Abstract
Although draft genome sequences of two of the major human schistosomes, Schistosoma japonicum and S. mansoni are available, the structures and characteristics of most genes and the influence of exogenous genes on the metabolism of schistosomes remain uncharacterized. Furthermore, which functional genomics approaches will be tractable for schistosomes are not yet apparent. Here, the vesicular stomatitis virus glycoprotein (VSVG)-pseudotyped pantropic retroviral vector pBABE-puro was modified to incorporate the human telomerase reverse transcriptase gene (hTERT) as a reporter, under the control of the retroviral long terminal repeat (LTR). Pseudotyped virions were employed to transduce S. japonicum to investigate the utility of retrovirus-mediated transgenesis of S. japonicum and the activity of human telomerase reverse transcriptase as a reporter transgene in schistosomes. Schistosomules perfused from experimentally infected rabbits were cultured for six days after exposure to the virions after which genomic DNAs from virus-exposed and control worms were extracted. Analysis of RNA from transduced parasites and immunohistochemistry of thin parasite sections revealed expression of hTERT in the transduced worms. Expression of hTERT was also confirmed by immunoblot analysis. These findings indicated that S. japonicum could be effectively transduced by VSVG pseudotyped retrovirus carrying the hTERT gene. Given the potential of hTERT to aid in derivation of immortalized cells, these findings suggest that this pantropic retroviral approach can be employed to transduce cells from specific tissues and organs of schistosomes to investigate the influence of transgene hTERT on growth and proliferation of schistosome cells.
Keywords: Schistosoma japonicum, glycoprotein of vesicular stomatitis virus (VSVG), pantropic retrovirus, murine leukemia virus, transgene, telomerase
1. Introduction
Schistosomiasis, which is caused mainly by three of the major human schistosomes, Schistosoma mansoni, S. haematobium and S. japonicum, is a debilitating, chronic and widespread disease. It is estimated that as many as 200 million people in 76 countries are infected, and that another 600 million live at risk of infection with schistosomes [1,2]. S. japonicum, a zoonotic species endemic in regions of East Asia including southern China and the Philippines, is considered the most pathogenic of the major human schistosomes.
Establishment of transgenic schistosomes can be expected to facilitate research on the biology, genetics and immunology of schistosomes and to enhance our understanding of the host-parasite relationship [3]. However, derivation of transgenic schistosome lines, as well as immortalized schistosome cell lines, may depend on transduction of an appropriate exogenous gene(s). Although the draft genome sequences for S. japonicum and S. mansoni are now available [4,5], the structure and characteristics of most schistosome genes, the value of their expression products as intervention targets, and the influence of exogenous genes on the metabolism of schistosomes remain to be established.
The development of molecular tools for functional genomics studies in schistosomes is still at an exploratory phase. Utilizing existing research resources and gene sequence information for schistosomes to explore and develop tools for effective transgenesis may be a key to solving many challenges in schistosome pathobiology. Thus far, transgenics technology has been developed for a number of medically and economically important pathogens [6-9] but the approach for studying parasitic helminths is not well advanced, although there are some reports of transgenesis in schistosomes. By utilization of biolistics, Davis et al [10] pioneered schistosome transgenesis; they bombarded adult S. mansoni parasites with mRNA encoding firefly luciferase and subsequently detected the enzyme activity in transformed worms. Thereafter, other investigators employed electroporation [11-13], biolistics (particle bombardment) [14-20] or lipofection [13] to transfer exogenous RNAs or plasmid DNAs into miracidia, sporocysts, schistosomules, adult worms and cultured cells; gene expression could be detected. Usually in these cases, the exogenous genes were episomal in location, and were expressed transiently or for only short terms [11-20]. It is unlikely that plasmid-based transgenes, introduced for example by biolistics or electroporation, would result in transgene-integration into the schistosome genome and produce a heritable transgenic schistosome line [3]. More recently, approaches employing mobile genetic elements including the piggyBac transposon and pseudotyped murine leukemia virus have been described that resulted in the integration of reporter transgenes into the chromosomes of S. mansoni [21-23].
Persistent, stable and heritable expression of transgenes in worms is an important step for the study of long-term transgene model expression, gene function in schistosomes, the exploration of the influence of schistosome gene expression and regulation, investigation of the effect on schistosome cells by exogenous genes, building a transgenic worm line, and developing insertional mutagenesis approaches [3]. The pantropic retroviral vector pseudotyped with vesicular stomatitis virus glycoprotein (VSVG) is widely used to transduce exogenous genes into a broad range of host cell types. As it is able to infect not only mammalian cells but also non-mammalian vertebrate and invertebrate cells [24] which are difficult to infect with amphotropic retroviral vectors, the pantropic vector approach has significant potential as a tool in transgenic research [6,7,24-31]. However, to date, the reporter genes inserted as cargo into retroviral vectors consist of only short sequences, such as genes encoding jellyfish green fluorescent protein (<800 bp) [32-34] or firefly luciferase (<560 bp) [35]. To test whether the pantropic retroviral vector, inserted with a long fragment (3500 bp in length) of a functional and exogenous gene encoding human telomerase reverse transcriptase (hTERT), was able to transduce S. japonicum, with the transgene transcribed and expressed under the control of the viral long terminal repeat (LTR) promoter, a protocol was designed and is described in this study. VSVG pseudotyped, replication-defective retroviral particles were produced in mammalian GP2-293 cells co-transfected with the plasmids pBABE-puro-hTERT and pVSV-G. Thereafter, in vitro cultured schistosomules were transduced with these virions. Active transcription and successful protein expression of the reporter transgenes hTERT and puror were evident within tissues of the schistosomes.
2. Materials and Methods
2.1 Schistosomes, snails, rabbits
A Chinese mainland strain of S. japonicum was used in this study. Schistosome cercariae were shed from Oncomelania hupensis snails provided by the Institute of Parasitic Diseases, Yueyang, Hunan Province, People’s Republic (P.R.) of China. White hybrid rabbits were purchased from the Central South University Animal Unit, Changsha, Hunan Province, P.R. China.
2.2 Cell lines
GP2-293 packaging cells were purchased from BD Biosciences (Clontech, Palo Alto, USA), and cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, Carlsbad, CA, USA) supplemented with 100 units/ml penicillin G, 100 μg/ml streptomycin (Sigma, St. Louis, MO, USA), 4 mM L-glutamine (Amresco, Solon, OH, USA), 1 mM sodium pyruvate (Sigma) and 10% (v/v) fetal bovine serum (FBS, Gibco) at 37 °C under 5% CO2 in air according to the manufacturer’s recommendations. The NIH3T3 mouse fibroblast cell line was kindly provided by the Cell Center of the Central South University, P.R. China, and cultured in DMEM (Gibco) with 4 mM L-glutamine, 1.5 g/L sodium bicarbonate, 4.5 g/L glucose, and 10% bovine calf serum (BCS, Gibco).
2.3 Preparation of schistosomules
S. japonicum schistosomules were recovered by perfusion of mesenteric blood vessels of rabbits twelve days after percutaneous exposure to ~4,000 cercariae. The schistosomules were thoroughly washed three times with RPMI 1640 medium (Gibco) containing 1,000 U/ml penicillin G, 1,000 μg/ml streptomycin (Sigma) and heparin (10 U/ml, Sigma) to remove contaminating host tissues, then cultured in modified Basch’s medium at 37°C in a humidified chamber under 5% CO2 in air [36].
