Abstract
The goals of this study were to investigate the effects of hypoxia on cochlear hair cell damage, and to explore the role of sirtuin1 in hypoxia-induced hair cell damage. Cochlear organotypic cultures from postnatal day 4 rats were used in this study. Hypoxia was induced by treating cochlear explants with CoCl2. Cochlear cultures were treated with CoCl2 alone or in combination with the sirtuin1 activator resveratrol and the sirtuin1 inhibitor sirtinol. Hair cell damage was identified by phalloidin staining and imaged using scanning electron microscopy. RT-PCR and Western blot analyses were used to detect the expression of sirtuin1 and acetylated nuclear factor-κB (NF-κB). Low concentrations of CoCl2 (100–200 μM) did not cause an obvious change in the number and morphology of hair cells, whereas higher concentrations of CoCl2 (300–400 μM) induced swelling of hair cells, accompanied by cell loss. Increased sirtuin1 expression was induced by CoCl2 at 100 to 200 μM, but not at 400 μM. NF-κB acetylation was significantly increased in explants treated with 400 μM CoCl2. Pretreatment with resveratrol prevented CoCl2-induced hair cell loss and acetylation of NF-κB. The protective effect of resveratrol was significantly reduced by sirtinol. CoCl2 induces hair cell damage in organotypic cochleae cultures. Resveratrol attenuates CoCl2-induced cochlear hair cell damage possibly via activation of sirtuin1, which deacetylates NF-κB.
Introduction
Neonatal hypoxic ischemic encephalopathy, a common disease in perinatal neonates, can cause various neurological sequelae, including epilepsy, cerebral palsy, visual impairment, and mental retardation. Sensorineural hearing loss is a severe complication that is associated with perinatal hypoxia and ischemia [1,2]. Jiang et al. reported that 16.2% of infants with perinatal hypoxia-ischemia also presented with impaired cochlear hair cell function [3]. Ischemia and hypoxia in the inner ear contribute to sudden deafness, acute acoustic trauma, and presbyacusis [4–6].
Cochlear tissues are very sensitive to hypoxia. Complex action potentials and endocochlear potentials have been shown to be reduced after transient asphyxia for 45 s in bilateral adrenalectomized rats [7]. Hearing thresholds were elevated in animal models of hypoxic-ischemic encephalopathy, accompanied by apoptosis in the organ of Corti, spiral ganglion cells, and brainstem neurons [8]. Hair cell loss and neuronal apoptosis have been found in cochlear organotypic cultures in in vitro models of hypoxia and ischemia [9–11]. Mitochondrial cristae in cochlear inner hair cells (IHCs) disappeared in rats weaned from mechanical ventilation for 10 min. Huge vacuoles occurred in the IHCs, accompanied by swollen cell bodies after hypoxia for 30 min. Most IHCs exhibited ruptured cell membranes with a leakage of intracellular contents, accompanied by vacuoles in IHCs after hypoxia for 60 min [12].
The mechanism of hypoxia-induced IHC damage remains unclear. Hypoxia may induce hair cell loss by increasing intracellular Ca2+ levels due to disruption of ATP-dependent Ca2+ regulatory mechanisms [13]. Hypoxia can also induce the expression and secretion of proinflammatory cytokines in explanted cochlear tissues [14], suggesting that inflammation may contribute to hypoxia-induced hair cell loss.
Silence information regulation 1 (Sirt1), a NAD-dependent histone deacetylase, is extensively expressed in the cytoplasm and nucleus. Sirtuin1 plays an important role in cellular transcription and metabolism, and it has anti-apoptotic and anti-aging properties. Sirtuin1 overexpression was found to protect neurons from oxidative damage and inhibit neurodegeneration by promoting the transcriptional activity of PGC-1α and increasing mitochondrial density [15]. Sirtuin1 was also found to inhibit cell apoptosis by regulating many non-histone substrates, such as FOXO, tumor necrosis factor (TNF)-α, nuclear factor-κB (NF-κB), and p53 [16,17]. Sirtuin1 binds to the RelA/p65 subunit of NF-κB and inhibits acetylation of RelA/p65 at lysine 310, thereby decreasing NF-kB activation [18,19]. Thus, regulation of p65 acetylation by sirtuin1 may be a potential target for the treatment of inflammatory injury.
The role of sirtuin1 and acetylation of NF-κB p65 in hypoxia-induced cochlea injury remain unclear. In this study, we investigated hypoxia-induced hair cell damage in cochlear organotypic cultures from postnatal day 4 (P4) rats, using a CoCl2-induced hypoxic model. The aims of this study were to investigate the effects of hypoxia on the cochlear hair cell damage, and to explore the role of sirtuin1 in hypoxia-induced hair cell damage.
