield N. Identification of a family of human centromere proteins using autoimmune sera from patients with scleroderma. Chromosoma. 1985;91(3-4):313–321. doi: 10.1007/BF00328227. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Sullivan K. F., Machlin P. S., Cooke C. A., Kaiser D. A., Pollard T. D., Rothfield N. F., Cleveland D. W. Molecular cloning of cDNA for CENP-B, the major human centromere autoantigen. J Cell Biol. 1987 Apr;104(4):817–829. doi: 10.1083/jcb.104.4.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbsky G. J. Kinetochores, microtubules and the metaphase checkpoint. Trends Cell Biol. 1995 Apr;5(4):143–148. doi: 10.1016/s0962-8924(00)88968-0. [DOI] [PubMed] [Google Scholar]
- Gorbsky G. J., Ricketts W. A. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J Cell Biol. 1993 Sep;122(6):1311–1321. doi: 10.1083/jcb.122.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jameson B. A., Wolf H. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput Appl Biosci. 1988 Mar;4(1):181–186. doi: 10.1093/bioinformatics/4.1.181. [DOI] [PubMed] [Google Scholar]
- Kitagawa K., Masumoto H., Ikeda M., Okazaki T. Analysis of protein-DNA and protein-protein interactions of centromere protein B (CENP-B) and properties of the DNA-CENP-B complex in the cell cycle. Mol Cell Biol. 1995 Mar;15(3):1602–1612. doi: 10.1128/mcb.15.3.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knehr M., Poppe M., Enulescu M., Eickelbaum W., Stoehr M., Schroeter D., Paweletz N. A critical appraisal of synchronization methods applied to achieve maximal enrichment of HeLa cells in specific cell cycle phases. Exp Cell Res. 1995 Apr;217(2):546–553. doi: 10.1006/excr.1995.1121. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li X., Nicklas R. B. Mitotic forces control a cell-cycle checkpoint. Nature. 1995 Feb 16;373(6515):630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- Liao H., Li G., Yen T. J. Mitotic regulation of microtubule cross-linking activity of CENP-E kinetochore protein. Science. 1994 Jul 15;265(5170):394–398. doi: 10.1126/science.8023161. [DOI] [PubMed] [Google Scholar]
- Liao H., Winkfein R. J., Mack G., Rattner J. B., Yen T. J. CENP-F is a protein of the nuclear matrix that assembles onto kinetochores at late G2 and is rapidly degraded after mitosis. J Cell Biol. 1995 Aug;130(3):507–518. doi: 10.1083/jcb.130.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombillo V. A., Nislow C., Yen T. J., Gelfand V. I., McIntosh J. R. Antibodies to the kinesin motor domain and CENP-E inhibit microtubule depolymerization-dependent motion of chromosomes in vitro. J Cell Biol. 1995 Jan;128(1-2):107–115. doi: 10.1083/jcb.128.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto H., Masukata H., Muro Y., Nozaki N., Okazaki T. A human centromere antigen (CENP-B) interacts with a short specific sequence in alphoid DNA, a human centromeric satellite. J Cell Biol. 1989 Nov;109(5):1963–1973. doi: 10.1083/jcb.109.5.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay S., Thomson E., Cooke H. Sequence homologies and linkage group conservation of the human and mouse Cenpc genes. Genomics. 1994 Jul 1;22(1):36–40. doi: 10.1006/geno.1994.1342. [DOI] [PubMed] [Google Scholar]
- Mitchison T. J., Kirschner M. W. Properties of the kinetochore in vitro. II. Microtubule capture and ATP-dependent translocation. J Cell Biol. 1985 Sep;101(3):766–777. doi: 10.1083/jcb.101.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroi Y., Peebles C., Fritzler M. J., Steigerwald J., Tan E. M. Autoantibody to centromere (kinetochore) in scleroderma sera. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1627–1631. doi: 10.1073/pnas.77.3.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy L. D., Herzog C. E., Rudick J. B., Fojo A. T., Bates S. E. Use of the polymerase chain reaction in the quantitation of mdr-1 gene expression. Biochemistry. 1990 Nov 13;29(45):10351–10356. doi: 10.1021/bi00497a009. [DOI] [PubMed] [Google Scholar]
