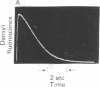
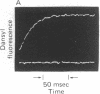
Abstract
Rigorous definition of the elementary steps of an enzymatic reaction requires visualization of transient enzyme—substrate (ES) complexes. Measurement of radiationless energy transfer (RET) between enzyme tryptophan residues and a fluorescent dansyl (5-dimethylaminonaphthalene-1-sulfonyl) substrate provides a sensitive means to observe ES complexes directly. Analysis of the rate of formation and breakdown of ES complexes by RET can serve as the basis of a rapid kinetic approach to enzyme mechanisms. Both pre-steady-state and steady-state kinetics can be performed in the same RET experiment. Analysis at steady state precisely determines kcat and Km values by multiple means. Analysis at pre-steady state determines the number of intermediates, the type of reaction mechanism, and all the individual binding and rate constants. Chymotrypsin was chosen as a standard of reference for RET kinetics because extensive investigations have established both the existence of transient intermediates in the course of its catalytic process and the range of values to be expected for pertinent kinetic constants. As predicted, RET kinetics readily detects the two known intermediates in the α-chymotrypsincatalyzed hydrolysis of specific ester substrates. The results are both qualitatively and quantitatively in accord with data derived for this enzyme from classical kinetics. Hence, this experimental study both validates and demonstrates the theoretical advantages and potential of RET kinetics. The generality of the approach has been investigated by synthesizing a family of dansyl-labeled substrates designed to meet the specificity requirements of a number of metallo- and nonmetallo- exo- and endopeptidases. In all cases, the ES complex is observed readily at micromolar or lower concentrations of enzyme under stopped-flow conditions. The success of the RET kinetic approach on proteolytic enzymes shows its broad utility.