Materials and Methods
Cochlear organotypic culture
This study was performed in accordance with the institutional recommendations for animal care of Jilin University. The care and use of the animals in this study was approved by the Jilin University Animal Care and Use Committee. All Wistar rats were purchased from the experimental animal center of Basic Medical College of Jilin University. The Institutional Animal Care and Use Committee of Jilin University approved this study.
Wistar rats of postnatal day 4 (P4) were prepared by inhalation anesthesia with 4% halothane. The skull was opened, and the temporal bones were harvested under sterile conditions. Cochleae containing the organs of Corti were removed by dissection of the spiral ligament and internal nerve fibers. The basilar membrane was placed on poly-lysine–coated culture dishes, with care taken to maintain the natural curvature of the cochlea.
Cochleae were cultured in DMEM/F12 culture medium (Invitrogen, USA) with N2 supplements containing 10% fetal bovine serum (FBS, Invitrogen, USA). After 24 h in culture, the cochleae were treated with 100, 200, 300, and 400 μM CoCl2 (dissolved in DMEM/F12 culture medium) for 24 or 48 h. DMEM/F12 culture medium alone was used as a vehicle control. To investigate the effects of resveratrol on CoCl2-induced activation of sirtuin1, cochleae were pretreated with 50 μM resveratrol for 1 h and cultured in DMEM/F12 medium containing 400 μM CoCl2 for 24 h.
Phalloidin staining
To visualize the cellular structure of the cochleae, F-actin was stained with TRITC-labeled phalloidin. Briefly, after culture for 24 h, cochleae were fixed in 10% formaldehyde in PBS overnight at room temperature. Cochleae were then washed with 0.1 M PBS three times, and treated with 0.25% Triton X-100 for 10 min. They were stained with TRITC-labeled phalloidin (1: 200, Sigma-Aldrich, USA) for 30 min. After three washes with PBS, the specimens were counterstained with Hoechst 33342, and viewed using confocal microscopy.
Hair cells were counted in a 160-μm-long section in three different zones located at the apical, middle, and basal turns of each organ of Corti. Three sections of each zone were counted, and the average of the three zones was determined. Hair cell loss was calculated as the percentage of missing hair cells to the total hair cells in each zone.
Scanning electron microscopy
The basilar membrane of each cochlea was placed on a coverslip and cultured in DMEM/F12 culture medium, alone or with 400 μM CoCl2, in a 35-mm culture dish. Samples for scanning electron microscopy (SEM) were prepared as previously described with some modifications [20]. Briefly, after culture, the basilar membrane of cochleae were washed with PBS for 5 min and fixed with 2.5% glutaraldehyde for 24 h. After three 10-min washes with 0.13 M PBS, they were postfixed in 1% OsO4 for 2 h, dehydrated in a graded series of ethanol (50%, 70%, 80%, 90%, and 100% for 10 min each), and then incubated in tert-butoxide for 10 min. Samples were critical-point dried with CO2, coated with gold (Au) using ion sputter coater, and viewed and imaged with a scanning electron microscope.
Real-time quantitative RT-PCR
Total RNA was isolated from cochlear organotypic cultures by using Trizol reagent (Invitrogen, USA) according to manufacturer’s protocol. RNA was reverse-transcribed into complementary DNA (cDNA) using the Quantscript reverse transcription kit (Tiangen, Beijing), according to the manufacturer’s instructions. RT-PCR was performed in a final volume of 20 μl mixture containing 5 μl of cDNA, 5 μl of each primer, and 10 μl of Mater SYBRGreen I mix (Roche, USA). Primers were selected as previously described [8]. Primers were 5’-TGTTTCCTGT GGGATACCTG A-3’ (forward) and 5’- TGAAGAATGG TCTTGGGTCT TT-3’ (reverse) for sirtuin1 (size, 137 bp). β-actin was used as an internal control. The reaction conditions were as follows: 95 °C for 10 min; 94 °C for 30 s, 60 °C for 30 s, and 72 °C for 45 s with 35 cycles; and 72 °C for 7 min. Melting curve analyses were performed to verify the amplification specificity. The gene expression ΔCt values of sirtuini1 mRNAs from each sample were calculated by normalizing with internal control actin. The relative expression of sirtuin1 was calculated using 2-ΔΔCT method [20].
