Abstract
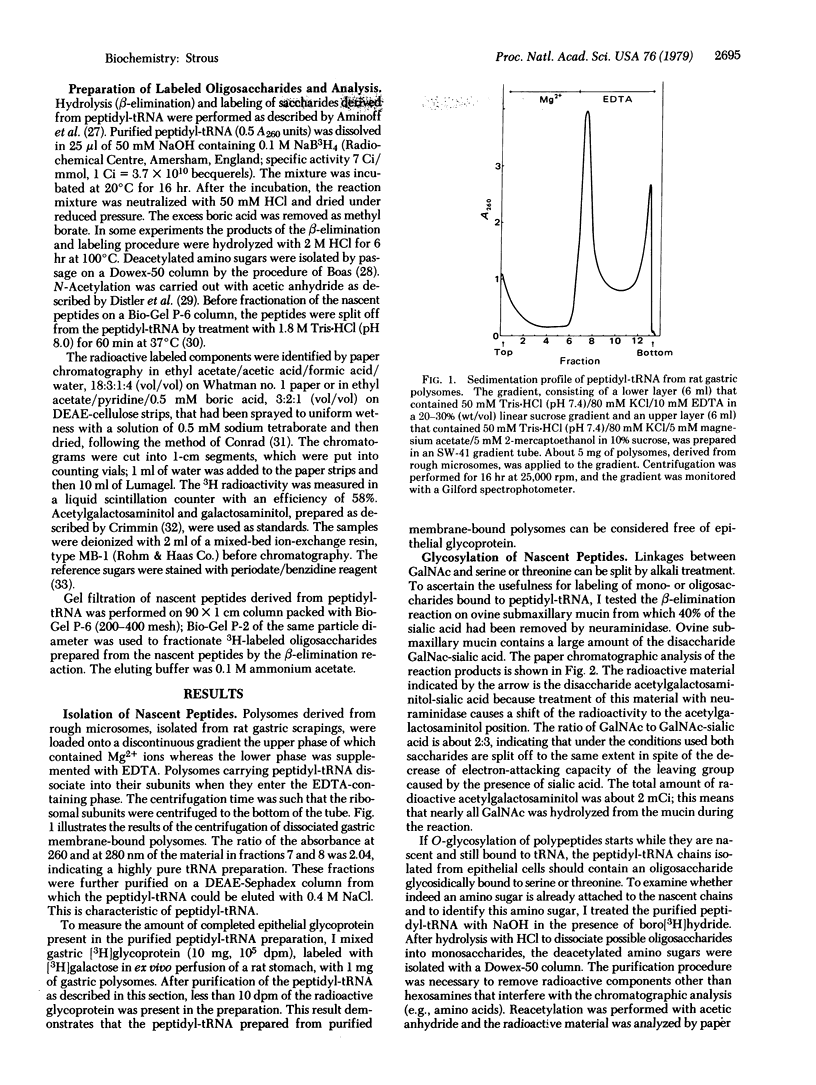
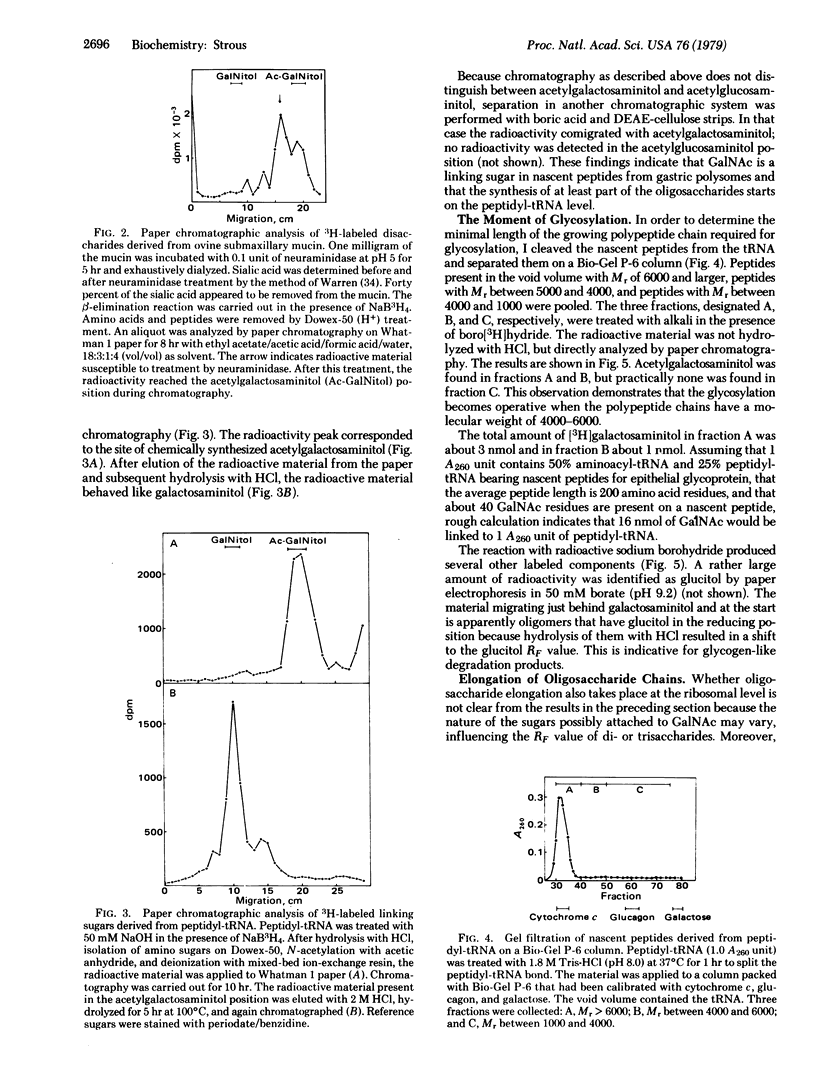
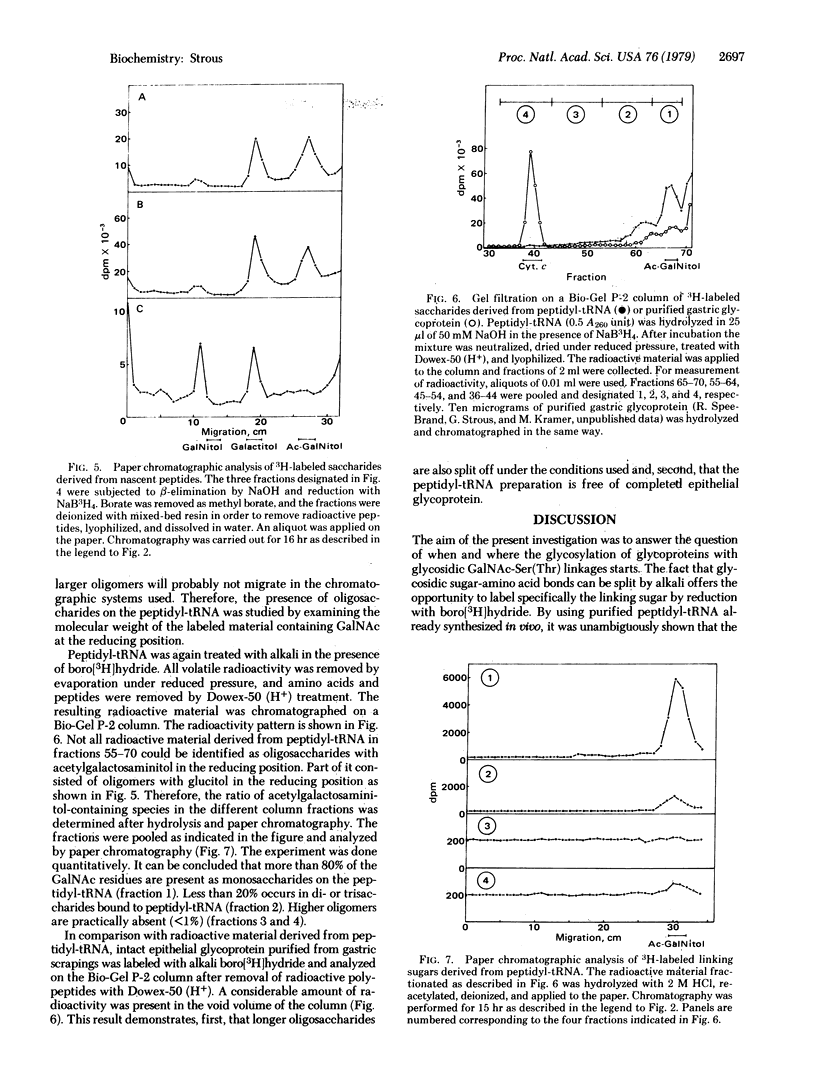
Epithelial glycoprotein like that produced by the gastric surface consists of a polypeptide chain rich in serine and threonine; to these amino acid residues oligosaccharide chains of variable length are linked. The linking sugar is acetylgalactosamine. To find out whether the initial glycosylation takes place at the ribosomal level. I treated purified peptidyl-tRNA, derived from rat gastric membrane-bound polysomes, with alkali in the presence of boro[3H]hydride. Alkali specifically splits glycosidic bonds between serine or threonine and oligosaccharide side chains (beta-elimination reaction). The linking sugar is converted to an alditol and simultaneously labeled. GalNAc was identified as the linking sugar by paper chromatography. Furthermore, nascent peptides with lengths between 40 and 60 amino acid residues already contained this linking sugar. Gel filtration on Bio-Gel P-2 of 3H-labeled saccharides revealed that nascent chains contained mainly monosaccharides, but some di- or trisaccharides were found with GalNAc as the linkage sugar. These findings demonstrate that initial glycosylation of epithelial glycoprotein occurs at the ribosomal level rather shortly after the nascent peptide chain has reached the cisternal lumen of the endoplasmic reticulum.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima T., Spiro M. J., Spiro R. G. Studies on the carbohydrate units of thyroglobulin. Evaluation of their microheterogeneity in the human and calf proteins. J Biol Chem. 1972 Mar 25;247(6):1825–1835. [PubMed] [Google Scholar]
- BOAS N. F. Method for the determination of hexosamines in tissues. J Biol Chem. 1953 Oct;204(2):553–563. [PubMed] [Google Scholar]
- Bahl O. P. Human chorionic gonadotropin. II. Nature of the carbohydrate units. J Biol Chem. 1969 Feb 25;244(4):575–583. [PubMed] [Google Scholar]