2.4 Constructs - retroviral vector and pVSV-G plasmids
The retroviral vector (plasmid) pBABE-puro-hTERT was kindly provided by Dr. Dongming Zhou, the Wistar Institute, Philadelphia, PA. It was assembled from the pBABE-puro plasmid (Supplementary Figure 1A) (Fig. S1A), which is derived from Moloney murine leukemia virus (MMLV), designed for retroviral gene delivery and expression [37,38]. Based on transfection of viral packaging cell lines including PA317 or GP2-293 cells, the pBABE-puro-hTERT retrovirus vector can transiently express, or integrate and stably express a transcript encoding the viral packaging signal ψ+, the puromycin resistance selection marker (puror) and the reporter gene encoding human telomerase reverse transcriptase (hTERT). The 5′-retrovirus long terminal repeat (LTR) drives expression of the hTERT gene (Fig. S1B), and a puromycin-resistant gene (puror), which allows for the antibiotic selection of transduced eukaryotic cells in puromycin-containing culture media, is expressed from the simian virus 40 immediate early promoter (SV40 IEP) (Fig. S1B) [37-39]. The pVSV-G plasmid (Clontech) expresses the glycoprotein of the vesicular stomatitis virus (VSV-G) under the control of the cytomegalovirus (CMV) immediate-early promoter (Fig. S2). Restriction endonuclease cleavages with Sal I or EcoR I were performed to identify the plasmids and authenticate insert fragments; digestion products were separated by electrophoresis through 1% (w/v) agarose gels containing ethidium bromide, visualized under UV light, and digital images recorded (Upland, Newport, WA , USA).
2.5 Production and titer determination of VSVG-pseudotyped retrovirus
GP2-293 packaging cells modified to express the MMLV gag and pol gene products (Clontech) were transfected with the pBABE-puro-hTERT and pVSV-G plasmids aided by Lipofectamine™ 2000 (Invitrogen, Carlsbad, CA, USA) and chloroquine (25μM) (Sigma). Transfected GP2-293 cells were cultured in DMEM supplemented with 10% (v/v) fetal bovine serum (FBS) (Gibco), 100 units/ml penicillin G, 100 μg/ml streptomycin (Sigma), 4 mM L-Glutamine (Sigma) and 1mM sodium pyruvate (Sigma) at 37 °C under 5% CO2 in air. Culture media were replaced 8 h after transfection to remove the Lipofectamine™ 2000, chloroquine, and residual plasmids, and following a further 36 h culture, supernatants containing the replication incompetent virions were harvested, centrifuged at 500g for 10 min to remove cellular debris and filtered through 0.45μm pore size membranes (Pall Life Sciences, Port Washington, NY, USA). Subsequently, virus-containing supernatants were concentrated by high speed centrifugation (Beckman, Fullerton, CA, USA), 50,000×g, 90 min, 4°C. The pellet of concentrated virions was resuspended to 1% of the original volume in 50 mM Tris, 130 mM NaCl, 1 mM EDTA, pH 7.8 (TNE) at 4°C overnight, after which the virions in TNE were aliquoted, and stored at −80 °C. Functional titers of concentrated virions were determined as described [40].
2.6 Infection with pantropic retrovirus
Both schistosomules recovered from rabbits, and NIH-3T3 cells, were exposed to the concentrated virions in the presence of the cation polybrene (Sigma), which can enhance the effectiveness of retroviral transduction [41]. After washing twice with serum-free RPMI 1640 (Gibco), a total mixture of 200 μl of concentrated retrovirus virions, 1.8 ml modified Basch’s medium [36] and polybrene (8 μg/ml) was added to cultures of 50-100 schistosomules per well in 6-well plates (Corning, Lowell, MA ,USA). Subsequently, the schistosomules were incubated for 3 h at 37 °C, mixed gently by rocking the plate back and forth for 30 min, and then 2 ml modified Basch’s medium was added. Following a further 24 h incubation, media were replaced with 2 ml modified Basch’s medium, after which the schistosomules exposed to virions were cultured for 6 days. During cultivation, media were replaced daily.
Infection of NIH3T3 cells was undertaken as a positive control to determine the infectivity of the virions. Target NIH3T3 cells were plated to a density of 2×105 cells in 6-well plates and cultured in the complete growth medium, as above. When cells reached ~70% confluence, media were removed and the cells washed twice with serum-free DMEM. In the presence of polybrene (8 μg/ml), a mixture of 200 μl concentrated retrovirus and 800 μl serum-free DMEM was added and the cells incubated for 3 h at 37 °C, after which 2 ml complete medium was added. The media were replaced 24 h later with 2 ml conditioned medium supplemented with puromycin (3 μg/ml). After 10-12 days of puromycin selection, puromycin-resistant clones were obtained, and one of these was randomly selected to culture for up to three subsequent generations in conditioned media containing puromycin (3μg/ml). Negative controls included schistosomules and NIH3T3 cells, not exposed to virions, cultured in modified Basch’s medium and complete growth medium, respectively.
2.7 PCR based detection of reporter hTERT and puror genes in schistosomules and NIH3T3 cells
Transduced and negative control schistosomules were harvested after 6-day cultivation, and washed three times with centrifugation, 800g ×2 min, in ice cold PBS to remove unbound virus. At the time of harvest of the cultured schistosomules, an aliquot of culture media was also collected for analysis to investigate the presence of residual, contaminating pBABE-puro-hTERT plasmids carried over in virion inocula. Puromycin-resistant and negative control NIH3T3 cells after passage for three generations were trypsinized and collected, washed three times in ice cold PBS (500g ×5 min). Total genomic DNAs (gDNAs) were extracted from schistosomules and NIH3T3 cells using the illustra tissue and cells genomicPrep Mini Spin Kit (GE Healthcare, Piscataway, NJ, USA). Primers specific for the hTERT gene, forward-CGGAAGAGTGTCT GGAGCAA and reverse-GGATGAAGCGGAGTCTGGA, and primers for the puror gene, which were designed by Primer 3 (v.0.4.0) program (http:// frodo.wi.mit.edu/) according to the coding sequences of the puror gene (GenBank accession DQ322642), forward-GTCACCGAGCTGCA AGAACT and reverse-CAGGAGGCCTTCCATCTGT, were employed to amplify proviral transgenes. PCRs were carried out using Go Taq Green Master Mix (Promega, Madison, WI, USA), and amplification for the hTERT gene was one cycle at 94 °C for 5 min, 30 cycles at 94 °C, 30s, 58 °C, 45s, 72 °C, 40s, followed by a 5-min extension at 72 °C, as well as 30 thermal cycles for the puror gene of 94 °C for 30s, 61.7 °C for 45s, and 72 °C for 40s. Negative controls included DNAs isolated from schistosomules and NIH3T3 cells not exposed to virions. The pBABE-puro-hTERT plasmid was employed as the positive control template. PCR products were separated by electrophoresis through 2% (w/v) agarose gels, stained with ethidium bromide, visualized under UV illumination and digital images captured (UpLand). Predicted sizes for PCR products from the hTERT and puror genes were 145 bp [42] and 204 bp, respectively.