Western blot
After culture for 24 h, the basilar membrane of the cochlea was homogenized on ice in lysis buffer. Lysates were centrifuged at 12,000 rpm at 4 °C for 15 min. Protein concentrations were determined using the BCA method. Proteins (30 μg) were resolved by SDS–PAGE and transferred onto polyvinylidene fluoride membranes by electroblotting. Membranes were incubated with 5% fat-free milk for 2 h, followed by primary antibodies against sirtuin1 (dilution 1:500, Santa Cruz, USA) and acetylated NF-κB (dilution 1:1000, Cell Signaling, USA) at 4 °C, overnight. β-actin was used as a loading control. Membranes were incubated with horseradish peroxidase-linked goat anti-rabbit secondary antibodies (dilution 1:2000) at room temperature, for 2 h. Bands were visualized using a chemiluminescence detection system, and analyzed using Quantity One software (version 4.52).
Statistical analyses
Analyses were performed using SPSS (version 17.0). All values are presented as mean and standard deviation (SD). One-way or two-way analysis of variance (ANOVA) was used to compare differences in the percentage of hair cell loss, or the expression of sirtuin1 and NF-κB among two or more groups, respectively, followed by Turkey’s post hoc tests. Probability values less than 0.05 were considered statistically significant.
Results
CoCl2 induces hair cell damage in the organotypic culture of the cochlea
Organ of Corti explants from P4 rat cochlea were treated with different concentrations of CoCl2 for 24 h, stained with TRITC-labeled phalloidin, and observed using a confocal microscope (Figure 1). Control explants exhibited a normal pattern of three rows of outer hair cells (OHCs) and a single row of IHCs (Figure 1). Hair cells were arranged in an orderly manner, and the structure of the sterocilla bundles was clearly observed.
Figure 1. Hair cell death induced by CoCl2 exposure.
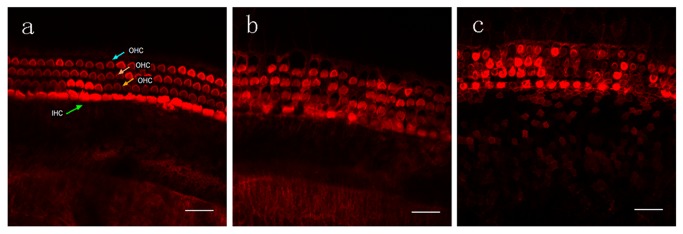