Keywords: fluorescence, proteolytic enzymes, stopped-flow kinetics, metalloenzymes, transient enzyme-substrate complexes
Full text
PDF
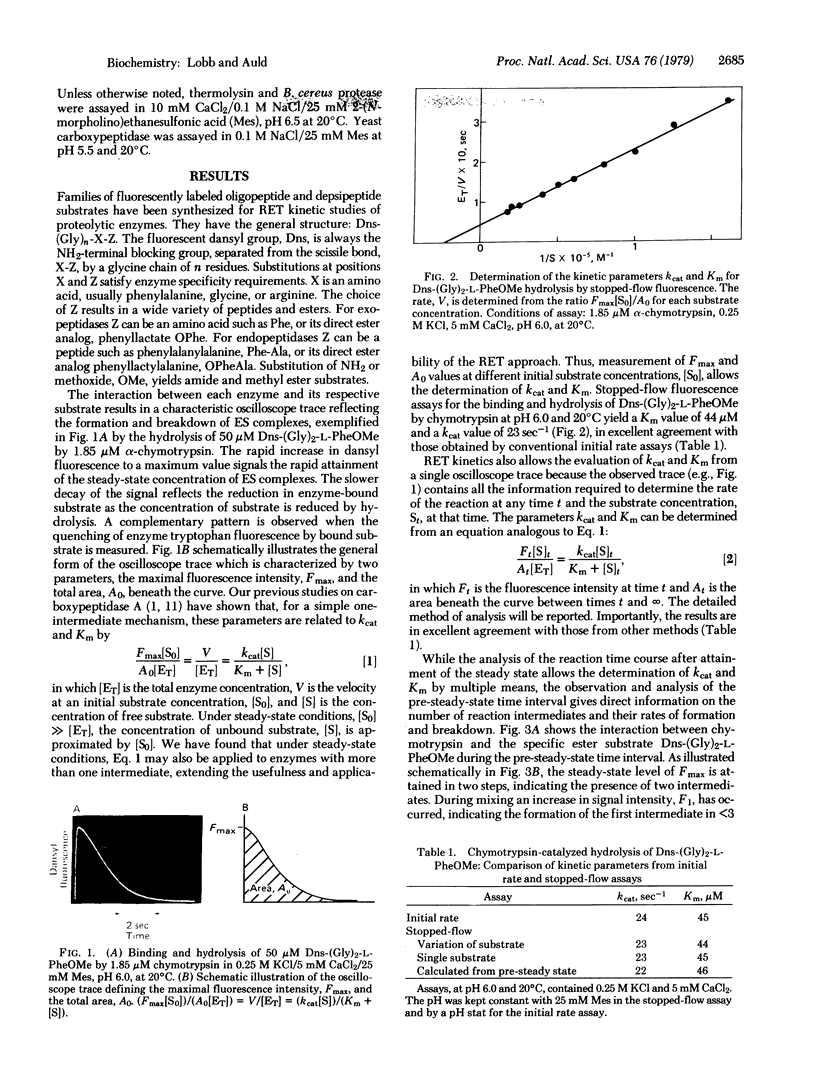
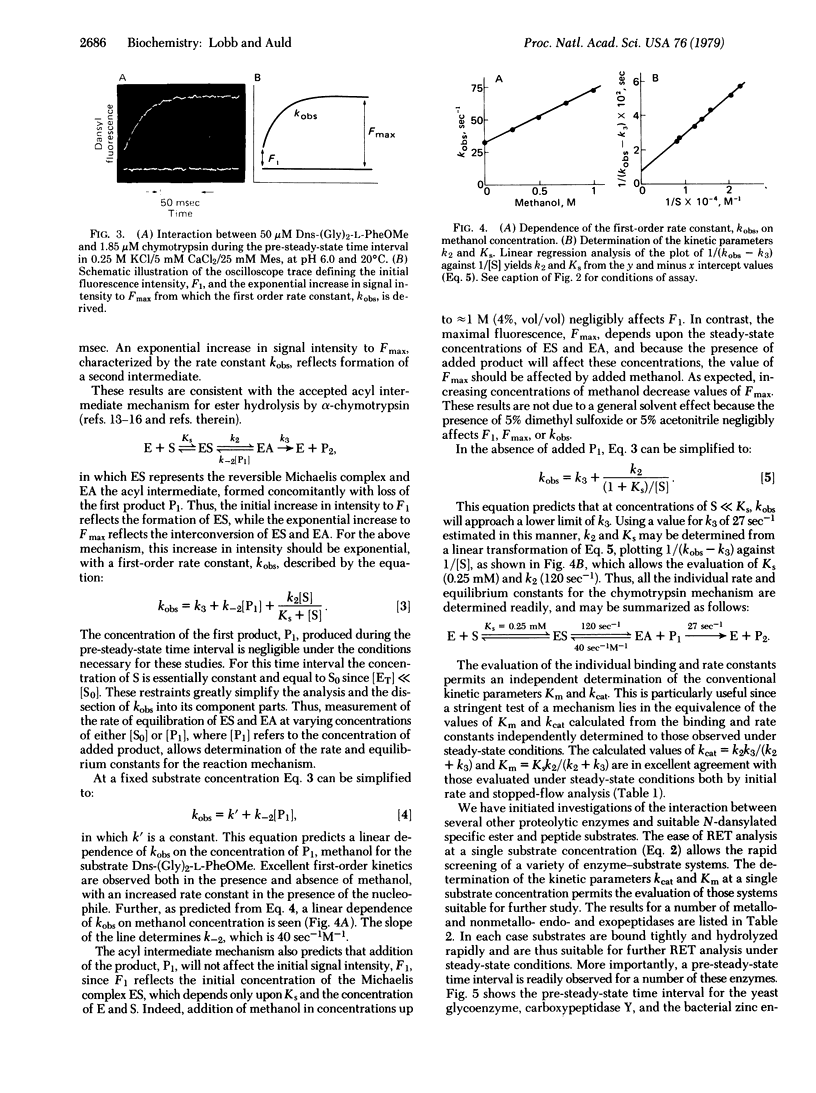
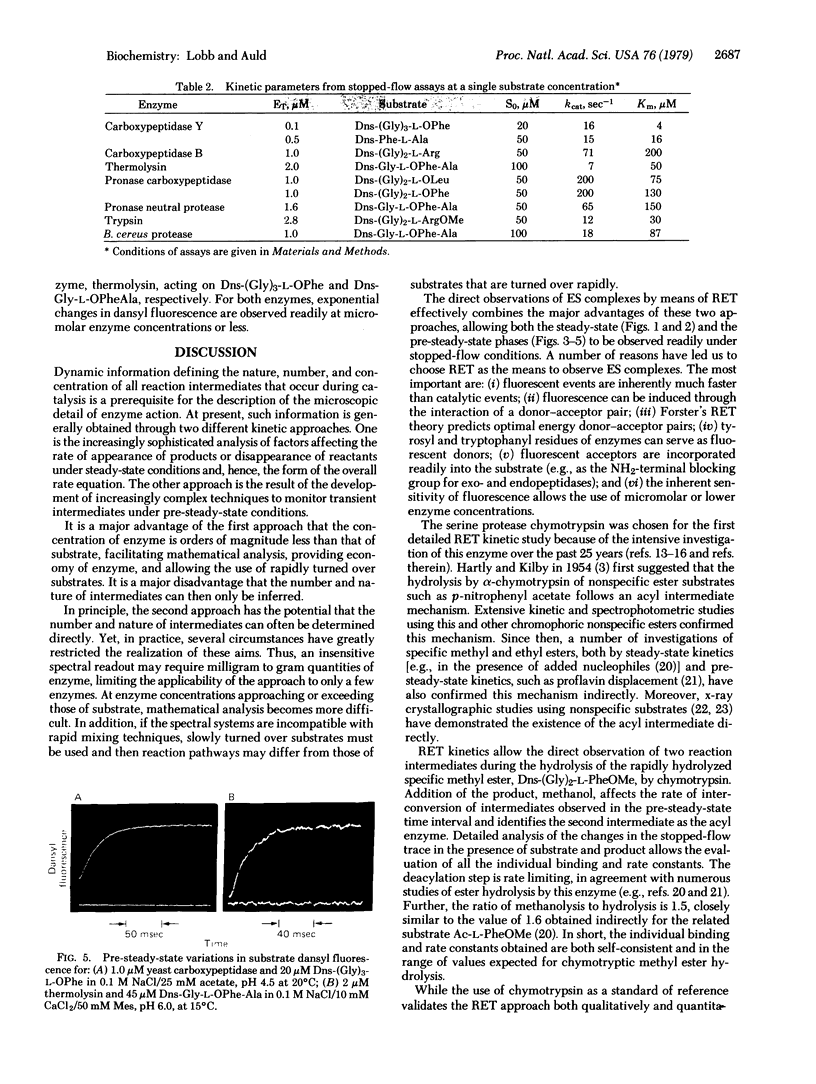

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auld D. S., Holmquist B. Carboxypeptidase A. Differences in the mechanisms of ester and peptide hydrolysis. Biochemistry. 1974 Oct 8;13(21):4355–4361. doi: 10.1021/bi00718a018. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Latt S. A., Vallee B. L. An approach to inhibition kinetics. Measurement of enzyme-substrate complexes by electronic energy transfer. Biochemistry. 1972 Dec 19;11(26):4994–4999. doi: 10.1021/bi00776a019. [DOI] [PubMed] [Google Scholar]
- Auld D. S., Vallee B. L. Kinetics of carboxypeptidase A. II. Inhibitors of the hydrolysis of oligopeptides. Biochemistry. 1970 Feb 3;9(3):602–609. doi: 10.1021/bi00805a022. [DOI] [PubMed] [Google Scholar]
- Bernhard S. A., Gutfreund H. The optical detection of transients in trypsin- and chymotrypsin-catalyzed reactions. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1238–1243. doi: 10.1073/pnas.53.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt K. G., Himoe A., Hess G. P. Investigations of the chymotrypsin-catalyzed hydrolysis of specific substrates. 3. Determination of individual rate constants and enzyme-substrate binding constants for specific amide and ester substrates. J Biol Chem. 1967 Sep 10;242(17):3973–3982. [PubMed] [Google Scholar]
- Fife W. K. Phosphorylation of alkaline phosphatase (E. coli) with o- and p-nitrophenyl phosphate at pH below 6. Biochem Biophys Res Commun. 1967 Aug 7;28(3):309–317. doi: 10.1016/0006-291x(67)90310-5. [DOI] [PubMed] [Google Scholar]