2.8 Reverse transcription-polymerase chain reaction (RT-PCR)
Transduced and non-transduced control schistosomules were obtained, as above. Total RNA was isolated from the worms using Trizol Reagent (Gibco-BRL) according to the manufacturer’s protocol, and the RNA incubated with RNase-free DNase I in the presence of Ribonuclease inhibitor (Fermentas, Burlington, Canada) to remove any residual, contaminating genomic DNA (gDNA). cDNA synthesis was accomplished using 5 μg DNase I-treated RNA, 1 μl random primer, 2 μl dNTPs, and 200 U M-MuLV Reverse Transcriptase using the First Strand cDNA Synthesis Kit (Fermentas), and the hTERT and puror genes amplified using the primers described above. Simultaneously, the control S. japonicum housekeeping gene α-tubulin was amplified using the primers 5′-CTGGAGTTCAAATGGGCAAT-3′ and 5′-TACCTCCACCGAA AGAATGG-3′; this produced a 399 bp amplicon [43], and this was taken as an internal control to confirm the integrity of the schistosomule RNAs and the presence of amplified cDNAs in all samples. Thermal cycling conditions involved denaturation at 94 °C for 5 min followed by 25 cycles of amplification at 94 °C for 30 s, 55°C for 45 s, 72 °C for 40 s, and a final extension at 72 °C for 5 min. Amplification products were separated by electrophoresis through 2% (w/v) agarose containing ethidium bromide, visualized under UV illumination, and digital images recorded. Negative controls included cDNA from non-virus exposed schistosomes, RNA, which was not reverse transcribed into cDNA, from virus-exposed schistosomes and reactions in which water replaced the template nucleic acids. The plasmid pBABE-puro-hTERT plasmid was included as a positive control.
2.9 In situ staining of hTERT protein in schistosomes
To confirm the expression of hTERT protein in S. japonicum and NIH3T3 cells transduced by pBABE-puro-hTERT retrovirus, immunohistochemical staining was performed. Transduced and control schistosomules were harvested 6 days following exposure to virions, rinsed and fixed with 4% (v/v) paraformaldehyde (Sigma) for 3-5 h at room temperature. Following rinsing in PBS, worms were embedded with 3% (w/v) agarose and dehydrated through an ethanol series. Subsequently, agarose-embedded worms were embedded with paraffin, cut in serial sections of 5 μm, deparaffinized in xylene and rehydrated. In parallel, puromycin-resistant and control NIH3T3 cells were mounted on slides and fixed with 4% (v/v) paraformaldehyde for 3-5 h at room temperature. Both the sections of worms and slides of NIH3T3 cells were immersed in 10 mM citrate (pH 6.0), and incubated at 80 °C for 20 min to retrieve antigenicity. Endogenous peroxidase was quenched with 3% (v/v) hydrogen peroxide (H2O2) and 0.3% (v/v) Triton X-100 in PBS for 10 minutes at 37 °C. After incubation in 10% normal goat serum and 0.3% (v/v) Triton X-100 in PBS for 60 min to block nonspecific immunoglobulin (Ig) binding, the slides were incubated in polyclonal rabbit anti-hTERT serum (Boster Biotechnology, Wuhan, China) at a 1:200 dilution overnight at 4 °C. After rinsing three times in PBS, a SABC detection kit (Boster Biotechnology, Wuhan, China) comprising biotinylated secondary antibody and avidin-conjugated horseradish peroxidase, was employed to detect antibody binding. Following washing in PBS, the slides were incubated with 0.05% (w/v) 3,3-diaminobenzidine (DAB) for 5 min at room temperature, washed with distilled water, counterstained with hematoxylin (Sigma), dehydrated through an ethanol series, and coverslipped. Worm sections were examined microscopically, and schistosomes and schistosome tissues exhibiting positive, brown colored staining were counted and photographed.
2.10 Immunoblot analysis of reporter hTERT transgene expression
To investigate whether the exogenous hTERT transgene was expressed in schistosomes exposed to the VSVG pseudotyped virions, virus-treated and non-treated control worms were harvested after cultivation for 6 days, as described above. Protein lysates were prepared by ultrasonication (300W, 5 min) in the lysis buffer (50 mM Tris-HCl, pH 8.0; 1 mM EDTA, 2% (w/v) sodium dodecyl sulfate (SDS); 5 mM dithiothreitol (DTT); 10 mM phenylmethyl sulfonylfluoride (PMSF)), and then centrifuged at 10,000g for 10 min. The supernatant was collected as whole-worm lysate for western blot analysis. Protein concentration was determined using the BCA Protein Assay Reagent (Pierce, Rockford, USA). Protein extracts (50 μg/sample) were separated by 10% (w/v) SDS-polyacrylamide gel electrophoresis (SDS-PAGE) and electrotransferred to nitrocellulose (NC, Pall Corporation, USA). The membranes were blocked with 5% (w/v) nonfat milk in PBS with 0.05% (v/v) Tween-20 (PBST) for 2 h at room temperature. Subsequently, membranes were probed with polyclonal rabbit anti-hTERT serum (above) and monoclonal mouse anti-human beta actin (Abcam, San Francisco, CA, USA) as primary antibody overnight at 4°C. After rinsing in PBST, the membranes were incubated with HRP-conjugated goat anti-rabbit IgG (H+L) and goat anti-mouse IgG (H+L) (Santa Cruz, CA, USA) for 1 h at room temperature. Signals were detected with an enhanced chemiluminescence assay (Pierce). Both PA317/hTERT strain cells, which are derived from PA317 cells transfected with pBABE-puro-hTERT plasmid and which stably produce amphotropic retrovirus virions, and a puromycin-resistant NIH3T3 cell strain, following 30 passages after infection with pantropic pBABE-puro-hTERT retrovirus, were included as positive controls.
3. Results
3.1 Identification of infectious VSVG-pseudotyped pantropic retroviral particles
To test the viability of the retroviral particles, which were concentrated from culture supernatants of GP2-293 packaging cell line cotransfected with vector pBABE-puro-hTERT and pVSVG plasmid, target NIH3T3 murine fibroblasts were infected with retroviral particles and the titer of concentrated virions was determined. Following screening, puromycin-resistant clones were obtained and counted (Fig. S3); the titer was 3.2×108 colony forming units (cfu)/ml. Direct PCR analysis showed that two fragments of the predicted sizes of 145 and 204 bp were generated from the gDNAs of puromycin-resistant NIH3T3 cells (Fig. S4A, lane 3; S4B, lane 5), respectively, but not from uninfected control NIH3T3 cells (Fig. S4A, B lanes 4), indicating the presence of heterologous hTERT and puror gene in the genomes of the puromycin-resistant NIH3T3 cells. In addition, immunohistochemical staining revealed intense staining of the hTERT protein, localized to nuclei of puromycin-resistant NIH3T3 cells, demonstrating protein expression of transgene hTERT in transduced NIH3T3 cells (Fig. S5B), although a few control NIH3T3 cells showed faint staining for hTERT in nuclear regions (Fig. S5A), possibly as a consequence of cross-reaction between human and murine telomerase reverse transcriptase [44]. These findings verified that the virion preparation was active and therefore suitable for investigation of retroviral transduction of the schistosomules.