Cochlear explant cultures from P4 rats were treated with different concentrations of CoCl2 for 24 h. Explants were stained with TRITC-labeled phalloidin and observed using confocal microscopy. A. Representative micrograph of control explants showing an orderly row of IHCs and three rows of OHCs with stereocilia bundles. B. Representative micrograph of explants exposed to 300 μM CoCl2 showing loss of IHCs and OHCs. C. Representative micrograph of explants exposed to 400 μM CoCl2 showing loss of a large number of hair cells with widened intercellular spaces. Bar, 20 μm. IHC, inner hair cell; OHC, outer hair cell.
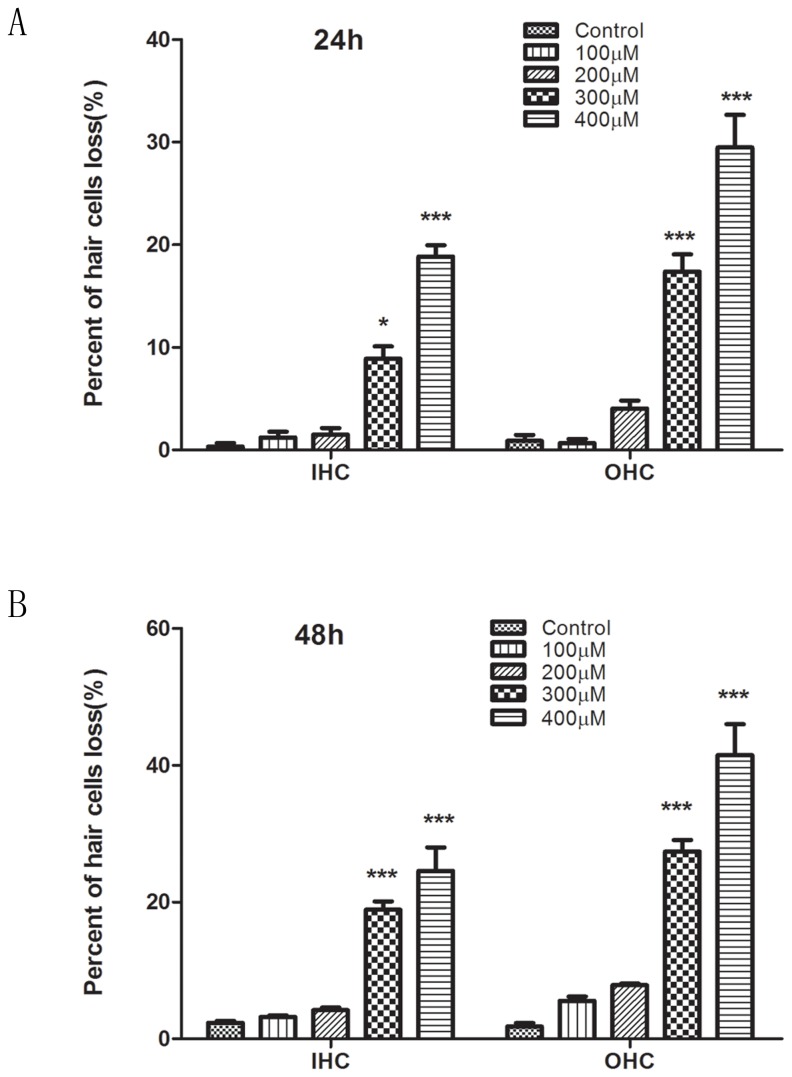
Low concentrations of CoCl2 (100–200 μM) did not cause obvious changes in the number or morphology of hair cells, whereas 300 μM CoCl2 induced swelling of OHCs accompanied by cell loss. After treatment with 400 μM CoCl2, a large number of hair cells were lost, and the orderly structure was disrupted. CoCl2 dose-dependently induced cell loss of both IHCs and OHCs (Figure 2). Exposure to 400 μM CoCo2 for 48 h significantly increased the percentage losses of IHCs (24.54%) and OHCs (41.48%) compared to exposure for 24 h (IHC loss, 18.84%; OHC loss, 29.48%). SEM results confirmed that CoCl2 induced more OHC loss than IHC loss (Figure 3). After 400 μM CoCl2 exposure for 24 h, most OHCs were lost, and the basilar membrane was fused, but most IHCs survived (Figure 3).
Figure 2. Quantitative analysis of hair cell loss in explants treated with CoCl2.
(A) CoCl2 treatment for 24h. (B) CoCl2 treatment for 48h. The percentage of IHC and OHC loss was calculated in the apical, middle and basal turns, and the average of the three zones were presented. N = 5 for each group. * P < 0.05, ** P < 0.01 versus control, 100 μM CoCl2 or 200 μM CoCl2.
Figure 3. Scanning electron microscopy results showing hair cells in the basal zone of control explants.
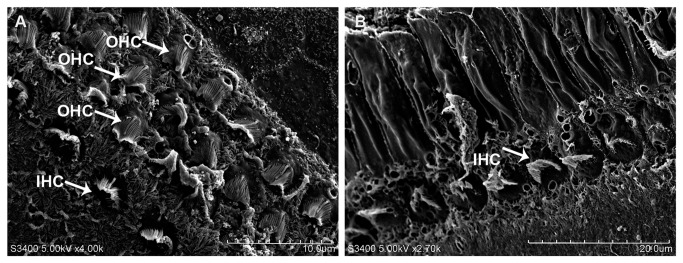
(A) and explants treated with 400 μM CoCl2 for 24 h (B). After CoCl2 treatment, most OHCs were lost, while the structure of most IHCs remained. The basilar membrane was fused to form a cord-like structure.