- Bergman L. W., Kuehl W. M. Addition of glucosamine and mannose to nascent immunoglobulin heavy chains. Biochemistry. 1977 Oct 4;16(20):4490–4497. doi: 10.1021/bi00639a025. [DOI] [PubMed] [Google Scholar]
- Blobel G., Sabatini D. D. Controlled proteolysis of nascent polypeptides in rat liver cell fractions. I. Location of the polypeptides within ribosomes. J Cell Biol. 1970 Apr;45(1):130–145. doi: 10.1083/jcb.45.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISTLER J. J., MERRICK J. M., ROSEMAN S. Glucosamine metabolism. III. Preparation and N-acetylation of crystalline D-glucosamine- and D-galactosamine-6-phosphoric acids. J Biol Chem. 1958 Jan;230(1):497–509. [PubMed] [Google Scholar]
- Hagopian A., Eylar E. H. Glycoprotein biosynthesis: studies on the receptor specificity of the polypeptidyl: N-acetylgalactosaminyl transferase from bovine submaxillary glands. Arch Biochem Biophys. 1968 Nov;128(2):422–433. doi: 10.1016/0003-9861(68)90048-9. [DOI] [PubMed] [Google Scholar]
- Hagopian A., Eylar E. H. Glycoprotein biosynthesis: the purification and characterization of a polypeptide. N-acetylgalactosaminyl transferase from bovine submaxillary glands. Arch Biochem Biophys. 1969 Feb;129(2):515–524. doi: 10.1016/0003-9861(69)90209-4. [DOI] [PubMed] [Google Scholar]
- Hill H. D., Jr, Schwyzer M., Steinman H. M., Hill R. L. Ovine submaxillary mucin. Primary structure and peptide substrates of UDP-N-acetylgalactosamine:mucin transferase. J Biol Chem. 1977 Jun 10;252(11):3799–3804. [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. S., Perdomo J., Nordberg J. Glycoprortein biosynthesis in small intestinal mucosa. I. A study of glycosyltransferases in microsomal subfractions. J Biol Chem. 1971 Sep 10;246(17):5466–5476. [PubMed] [Google Scholar]