3.2 Detection of puror and hTERT reporter genes in schistosomes
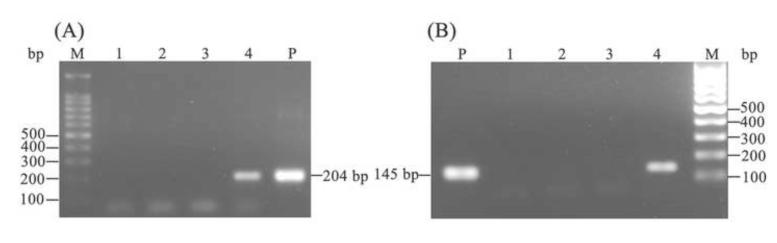
To determine whether VSVG-pseudotyped pantropic retrovirus could transduce S. japonicum and mediate transgene into schistosome cells, cultured schistosomules were exposed to pBABE-puro-hTERT virions. After 6 days, gDNA was extracted from the parasites and targeted by PCR with primers specific for the puror and hTERT genes. Amplicons of the predicted sizes of 204 bp and 145 bp were obtained from virion-treated schistosome gDNAs, respectively; the products stained intensely with ethidium (Fig. 1A, B, lanes 4). By contrast, products of 204 bp and 145 bp were not present in untreated schistosome gDNAs (Fig. 1A, B, lanes 3). The culture supernatants from schistosomules exposed to virions before harvesting also were investigated by PCR for the presence of contaminating pBABE-puro-hTERT plasmids; no puror or hTERT gene specific targets were amplified, indicating the absence of residual plasmid carried over in the virion inoculum (Fig. 1A, B, lanes 2). Based on these data, we infer that attachment, uncoating and entry into schistosome worms of VSVG-pseudotyped pantropic retrovirus can be supported in S. japonicum schistosomules, and that puror and hTERT reporter transgenes were present within the cells of these virus-exposed schistosomules.
Fig. 1. Proviral sequences detected in transduced Schistosoma japonicum by direct PCR.
(A) Identification of the puror transgene in schistosomules transduced with pseudotyped pBABE-puro-hTERT virions; (B) Identification of the hTERT transgene in schistosomules transduced with pseudotyped pBABE-puro-hTERT virions. Lanes: M, molecular size standards; P, positive control, pBABE-puro-hTERT plasmid; 1, negative control (water); 2, culture supernatants from schistosomules exposed to pBABE-puro-hTERT virions; 3, gDNA from non-transduced schistosomules; 4, gDNA from schistosomules exposed to pBABE-puro-hTERT virions.
3.3 Transcription activity for puror and hTERT gene in transduced schistosomes
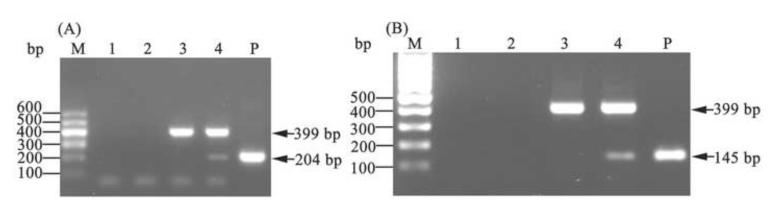
RT-PCR analysis was performed to investigate the transcriptional activity of the puror and hTERT transgenes within the schistosomules exposed to pBABE-puro-hTERT retrovirus. Products of the anticipated sizes of 204 bp and 145 bp, respectively, were amplified from virus-exposed worms (Fig. 2A, B, lanes 4) and the control plasmid pBABE-puro-hTERT (Fig. 2A, B, lanes P), but not from control, non-virus exposed parasites (Fig. 2A, B, lanes 3). The band at 204 bp (Fig. 2A, lane 4) was not intense, suggesting that although transcription of the puror transgene in schistosomules transduced by pBABE-puro-hTERT virions had taken place, the transcription level may not have been as high as observed in mammalian cells. Nonetheless, presence of the 145 bp product (Fig. 2B, lane 4) indicated that the transcription of the hTERT transgene was occurring in schistosomes exposed to pBABE-puro-hTERT virions, and that the transcription level was as high as observed within S. mansoni [22,23]. The product of predicted size of 399 bp of the housekeeping gene α-tubulin was amplified from all the cDNA preparations (Fig. 2A, B, lanes 3, 4), confirming the integrity of the schistosome RNA in all samples. A negative control of water as template yielded no product (Fig. 2A, B, lanes 1). Additional negative control reactions were included, utilizing RNA from virus-exposed schistosomes rather than cDNA as the template, to confirm the absence of gDNAs. No specific band was amplified, indicating that the RNA preparations were free of contaminating DNA (Fig. 2A, B, lanes 2). These findings indicated that the puror and hTERT transgenes in schistosome worm cells could be transcribed under the control of the MMLV LTR and SV40 IEP promoters respectively, although the transcription level of the puror transgene was modest compared to levels reported in mammalian cells [45,46].
Fig. 2. Assessment by RT-PCR of puror and hTERT transgene expression following transduction of Schistosoma japonicum schistosomules by pBABE-puro-hTERT retrovirus.
Total RNA samples were extracted from schistosomules and treated with DNase I. End point PCR was performed using reverse transcribed RNA/cDNA. The α-tubulin gene, amplified as an internal control to verify the integrity of the schistosome RNA and equivalent loadings of cDNAs, produced a 399 bp amplicon of the expected size. (A) Amplification using primers specific for the puror gene; (B) Amplification using primers specific for the hTERT gene. Lanes: M, molecular size markers; 1, water, negative control; 2, RNA not reverse transcribed into cDNA from schistosomules transduced by virions; 3, cDNA from non-virus exposed schistosomules; 4, cDNA from schistosomules transduced by virions; P, positive control, pBABE- puro-hTERT plasmid.
3.4 Human telomerase reverse transcriptase expressed in transduced schistosomules
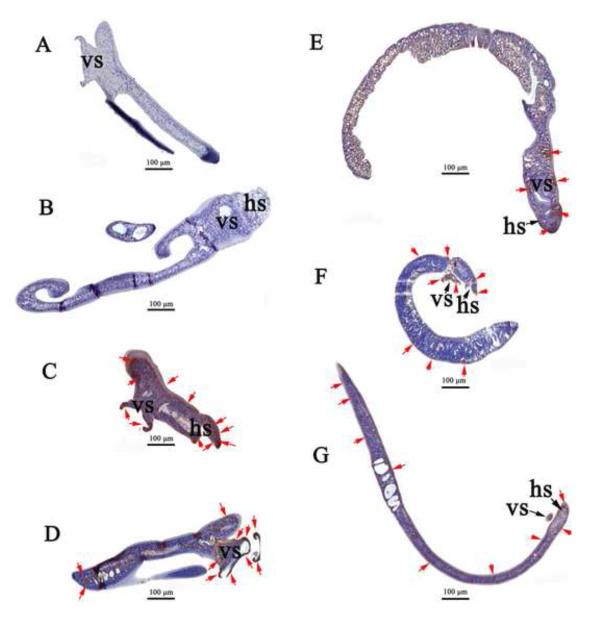
To determine whether the presence of the hTERT gene transcript led to hTERT protein expression, we analyzed the hTERT protein levels in virus-transduced schistosomules and in corresponding controls, using immunohistochemical approaches. The labeling revealed brown staining for the hTERT protein in 44% (15/34) of the worm sections that we examined from parasites exposed to pBABE-puro-hTERT retrovirus. Furthermore, intense staining was located in sub-tegumental regions at the anterior of the worms, especially in the oral (head) sucker (hs) and ventral sucker (vs), as well in tissues as at the posterior of the schistosomules (Fig. 3C, D, E, F, G). By contrast, control non-transduced schistosomules were negative for the hTERT protein (Fig. 3A, B). These findings confirmed that protein expression of the proviral hTERT transgene had taken place in virus-transduced worms, a result consistent with the RT-PCR analysis (Fig. 2).