CoCl2 induces upregulation of sirtuin1
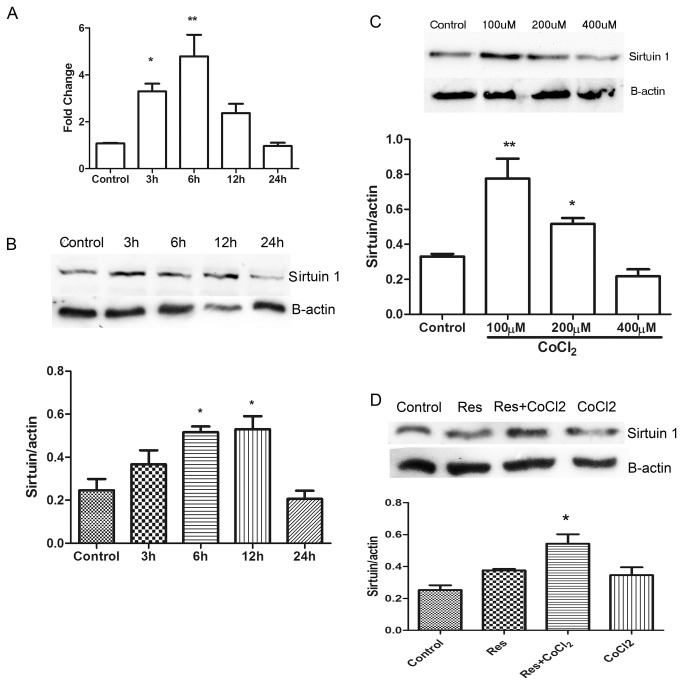
We investigated the effects of 400 μM CoCl2 treatment on the expression of sirtuin1 in organotypic cultured cochlea, using real-time RT-PCR and Western blot analyses. Compared with the control, the mRNA expression of sirtuin1 was significantly increased in explants treated with CoCl2 for 3 h. Sirtuin1 mRNA levels reached a peak in explants treated with CoCl2 for 6 h, and declined to a level lower than the control in explants treated with CoCl2 for 24 h (Figure 4A). Western blot analysis showed that the protein expression of sirtuin1 was significantly increased in explants treated with CoCl2 for 3 h, reached a peak in explants treated with CoCl2 for 12 h, and declined to a level lower that the control in explants treated with CoCl2 for 24 h (Figure 4B). We tested the effects of different concentrations of CoCl2 on the protein expression of sirtuin1. After exposure to CoCl2 for 24 h, low concentrations of CoCl2 (100 and 200 μM) significantly increased the expression of sirtuin1 compared with the control. However, the highest concentration of CoCl2 (400 μM) did not upregulate the expression of sirtuin1 (Figure 4C). In addition, resveratrol (50 μM) pretreatment significantly increased the expression of sirtuin1 in explants treated with 400 μM CoCl2 for 24 h (Figure 4D).
Figure 4. CoCl2 upregulates the mRNA and protein expressions of sirtuin1.
(A) Real-time quantitative RT-PCR showing relative mRNA expression of sirtuin1 in cochlear explants treated with 400 μM CoCl2 for 3, 6, 12, and 24 h. Expression of β-actin was determined as an internal control. * P < 0.05 versus control, ** P < 0.01 versus control. (B) Western blot showing the protein expression of sirtuin1 in cochlear explants treated with 400 μM CoCl2 for 3, 6, 12, and 24 h. * P < 0.05 versus control. (C) Representative Western blot showing the protein expression of sirtuin1 in cochlear explants treated with different concentrations of CoCl2 for 24 h. Quantitative Western blot analysis of the protein expression of sirtuin1. β-actin was used as a loading control. * P < 0.05, ** P < 0.01 versus control. (D) Representative Western blot showing the protein expression of sirtuin1 in cochlear explants pretreated with 50 μM Resveratrol for 1 h, followed by exposure to 400 μM CoCl2 for 24 h. * P < 0.05 versus CoCl2 treatment.
Resveratrol attenuates CoCl2-induced hair cell damage
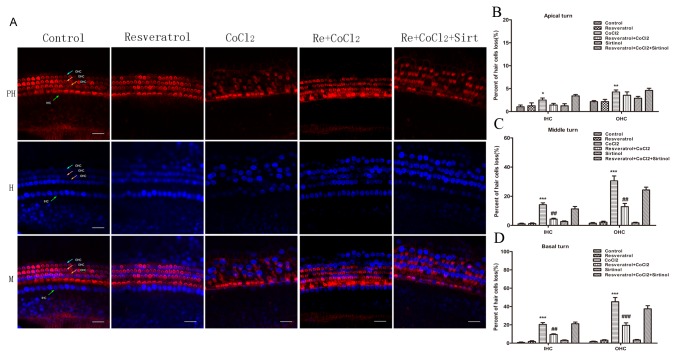
We investigated the effect of the sirtuin1 activator resveratrol and the sirtuin1 inhibitor sirtinol on CoCl2-induced hair cell damage. In cochlear explants treated with 400 μM CoCl2 for 24 h, hair cells exhibited a base-to-apex gradient damage, with the greatest degree of damage in the basal turn (Figure 5). The percentage losses of IHCs and OHCs were both <5% in the apical turn, 14.17% and 30.55% in the middle turn, and 20.53% and 45.19% in the basal turn, respectively.
Figure 5. Resveratrol attenuates CoCl2-induced hair cell damage.