- Lawford G. R., Schachter H. Biosynthesis of glycoprotein by liver. The incorporation in vivo of 14C-glucosamine into protein-bound hexosamine and sialic acid of rat liver subcellular fractions. J Biol Chem. 1966 Nov 25;241(22):5408–5418. [PubMed] [Google Scholar]
- Lingappa V. R., Lingappa J. R., Prasad R., Ebner K. E., Blobel G. Coupled cell-free synthesis, segregation, and core glycosylation of a secretory protein. Proc Natl Acad Sci U S A. 1978 May;75(5):2338–2342. doi: 10.1073/pnas.75.5.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire E. J., Roseman S. Enzymatic synthesis of the protein-hexosamine linkage in sheep submaxillary mucin. J Biol Chem. 1967 Aug 25;242(16):3745–3747. [PubMed] [Google Scholar]
- Pless D. D., Lennarz W. J. Enzymatic conversion of proteins to glycoproteins. Proc Natl Acad Sci U S A. 1977 Jan;74(1):134–138. doi: 10.1073/pnas.74.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Robinson G. B. The role of polyribosomes in the biosynthesis of glycoprotein. Biochem J. 1969 Dec;115(5):1077–1078. doi: 10.1042/bj1151077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman J. E., Lodish H. F. Synchronised transmembrane insertion and glycosylation of a nascent membrane protein. Nature. 1977 Oct 27;269(5631):775–780. doi: 10.1038/269775a0. [DOI] [PubMed] [Google Scholar]
- SARIN P. S., ZAMECNIK P. C. ON THE STABILITY OF AMINOACYL-S-RNA TO NUCLEOPHILIC CATALYSIS. Biochim Biophys Acta. 1964 Dec 16;91:653–655. doi: 10.1016/0926-6550(64)90018-0. [DOI] [PubMed] [Google Scholar]
- Schachter H., McGuire E. J., Roseman S. Sialic acids. 13. A uridine diphosphate D-galactose: mucin galactosyltransferase from porcine submaxillary gland. J Biol Chem. 1971 Sep 10;246(17):5321–5328. [PubMed] [Google Scholar]