Fig. 3. Immunohistochemical staining for human telomerase reverse transcriptase in Schistosoma japonicum schistosomules exposed to VSVG-pseudotyped pBABE-puro- hTERT virions.
(A, B) Control, non-virion exposed schistosomules (showing head sucker, ventral sucker and posterior parts of the worms): absence of reaction for hTERT in sub-tegumental regions. (C, D, E, F, G) Schistosomules exposed to virions: intense staining for hTERT mainly located in sub-tegumental regions of the head sucker, ventral sucker and posterior tissues of the parasites as indicated by the arrows (red). Scale bar, 100 μm. hs, head (oral) sucker; vs, ventral sucker.
3.5 Protein expression of hTERT gene in schistosomules
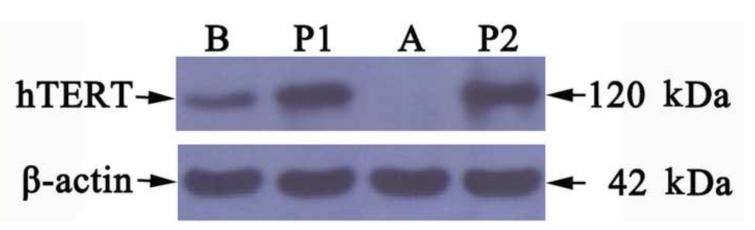
To confirm the expression of hTERT shown by immunohistochemistry (Fig. 3), we undertook an immunoblot analysis of proteins extracted from retrovirus-exposed schistosomules. When membranes were probed with polyclonal rabbit anti-hTERT antibody, a moderately intense band of ~120 kDa was observed in worms exposed to virions (Fig. 4, lane B). By contrast, no signal was seen with proteins from control, non-virus exposed worms (Fig. 4, lane A). The positive controls of PA317/hTERT and puromycin-resistant NIH3T3 cells showed intense bands at ~120 kDa (Fig. 4, lanes P1, P2). As a loading control, a specific band of ~42 kDa (β-actin) was present in all samples, indicating similar quantities in each lane. These results demonstrated that the hTERT transgene was active in the transduced schistosomules, and supported the findings from the RT-PCR and immunohistochemical analysis.
Fig. 4. Western blot analysis for expression of human telomerase reverse transcriptase in virion-transduced Schistosoma japonicum schistosomules.
Lane A, non-transduced worms; B, virus transduced schistosomules; P1, PA317/hTERT cell strain, derived from PA317 cells transfected with the pBABE-puro-hTERT plasmid, and which can stably produce amphotropic retrovirus virions; P2, puromycin-resistant NIH3T3 cell strain following 30 passages after infection with pantropic pBABE-puro-hTERT retrovirus.
4. Discussion
Approaches for transgenesis that involve the pantropic murine leukemia virus (MLV) have provided a tractable and effective means to introduce foreign genes into mammalian cells. With this vector, the coding sequences for the retrovirus structural gene (gag), the reverse transcriptase gene (pol) and envelope protein gene (env) are removed and replaced with exogenous genes under the control of the retroviral long terminal repeat (LTR) or endogenous promoters. Replication-defective retroviral vector particles that can transduce target cells can be produced by transfection into specific retroviral packaging cells, which provide the virus envelope protein, structural protein and reverse transcriptase needed for formation of infectious virions, and retroviral particles can be recovered by collection of culture supernatants from packaging cells. The retroviral provirus genome, along with reporter transgenes, can integrate into the chromosomes of target cells. Subsequently, the integrated transgenes replicate, during mitosis of target cells, resulting in heritable changes in the phenotypes of target cells [39,47,48]. The pantropic retroviral vector is a gene transfer vector based on the Moloney murine leukemia virus, which has been pseudotyped with the envelope glycoprotein of vesicular stomatitis virus (VSVG) [24] substituted for the retroviral envelope protein. VSVG is able to bind to phospholipids on the target cell membrane to meet the requisition of virus for specific protein receptors on the membrane of target cells and mediate virus entry [49,50]. Furthermore, VSVG pseudotyped pantropic retroviral particles are more stable than amphotropic retrovirus, and the titer of virus can be concentrated to 109 CFU/ml after ultracentrifugation. Compared with normal amphotropic retroviral particles, which require binding of virus to specific protein receptors on the membrane of target cells, VSVG pseudotyped retroviral vectors possess a broad host cell range, are termed pantropic retroviruses, and have been widely employed for transgenesis of non-mammalian cells [24]. Pantropic retroviruses have been successively applied to transgenic research on numerous target species of non-mammalian vertebrates or invertebrates, including zebra fish [6], Xenopus [26], newts [25], oysters [27], surf clams [7], shrimp [28,29], mosquitoes [30], Drosophila [51]and amoebae [31].
Kines and co-workers [22,23] reported transduction of S. mansoni sporocysts, juvenile and adult worms using VSVG-pseudotyped retroviral vectors and that both EGFP (enhanced green fluorescence protein, EGFP) and firefly luciferase reporters could be detected in the genome of virus treated worms. These findings also demonstrated integration of transgenes into schistosome chromosomes, and they provided the impetus for this investigation into whether S. japonicum likewise could be transduced with VSVG pseudotyped MMLV virions. Here the MMLV vector pBABE-puro-hTERT, which includes an exogenous hTERT gene, was used as the gene transfer vector. A foreign (non-schistosome) gene hTERT was employed as the reporter or functional transgene under the control of the MLV viral long terminal repeat (LTR) sequence within the construct [37,38], and was expressed in some telomerase-negative cells to recover telomerase activity, extend cellular life span and, further, lead to cellular immortalization. This approach has been used to establish immortalized cell lines from a wide variety of cell types [38,52-54].
Although Kines et al [22,23] transduced several developmental stages of S. mansoni with pantropic retroviral vector containing reporter genes encoding EGFP and luciferase, the reporter genes were relatively short in length (<800 bp and <560 bp, respectively). We wondered whether similar results could be obtained using pantropic retrovirus containing a longer gene cargo. Here schistosomules of S. japonicum were selected as the developmental stage target and transduction carried out using a retroviral vector carrying the hTERT gene. PCR, targeting genomic DNAs isolated from worms cultured for 6 days after exposure to the retrovirus, indicated the presence of the transgenes hTERT and puror within the schistosome cells. Our findings indicated that VSVG pseudotyped retrovirus could adhere to and enter cells of S. japonicum schistosomules and that the successful infection of this schistosome had taken place. Although we have not provided direct evidence of retroviral integration into schistosome genomes, for instance by sequence analysis of integration junctions, preliminary Southern blot analysis (not shown) suggested that genome integration occurred although additional analysis is needed to confirm this phenomenon.