(A) Representative micrographs of cochlear explants stained with TRITC-labeled phalloidin (upper), Hoechest 33342 (middle), and their merged images (lower). Control explants cultured in normal culture medium for 24 h (n = 6); resveratrol, explants pretreated with 50 μM resveratrol for 1 h (n = 6); CoCl2, explants treated with 400 μM CoCl2 for 24 h (n = 6). Re + CoCl2, explants pretreated with 50 μM resveratrol for 1 h followed by exposure to 400 μM CoCl2 for 24 h (n = 6). Re + CoCl2 + Sirt, explants pretreated with 50 μM resveratrol for 1 h followed by exposure to 400 μM CoCl2 and 20 μM sirtinol. Bar, 20 μm. B-D. Percentage of IHC and OHC loss in the apical (B), middle (C), and basal (D) turns. Hair cells in three 160 μm-long sections at each zone were calculated. The average was used to calculate the percentage of IHC and OHC loss. * P < 0.05, ** P < 0.01, *** P < 0.001 versus control; ## P < 0.01, ### P < 0.001 versus CoCl2.
Pretreatment with resveratrol significantly decreased the CoCl2-induced IHC and OHC losses in the middle and basal turns. The protective effect of resveratrol was significantly reduced by sirtuin1 inhibitor sirtinol. These results suggest that the upregulation of sirtuin1 plays a protective role in CoCl2-induced hair cell loss.
Resveratrol inhibits CoCl2-induced NF-κB acetylation
Sirtuin1 can bind to and deacetylate NF-κB, leading to inhibition of NF-κB activity [19]. To explore the signaling pathway of sirtuin1 in reducing CoCl2-induced hair cell damage, we investigated the effect of resveratrol on NF-κB acetylation (Figure 6A). Compared with control explants, NF-κB acetylation was significantly increased in explants treated with 400 μM CoCl2 for 6 and 12 h. Pretreatment of resveratrol prevented CoCl2-induced acetylation of NF-κB. The effect of resveratrol on CoCl2-induced acetylation of NF-κB was significantly reduced by the sirtuin1 inhibitor sirtinol (Figure 6B).
Figure 6. Expression of acetylated NF-κB in explants treated with CoCl2.
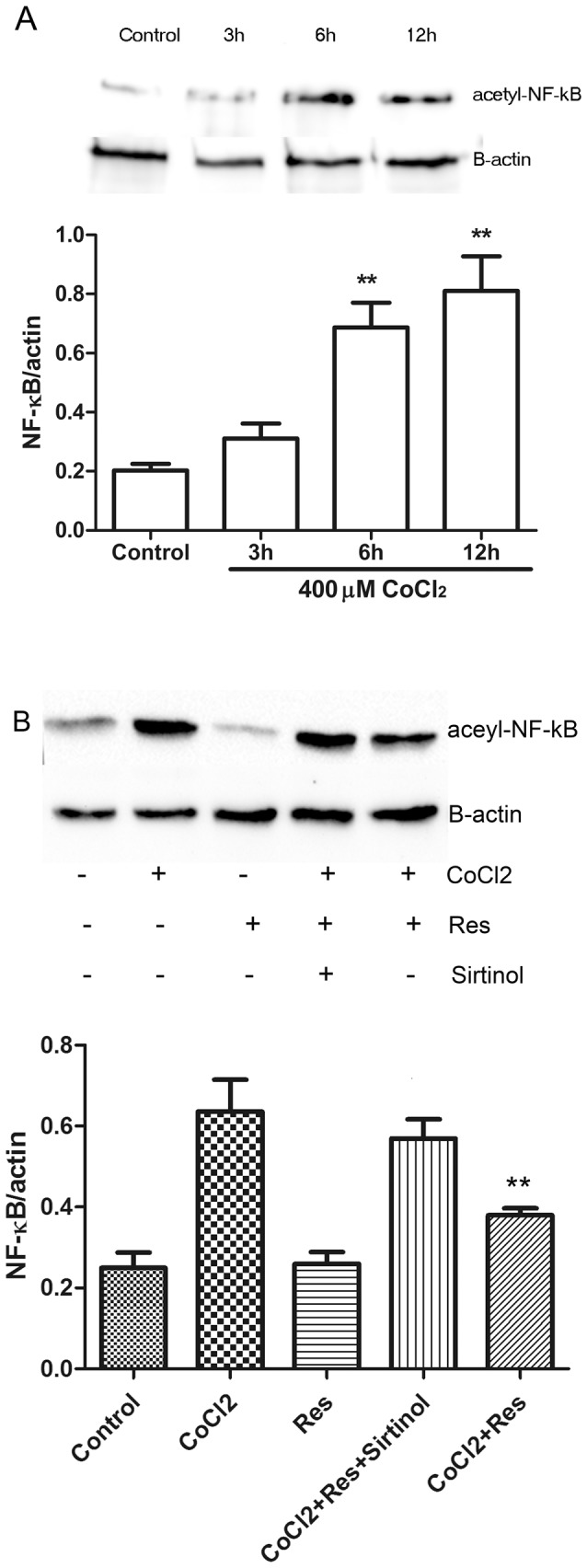
(A) Representative Western blot showing the expression of acetylated NF-κB in cochlear explants treated with 400 μM CoCl2 for 3, 6, and 12 h. Quantitative Western blot analysis of the protein expression of acetylated NF-κB in B. β-actin was used as a loading control. **P < 0.01 versus control. (B) Representative Western blot showing the expression of acetylated NF-κB in control explants, explants treated with 400 μM CoCl2 for 24 h, explants pretreated with 50 μM resveratrol for 1 h, explants pretreated with 50 μM resveratrol for 1 h, followed by exposure to 400 μM CoCl2 for 24 h, and explants pretreated with 50 μM resveratrol for 1 h, followed by exposure to 400 μM CoCl2 plus 20 μM sirtinol for 24 h. Quantitative Western blot analysis of the protein expression of acetylated NF-κB. β-actin was used as a loading control. **P < 0.01 versus CoCl2.
Discussion