- Gutfreund H., Sturtevant J. M. THE MECHANISM OF CHYMOTRYPSIN-CATALYZED REACTIONS. Proc Natl Acad Sci U S A. 1956 Oct;42(10):719–728. doi: 10.1073/pnas.42.10.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HARTLEY B. S., KILBY B. A. The reaction of p-nitrophenyl esters with chymotrypsin and insulin. Biochem J. 1954 Feb;56(2):288–297. doi: 10.1042/bj0560288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R. Structure of crystalline alpha-chymotrypsin. IV. The structure of indoleacryloyl-alpha-chyotrypsin and its relevance to the hydrolytic mechanism of the enzyme. J Mol Biol. 1970 Dec 14;54(2):341–354. doi: 10.1016/0022-2836(70)90434-1. [DOI] [PubMed] [Google Scholar]
- Hess G. P., McConn J., Ku E., McConkey G. Studies of the activity of chymotrypsin. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):89–104. doi: 10.1098/rstb.1970.0011. [DOI] [PubMed] [Google Scholar]
- Holmquist B. Characterization of the "microprotease" from Bacillus cereus. A zinc neutral endoprotease. Biochemistry. 1977 Oct 18;16(21):4591–4594. doi: 10.1021/bi00640a009. [DOI] [PubMed] [Google Scholar]
- Holmquist B., Vallee B. L. Esterase activity of zinc neutral proteases. Biochemistry. 1976 Jan 13;15(1):101–107. doi: 10.1021/bi00646a016. [DOI] [PubMed] [Google Scholar]
- KEZDY F. J., BENDER M. L. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1962 Nov;1:1097–1106. doi: 10.1021/bi00912a021. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Auld D. S., Valee B. L. Surveyor substrates: energy-transfer gauges of active center topography during catalysis. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1383–1389. doi: 10.1073/pnas.67.3.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latt S. A., Auld D. S., Vallee B. L. Distance measurements at the active site of carboxypeptidase A during catalysis. Biochemistry. 1972 Aug 1;11(16):3015–3022. doi: 10.1021/bi00766a013. [DOI] [PubMed] [Google Scholar]
- Robillard G. T., Powers J. C., Wilcox P. E. A chemical and crystallographic study of carbamyl-chymotrypsin A. Biochemistry. 1972 May 9;11(10):1773–1784. doi: 10.1021/bi00760a007. [DOI] [PubMed] [Google Scholar]