The contact interface between the schistosome and its host is the parasite surface which is the tegument composed of a syncytium of fused cells surrounding the entire worm with a single continuous double-bilayer membrane [55]. Consistent with other eukaryotic species, the lipid structure of the membrane is also composed of phospholipids [55-57]. It seems likely that transduction of S. japonicum schistosomules by pantropic retrovirus is also mediated by interaction between the VSVG envelope protein and phospholipid components of the worm surface [49,50].
Our analysis of RNA showed that there were transcripts of hTERT and puror in the transduced S. japonicum schistosomules. Immunohistochemical staining indicated expression of hTERT in regions below the tegument of the virus-exposed worms. Furthermore, hTERT protein expression was confirmed by immunoblot analysis. These findings demonstrated that there was mRNA transcription of the transgenes hTERT and puror under the control of the MMLV LTR or SV40 IEP promoter of Simian virus, protein expression of the hTERT gene in S. japonicum schistosomules, and that transgene silencing was not apparent. (Whether the transgenes in the schistosome cells are expressed continuously will be a key point in future studies. In some contexts, MMLV transgenes are silenced after integration into host chromosomes [58,59].) It has been widely reported that the LTR promoter of MMLV can drive strong transgene expression in diverse invertebrate species [7,22,23,28,30,31] and that the SV40 IEP promoter mediates high levels of expression in mammalian cells [45,46] and in the filarial nematode Litomosoides sigmodontis [60]. However, transcription levels from the SV40 IEP promoter were apparently not strong in these S. japonicum schistosomules. Intense expression of hTERT was localized to sub-tegumental sites at the anterior of the transduced worms, especially in the oral and ventral suckers, as well as at the posterior of the worms.
Dirks and Miller [61] reported that when VSVG pseudotyped MMLV virus infected cell lines of non-mammalian origin, including cells of both vertebrate and invertebrate species, there was frequent post-entry blocking whereby all or most viruses failed to complete reverse transcription and/or integration. They speculated that the possible reason for this phenomenon resulted from evolutionary divergence between mammals and non-mammals in cellular co-factors of requisite developmental steps in the retroviral life cycle. By contrast, Brindley and coworkers did not observe incapacitating blocks within S. mansoni [22,23], confirming that MLV was active, to at least some extent in invertebrates and in platyhelminths in particular. The low transduction efficiency in the current study may relate to adhesion of the virions to the cultured schistosomules or other factors. In any event, we propose to optimize the performance of the virions in future studies, including the use of repeated infection [62,63] and centrifugation infection protocols [64], aiming to enhance transduction efficiency. The retroviral vector also could be modified by introducing a powerful endogenous promoter, such as an actin gene promoter [12,23,65], to enhance the level of transcription and expression of the puror transgene.
This present study demonstrated that VSVG pseudotyped pantropic retroviral vectors can transduce long fragments of the exogenous hTERT gene into tissues of S. japonicum schistosomules and that this reporter transgene can be transcribed and expressed in schistosomes under the control of the MMLV retroviral LTR promoter. In overview, these findings confirmed that VSVG-MLV represents an effective gene transfer vector and tool for studying the functional genomics of schistosomes. We consider that VSVG-MLV will also be potentially useful for large-scale genetic analysis through insertional mutagenesis and for the establishment of lines of transgenic schistosome lines and, indeed, immortalized schistosome cell lines. In future studies, we plan to employ this pantropic retroviral vector containing the hTERT transgene to transduce cells from specific tissues and organs of schistosomes to investigate transgene integration into their chromosomes, the influence of the transgene hTERT on growth and proliferation of schistosome cells, and the vertical transmission and longevity of transgene activity.
Supplementary Material
Research highlights.
▶Schistosoma japonicum could be transduced by VSVG-pseudotyped pantropic retrovirus.
▶Transcription of transgene hTERT was evident within schistosomes post transduction.
▶Protein expression of transgene hTERT was detected in schistosome post infection.
Acknowledgements
We sincerely thank Dr. Dongming Zhou of the Wistar Institute, Philadelphia for the gift of retroviral plasmids, pBABE-puro and pBABE-puro-hTERT, and technical support. We also acknowledge Dr. Robert Weinberg for generously allowing us to use the pBABE-puro-hTERT construct. These studies were supported by Grant No. 30570952 from the Nature Science Foundation of China (NSFC), and in part by award number R01AI072773 (to PJB) from the NIAID-NIH, USA and the National Health and Medical Research Council of Australia (DPM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: a meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365:1561–69. doi: 10.1016/S0140-6736(05)66457-4. [DOI] [PubMed] [Google Scholar]
- [2].Hotez PJ, Brindley PJ, Bethony JM, et al. Helminth infections: the great neglected tropical diseases. J Clin Invest. 2008;118:1311–21. doi: 10.1172/JCI34261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brindley PJ, Pearce EJ. Genetic manipulation of schistosomes. Int J Parasitol. 2007;37:465–73. doi: 10.1016/j.ijpara.2006.12.012. [DOI] [PubMed] [Google Scholar]
- [4].Schistosoma japonicum genome sequencing and functional analysis consortium The Schistosoma japonicum genome reveals features of host-parasite interplay. Nature. 2009;460:345–51. doi: 10.1038/nature08140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Berriman M, Haas BJ, LoVerde PT, et al. The genome of the blood fluke. Nature. 2009;460:352–58. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lin S, Gaiano N, Culp P, et al. Integration and germ-line transmission of a pseudotyped retroviral vector in zebrafish. Science. 1994;265:666–69. doi: 10.1126/science.8036514. [DOI] [PubMed] [Google Scholar]
- [7].Lu JK, Chen TT, Allen SK, et al. Production of transgenic dwarf surfclams, Mulinia lateralis, with pantropic retroviral vectors. Proc Natl Acad Sci USA. 1996;93:3482–86. doi: 10.1073/pnas.93.8.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Jasinskiene N, Coates CJ, Benedict MQ, et al. Stable transformation of the yellow fever mosquito, Aedes aegypti, with the Hermes element from the housefly. Proc Natl Acad Sci USA. 1998;95:3743–47. doi: 10.1073/pnas.95.7.3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Crabb BS, Rug M, Gilberger TW, et al. Transfection of the human malaria parasite Plasmodium falciparum. Methods Mol Biol. 2004;270:263–76. doi: 10.1385/1-59259-793-9:263. [DOI] [PubMed] [Google Scholar]