Because in vivo animal models of hypoxia can induce hypoxia in many brain areas that may contribute to hypoxia-induced cochlear damage, investigators have adopted in vitro models of hypoxia using cochlear organotypic cultures [14,21]. CoCl2 is commonly used to induce a hypoxic environment by replacing Fe2+ in hemoglobin to form deoxygenated hemoglobin [22–24]. Co2+ has been shown to inhibit HIF-lα aryl hydrocarbon-hydroxylase activity and reduce HIF-lα degradation [25]. CoCl2 has been used for hypoxic preconditioning in many cell models of hypoxia, but very high concentrations of CoCl2 have been found to induce cellular damage by increasing intracellular reactive oxygen species (ROS), decreasing mitochondrial membrane potentials, and inducing cell apoptosis in many cells, especially neurons [26–28].
In the present study, we used CoCl2 to induce hypoxia in organotypic cultured cochlear explants. Higher concentrations of CoCl2 (300 and 400 μM) induced IHC and OHC loss, similar to findings made in an animal model of hypoxia [12]. Furthermore, CoCl2 induced more OHC than IHC loss, consistent with previous reports showing that mitochondrial swelling and intracellular vacuoles occurred earlier in OHCs than IHCs in an animal model of hypoxia [29]. However, Amarjargal et al. demonstrated that IHCs were more susceptible to hypoxia than OHCs in an in vitro model of oxygen-glucose deprivation-induced hypoxia [9]. This difference is likely due to the different hypoxic models used in the different studies. CoCl2-induced chemical hypoxia is thought to be associated with oxidative stress [30,31], which leads to production of a large amount of ROS, subsequent cell apoptosis, and hair cell death. The higher sensitivity of OHCs to hypoxia may be caused by a lower concentration of glutathione in OHCs compared to IHCs, thereby resulting in a weaker capacity to clear ROS [32].We also examined the expression of sirtuin1 in cochlear explants treated with CoCl2 over various durations. The mRNA expression of sirtuin1 was significantly increased in explants treated with CoCl2 for 3 h, peaked in explants treated for 6 h, and declined in explants treated for 12 h to a level higher than that in controls. Sirtuin1 protein expression patterns showed a similar time course as the mRNA expression, with a delayed peak expression. The protein expression of sirtuin1 increased after treatment with lower concentrations of CoCl2 (< 200 μM), but decreased after treatment with the highest concentration of CoCl2 used (400 μM). These results suggest that hypoxic preconditioning induces the upregulation of sirtuin1 in the cochlea. In addition, we found that 400 μM CoCl2 significantly induced IHC and OHC loss, accompanied by a significant down regulation of sirtuin1. This suggested that sirtuin1 down regulation is the main mechanism underlying CoCl2-induced hair cell loss. Furthermore, we found that 200 μM CoCl2 significantly increased sirtuin1 expression, and induced more (but not significantly more) hair cell loss compared with controls, suggesting that CoCl2 may induce hair cell loss via other signaling pathways that do not involve sirtuin1. For example, it has been reported that CoCl2 induces PC12 cell apoptosis via p53 stability and regulating UCN5B [33]. Further studies are needed to confirm the presence of these signaling pathways in hair cells.