- Nicklas R. B. The motor for poleward chromosome movement in anaphase is in or near the kinetochore. J Cell Biol. 1989 Nov;109(5):2245–2255. doi: 10.1083/jcb.109.5.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer D. K., O'Day K., Trong H. L., Charbonneau H., Margolis R. L. Purification of the centromere-specific protein CENP-A and demonstration that it is a distinctive histone. Proc Natl Acad Sci U S A. 1991 May 1;88(9):3734–3738. doi: 10.1073/pnas.88.9.3734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paweletz N., Finze E. M., Schroeter D., Enulescu M., Knehr M. Immunoelectron microscopic studies on centromere-kinetochore complexes detached from chromosomes. Chromosome Res. 1995 Jun;3(4):235–238. doi: 10.1007/BF00713048. [DOI] [PubMed] [Google Scholar]
- Paweletz N., Schroeter D., Finze E. M. Making first contacts between the spindle and the chromosomes in HeLa cells. Chromosome Res. 1994 Mar;2(2):115–122. doi: 10.1007/BF01553490. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner J. B., Rao A., Fritzler M. J., Valencia D. W., Yen T. J. CENP-F is a .ca 400 kDa kinetochore protein that exhibits a cell-cycle dependent localization. Cell Motil Cytoskeleton. 1993;26(3):214–226. doi: 10.1002/cm.970260305. [DOI] [PubMed] [Google Scholar]
- Rechsteiner M. PEST sequences are signals for rapid intracellular proteolysis. Semin Cell Biol. 1990 Dec;1(6):433–440. [PubMed] [Google Scholar]
- Rieder C. L., Schultz A., Cole R., Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994 Dec;127(5):1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieder C. L. The formation, structure, and composition of the mammalian kinetochore and kinetochore fiber. Int Rev Cytol. 1982;79:1–58. doi: 10.1016/s0074-7696(08)61672-1. [DOI] [PubMed] [Google Scholar]
- Rogers S., Wells R., Rechsteiner M. Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis. Science. 1986 Oct 17;234(4774):364–368. doi: 10.1126/science.2876518. [DOI] [PubMed] [Google Scholar]
- Saitoh H., Tomkiel J., Cooke C. A., Ratrie H., 3rd, Maurer M., Rothfield N. F., Earnshaw W. C. CENP-C, an autoantigen in scleroderma, is a component of the human inner kinetochore plate. Cell. 1992 Jul 10;70(1):115–125. doi: 10.1016/0092-8674(92)90538-n. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Hagishita Y., Himeno M. Functional domain structure of human centromere protein B. Implication of the internal and C-terminal self-association domains in centromeric heterochromatin condensation. J Biol Chem. 1994 Sep 30;269(39):24271–24276. [PubMed] [Google Scholar]
- Sullivan K. F., Hechenberger M., Masri K. Human CENP-A contains a histone H3 related histone fold domain that is required for targeting to the centromere. J Cell Biol. 1994 Nov;127(3):581–592. doi: 10.1083/jcb.127.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Kanaoka Y., Jinno S., Nagata A., Ogiso Y., Shimizu K., Hayakawa T., Nojima H., Okayama H. Cyclin G: a new mammalian cyclin with homology to fission yeast Cig1. Oncogene. 1993 Aug;8(8):2113–2118. [PubMed] [Google Scholar]
- Tomkiel J., Cooke C. A., Saitoh H., Bernat R. L., Earnshaw W. C. CENP-C is required for maintaining proper kinetochore size and for a timely transition to anaphase. J Cell Biol. 1994 May;125(3):531–545. doi: 10.1083/jcb.125.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vig B. K., Paweletz N. Kinetochores, centromeres, spindles and the induction of aneuploidy. Mutat Res. 1988 Oct;201(2):259–269. doi: 10.1016/0027-5107(88)90015-2. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong H., Anderson W. D., Cheng T., Riabowol K. T. Monitoring mRNA expression by polymerase chain reaction: the "primer-dropping" method. Anal Biochem. 1994 Dec;223(2):251–258. doi: 10.1006/abio.1994.1581. [DOI] [PubMed] [Google Scholar]
- Wordeman L., Mitchison T. J. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol. 1995 Jan;128(1-2):95–104. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. J., Compton D. A., Wise D., Zinkowski R. P., Brinkley B. R., Earnshaw W. C., Cleveland D. W. CENP-E, a novel human centromere-associated protein required for progression from metaphase to anaphase. EMBO J. 1991 May;10(5):1245–1254. doi: 10.1002/j.1460-2075.1991.tb08066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T. J., Li G., Schaar B. T., Szilak I., Cleveland D. W. CENP-E is a putative kinetochore motor that accumulates just before mitosis. Nature. 1992 Oct 8;359(6395):536–539. doi: 10.1038/359536a0. [DOI] [PubMed] [Google Scholar]