- [10].Davis RE, Parra A, LoVerde PT, Ribeiro E, Glorioso G, Hodgson S. Transient expression of DNA and RNA in parasitic helminths by using particle bombardment. Proc Natl Acad Sci USA. 1999;96:8687–92. doi: 10.1073/pnas.96.15.8687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Correnti JM, Pearce EJ. Transgene expression in Schistosoma mansoni: introduction of RNA into juvenile adult somules by electroporation. Mol Biochem Parasitol. 2004;137:75–79. doi: 10.1016/j.molbiopara.2004.04.015. [DOI] [PubMed] [Google Scholar]
- [12].Correnti JM, Jung E, Freitas TC, Pearce EJ. Transfection of Schistosoma mansoni by electroporation and the description of a new promoter sequence for transgene expression. Int J Parasitol. 2007;37:1107–15. doi: 10.1016/j.ijpara.2007.02.011. [DOI] [PubMed] [Google Scholar]
- [13].Yuan XS, Shen JL, Wang XL, et al. Schistosoma japonicum: a method for transformation by electroporation. Exp Parasitol. 2005;111:244–49. doi: 10.1016/j.exppara.2005.08.010. [DOI] [PubMed] [Google Scholar]
- [14].Wippersteg V, Kapp K, Kunz W, Jackstadt WP, Zahner H, Grevelding CG. HSP70-controlled GFP expression in transiently transformed schistosomes. Mol Biochem Parasitol. 2002;120:141–50. doi: 10.1016/s0166-6851(01)00446-7. [DOI] [PubMed] [Google Scholar]
- [15].Wippersteg V, Ribeiro F, Liedtke S, Kusel JR, Grevelding CG. The uptake of Texas Red-BSA in the excretory system of schistosomes and its colocalisation with ER60 promoter-induced GFP in transiently transformed adult males. Int J Parasitol. 2003;33:1139–43. doi: 10.1016/s0020-7519(03)00168-1. [DOI] [PubMed] [Google Scholar]
- [16].Wippersteg V, Sajid M, Walshe D, et al. Biolistic transformation of Schistosoma mansoni with 5′ flanking regions of two peptidase genes promotes tissue-specific expression. Int J Parasitol. 2005;35:583–89. doi: 10.1016/j.ijpara.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [17].Heyers O, Walduck AK, Brindley PJ, et al. Schistosoma mansoni miracidia transformed by particle bombardment infect Biomphalaria glabrata snails and develop into transgenic sporocysts. Exp Parasitol. 2003;105:174–78. doi: 10.1016/j.exppara.2003.11.001. [DOI] [PubMed] [Google Scholar]
- [18].Rossi A, Wippersteg V, Klinkert MQ, Grevelding CG. Cloning of 5′ and 3′ flanking regions of the Schistosoma mansoni calcineurin A gene and their characterization in transiently transformed parasites. Mol Biochem Parasitol. 2003;130:133–38. doi: 10.1016/s0166-6851(03)00158-0. [DOI] [PubMed] [Google Scholar]
- [19].Grevelding CG. Transgenic flatworms. In: Maule AG, Marks NJ, editors. Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology. CAB International; Wallingford: 2006. pp. 149–73. [Google Scholar]
- [20].Osman A, Niles EG, Verjovski-Almeida S, LoVerde PT. Schistosoma mansoni TGF-beta receptor II: role in host ligand-induced regulation of a schistosome target gene. PLoS Pathog. 2006;2:e54. doi: 10.1371/journal.ppat.0020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Morales ME, Mann VH, Kines KJ, et al. piggyBac transposon mediated transgenesis of the human blood fluke, Schistosoma mansoni. FASEB J. 2007;21:3479–89. doi: 10.1096/fj.07-8726com. [DOI] [PubMed] [Google Scholar]
- [22].Kines KJ, Mann VH, Morales ME, et al. Transduction of Schistosoma mansoni by vesicular stomatitis virus glycoprotein-pseudotyped Moloney murine leukemia retrovirus. Exp Parasitol. 2006;112:209–20. doi: 10.1016/j.exppara.2006.02.003. [DOI] [PubMed] [Google Scholar]
- [23].Kines KJ, Morales ME, Mann VH, Gobert GN, Brindley PJ. Integration of reporter transgenes into Schistosoma mansoni chromosomes mediated by pseudotyped murine leukemia virus. FASEB J. 2008;22:2936–48. doi: 10.1096/fj.08-108308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Burns JC, Friedmann T, Driever W, Burrascano M, Yee JK. Vesicular stomatitis virus G glycoprotein pseudotyped retroviral vectors; concentration to very high titer and efficient gene transfer into mammalian and non-mammalian cells. Proc Natl Acad Sci USA. 1993;90:8033–37. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Burns JC, Matsubara T, Lozinski G, et al. Pantropic retroviral vector mediated gene transfer, integration, and expression in cultured newt limb cells. Dev Biol. 1994;165:285–89. doi: 10.1006/dbio.1994.1253. [DOI] [PubMed] [Google Scholar]
- [26].Burns JC, McNeill L, Shimizu C, et al. Retroviral gene transfer in Xenopus cell lines and embryos. In Vitro Cell Dev Biol Anim. 1996;32:78–84. doi: 10.1007/BF02723038. [DOI] [PubMed] [Google Scholar]
- [27].Boulo V, Cadoret JP, Shike H, Shimizu C, Miyanohara A, Burns JC. Infection of cultured embryo cells of the pacific oyster, Crassostrea gigas, by pantropic retroviral vectors. In Vitro Cell Dev Biol Anim. 2000;36:395–99. doi: 10.1290/1071-2690(2000)036<0395:IOCECO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [28].Shike H, Shimizu C, Klimpel KS, et al. Expression of foreign genes in primary cultured cells of the blue shrimp Penaeus stylirostris. MAR BIOL. 2000;137:605–11. [Google Scholar]
- [29].Hu GB, Wang D, Wang CH, Yang KF. A novel immortalization vector for the establishment of penaeid shrimp cell lines. In Vitro Cell Dev Biol Anim. 2008;44:51–56. doi: 10.1007/s11626-007-9076-7. [DOI] [PubMed] [Google Scholar]
- [30].Matsubara T, Beeman RW, Shike H, et al. Pantropic retroviral vectors integrate and express in cells of the malaria mosquito, Anopheles gambiae. Proc Natl Acad Sci USA. 1996;93:6181–85. doi: 10.1073/pnas.93.12.6181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Que X, Kim D, Alagon A, et al. Pantropic retroviral vectors mediate gene transfer and expression in Entamoeba histolytica. Mol Biochem Parasitol. 1999;99:237–45. doi: 10.1016/s0166-6851(99)00021-3. [DOI] [PubMed] [Google Scholar]
- [32].Prasher DC, Eckenrode VK, Ward WW, Prendergast FG, Cormier MJ. Primary structure of the Aequorea victoria green-fluorescent protein. Gene. 1992;111:229–33. doi: 10.1016/0378-1119(92)90691-h. [DOI] [PubMed] [Google Scholar]
- [33].Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–5. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- [34].Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- [35].Grentzmann G, Ingram JA, Kelly PJ, Gesteland RF, Atkins JF. A dual-luciferase reporter system for studying recoding signals. RNA. 1998;4:479–86. [PMC free article] [PubMed] [Google Scholar]