- Schenkein I., Uhr J. W. Immunoglobulin synthesis and secretion. I. Biosynthetic studies of the addition of the carbohydrate moieties. J Cell Biol. 1970 Jul;46(1):42–51. doi: 10.1083/jcb.46.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrager J., Oates M. D. The isolation and partial characterization of a glycoprotein isolated from human gastric aspirates and from extracts of gastric mucosae. Biochim Biophys Acta. 1974 Nov 4;372(1):183–195. doi: 10.1016/0304-4165(74)90086-5. [DOI] [PubMed] [Google Scholar]
- Stanley P., Narasimhan S., Siminovitch L., Schachter H. Chinese hamster ovary cells selected for resistance to the cytotoxicity of phytohemagglutinin are deficient in a UDP-N-acetylglucosamine--glycoprotein N-acetylglucosaminyltransferase activity. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3323–3327. doi: 10.1073/pnas.72.9.3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkey B. J., Snary D., Allen A. Characterization of gastric mucoproteins isolated by equilibrium density-gradient centrifugation in caesium chloride. Biochem J. 1974 Sep;141(3):633–639. doi: 10.1042/bj1410633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strous G. J., Berns A. J., Bloemendal H. N-terminal acetylation of the nascent chains of alpha-crystallin. Biochem Biophys Res Commun. 1974 Jun 4;58(3):876–884. doi: 10.1016/s0006-291x(74)80498-5. [DOI] [PubMed] [Google Scholar]
- Strous G. J., Kramer M. F. Glycoprotein synthesis in gastric epithelial cells of the rat. Properties of microsomal glycoprotein glycosyltransferases. Biochim Biophys Acta. 1976 Nov 18;451(1):201–211. doi: 10.1016/0304-4165(76)90271-3. [DOI] [PubMed] [Google Scholar]
- Thomas D. B., Winzler R. J. Structure of glycoproteins of human erythrocytes. Alkali-stable oligosaccharides. Biochem J. 1971 Aug;124(1):55–59. doi: 10.1042/bj1240055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Waechter C. J., Lennarz W. J. The role of polyprenol-linked sugars in glycoprotein synthesis. Annu Rev Biochem. 1976;45:95–112. doi: 10.1146/annurev.bi.45.070176.000523. [DOI] [PubMed] [Google Scholar]