- [36].Basch PF. Cultivation of Schistosoma mansoni in vitro. I. Establishment of cultures from cercariae and development until pairing. J Parasitol. 1981;67:179–85. [PubMed] [Google Scholar]
- [37].Morgenstern JP, Land H. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 1990;18:3587–96. doi: 10.1093/nar/18.12.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Counter CM, Meyerson M, Eaton EN, et al. Dissociation among in vitro telomerase activity, telomere maintenance, and cellular immortalization. Proc Natl Acad Sci USA. 1998;95:14723–28. doi: 10.1073/pnas.95.25.14723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Gary L, Buchschacher Introduction to retroviruses and retroviral vectors. Somat Cell Mol Genet. 2001;26:1–11. doi: 10.1023/a:1021014728217. [DOI] [PubMed] [Google Scholar]
- [40].Cepko C. Lineage analysis and immortalization of neural cells via retrovirus vectors. In: Boulton AA, Baker GB, Campagnoni AT, editors. Neuromethods: Molecular Neurobiological Techniques. Vol. 16. Humana Press; Clifton: 1989. pp. 367–92. [Google Scholar]
- [41].Palsson B, Andreadis S. The physico-chemical factors that govern retrovirus- mediated gene transfer. Exp Hematol. 1997;25:94–102. [PubMed] [Google Scholar]
- [42].Ulaner GA, Hu JF, Vu TH, Giudice LC, Hoffman AR. Telomerase activity in human development is regulated by human telomerase reverse transcriptase (hTERT) transcription and by alternate splicing of hTERT transcripts. Cancer Res. 1998;58:4168–72. [PubMed] [Google Scholar]
- [43].Fitzpatrick JM, Johansen MV, Johnston DA, Dunne DW, Hoffmann KF. Gender-associated gene expression in two related strains of Schistosoma japonicum. Mol Biochem Parasitol. 2004;136:191–209. doi: 10.1016/j.molbiopara.2004.03.014. [DOI] [PubMed] [Google Scholar]
- [44].Greenberg RA, Allsopp RC, Chin L, Morin GB, DePinho RA. Expression of mouse telomerase reverse transcriptase during development, differentiation and proliferation. Oncogene. 1998;16:1723–30. doi: 10.1038/sj.onc.1201933. [DOI] [PubMed] [Google Scholar]
- [45].Edwards M, Wong SC, Chotpadiwetkul R, Smirlis D, Phillips R, Shephard EA. Transfection of primary cultures of rat hepatocytes. Methods Mol Biol. 2006;320:273–82. doi: 10.1385/1-59259-998-2:273. [DOI] [PubMed] [Google Scholar]
- [46].Li XW, Lee DK, Chan AS, Alpar HO. Sustained expression in mammalian cells with DNA complexed with chitosan nanoparticles. Biochim Biophys Acta. 2003;1630:7–18. doi: 10.1016/j.bbaexp.2003.08.011. [DOI] [PubMed] [Google Scholar]
- [47].Robbins PD, Ghivizzani SC. Viral vectors for gene therapy. Pharmacol Ther. 1998;80:35–47. [PubMed] [Google Scholar]
- [48].McTaggart S, Al-Rubeai M. Retroviral vectors for human gene delivery. Biotechnol Adv. 2002;20:1–31. doi: 10.1016/s0734-9750(01)00087-8. [DOI] [PubMed] [Google Scholar]
- [49].Mastromarino P, Conti C, Goldoni P, Hauttecoeur B, Orsi N. Characterization of membrane components of the erythrocyte involved in vesicular stomatitis virus attachment and fusion at acidic pH. J Gen Virol. 1987;68:2359–69. doi: 10.1099/0022-1317-68-9-2359. [DOI] [PubMed] [Google Scholar]
- [50].Emi N, Friedmann T, Yee JK. Pseudotype formation of murine leukemia virus with the G protein of vesicular stomatitis virus. J Virol. 1991;65:1202–7. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Teysset L, Burns JC, Shike H, Sullivan BL, Bucheton A, Terzian C. A Moloney murine leukemia virus-based retroviral vector pseudotyped by the insect retroviral gypsy envelope can infect Drosophila cells. J Virol. 1998;72:853–56. doi: 10.1128/jvi.72.1.853-856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Bodnar AG, Ouellette M, Frolkis M, et al. Extension of life-span by introduction of telomerase into normal human cells. Science. 1998;279:349–52. doi: 10.1126/science.279.5349.349. [DOI] [PubMed] [Google Scholar]
- [53].Chiu CP. Telomerase-immortalized hTERT-RPE1 cell line. Clontechniques. 1999;14:2–3. [Google Scholar]
- [54].Hooijberg E, Ruizendaal JJ, Snijders PJ, Kueter EW, Walboomers JM, Spits H. Immortalization of human CD8+ T cell clones by ectropic expression of telomerase reverse transcriptase. J Immunol. 2000;165:4239–45. doi: 10.4049/jimmunol.165.8.4239. [DOI] [PubMed] [Google Scholar]
- [55].McLaren JM, Hockley DJ. Blood flukes have a double outer membrane. Nature. 1977;269:147–49. doi: 10.1038/269147a0. [DOI] [PubMed] [Google Scholar]
- [56].Rogers MV, McLaren DJ. Analysis of total and surface membrane lipids of Schistosoma mansoni. Mol Biochem Parasitol. 1987;22:273–88. doi: 10.1016/0166-6851(87)90058-2. [DOI] [PubMed] [Google Scholar]
- [57].Allan D, Payares G, Evans WH. The phospholipid and fatty acid composition of Schistosoma mansoni and of its purified tegumental membranes. Mol Biochem Parasitol. 1987;23:123–28. doi: 10.1016/0166-6851(87)90147-2. [DOI] [PubMed] [Google Scholar]
- [58].Maksakova IA, Romanish MT, Gagnier L, Dunn CA, van de Lagemaat LN, Mager DL. Retroviral elements and their hosts: insertional mutagenesis in the mouse germ line. PLoS Genet. 2006;2:e2. doi: 10.1371/journal.pgen.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nabekura T, Otsu M, Nagasawa T, Nakauchi H, Onodera M. Potent vaccine therapy with dendritic cells genetically modified by the gene-silencing-resistant retroviral vector GCDNsap. Mol Ther. 2006;13:301–9. doi: 10.1016/j.ymthe.2005.09.021. [DOI] [PubMed] [Google Scholar]
- [60].Jackstadt P, Wilm TP, Zahner H, Hobom G. Transformation of nematodes via ballistic DNA transfer. Mol Biochem Parasitol. 1999;103:261–66. doi: 10.1016/s0166-6851(99)00089-4. [DOI] [PubMed] [Google Scholar]
- [61].Dirks C, Miller AD. Many nonmammalian cells exhibit postentry blocks to transduction by gammaretroviruses pseudotyped with various viral envelopes, including vesicular stomatitis virus G glycoprotein. J Virol. 2001;75:6375–83. doi: 10.1128/JVI.75.14.6375-6383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Guven H, Konstantinidis KV, Alici E, et al. Efficient gene transfer into primary human natural killer cells by retroviral transduction. Exp Hematol. 2005;33:1320–28. doi: 10.1016/j.exphem.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [63].Paya M, Segovia JC, Santiago B, et al. Optimising stable retroviral transduction of primary human synovial fibroblasts. J Virol Methods. 2006;137:95–102. doi: 10.1016/j.jviromet.2006.06.005. [DOI] [PubMed] [Google Scholar]
- [64].Bahnson AB, Dunigan JT, Baysal BE, et al. Centrifugal enhancement of retroviral mediated gene transfer. J Virol Methods. 1995;54:131–43. doi: 10.1016/0166-0934(95)00035-s. [DOI] [PubMed] [Google Scholar]
- [65].Beckmann S, Wippersteg V, El-Bahay A, Hirzmann J, Oliveira G, Grevelding CG. Schistosoma mansoni: Germ-line transformation approaches and actin-promoter analysis. Exp Parasitol. 2007;117:292–303. doi: 10.1016/j.exppara.2007.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.