The sirtuin1 activator resveratrol improved survival of IHCs and OHCs. This effect was blocked by the sirtuin1 inhibitor sirtinol. These findings suggest that the activation of sirtuin1 protects cochlear hair cells from hypoxic injury induced by CoCl2. Although it has been reported that overexpression of sirtuin1 can result in increased apoptosis and hypertrophy in the heart [34], resveratrol use has also been found to prevent cisplatin-induced ototoxicity [35]. This protective role of resveratrol may be mediated by its ability to decrease the intracellular ROS content in IHCs and OHCs [36]. However, it remains to be determined whether resveratrol improves hair cell survival via its anti-oxidative property.
NF-κB is a target of sirtuin1, and NF-κB acetylation is critical for its activity, especially during inflammation [37]. NF-κB activation promotes inflammatory cytokine production during inflammation, and hypoxia can induce inflammation [38]. Sirtuin1 physically interacts with the RelA/p65 subunit of NF-κB and inhibits transcription by deacetylation of RelA/p65 at lysine 310 [39]. Acetylation of RelA/p65 at lysine 310 is required for the full transcriptional activity of NF-κB during inflammation [40]. In the present study, we found that NF-κB acetylation was significantly increased in explants treated with 400 μM CoCl2 for 6 and 12 h, and pretreatment with resveratrol prevented CoCl2-induced acetylation of NF-κB. This finding suggests that sirtuin1 activation deacetylates NF-κB and reduces the hypoxia-induced increase in NF-κB activity, thereby protecting cochlear hair cells from hypoxic injury.
In summary, we found that CoCl2-induced hypoxia resulted in cochlear hair cell loss in cochlear organotypic cultures from P4 rats. CoCl2-induced hair cell loss was prevented by pretreatment of sirtuin1 activator resveratrol, possibly via NF-κB deacetylation. Sirtuin1 activation may represent a new therapeutic target for the prevention or treatment of hypoxia-induced cochlear hair cell injury.
Acknowledgments
We thank Medjaden Bioscience Limited for assisting in the preparation of this manuscript.
Funding Statement
This study was supported by the National Science Foundation of China (Grant No. 81260422). The above funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Medjaden Bioscience Limited assisted in the preparation of this manuscript.
References
- 1. Borg E (1997) Perinatal asphyxia, hypoxia, ischemia and hearing loss. An overview. Scand Audiol 26: 77-91. doi: 10.3109/01050399709074979. PubMed: 9187000. [DOI] [PubMed] [Google Scholar]
- 2. Jiang ZD, Wang J, Brosi DM, Shao XM, Wilkinson AR (2004) One-third of term babies after perinatal hypoxia-ischaemia have transient hearing impairment: dynamic change in hearing threshold during the neonatal period. Acta Paediatr 93: 82-87. doi: 10.1111/j.1651-2227.2004.tb00679.x. PubMed: 14989445. [DOI] [PubMed] [Google Scholar]
- 3. Jiang ZD, Zhang Z, Wilkinson AR (2005) Distortion product otoacoustic emissions in term infants after hypoxia-ischaemia. Eur J Pediatr 164: 84-87. doi: 10.1007/s00431-004-1569-8. PubMed: 15703978. [DOI] [PubMed] [Google Scholar]
- 4. Murphy-Lavoie H, Piper S, Moon RE, Legros T (2012) Hyperbaric oxygen therapy for idiopathic sudden sensorineural hearing loss. Undersea Hyperb Med 39: 777-792. PubMed: 22670557. [PubMed] [Google Scholar]
- 5. Kim YJ, Kang HH, Ahn JH, Chung JW (2007) Hypoxic changes in the central nervous system of noise-exposed mice. Acta Otolaryngol Suppl: 73-77. PubMed: 17882574. [DOI] [PubMed] [Google Scholar]
- 6. Dai P, Yang W, Jiang S, Gu R, Yuan H et al. (2004) Correlation of cochlear blood supply with mitochondrial DNA common deletion in presbyacusis. Acta Otolaryngol 124: 130-136. doi: 10.1080/00016480410016586. PubMed: 15072414. [DOI] [PubMed] [Google Scholar]
- 7. Gerhardt KJ, Ma YL, Rybak LP, Rarey KE (1998) Interaction of methylprednisolone and transient asphyxia on the inner ear of the adrenalectomized rat. Otolaryngol Head Neck Surg 118: 338-343. doi: 10.1016/S0194-5998(98)70312-2. PubMed: 9527114. [DOI] [PubMed] [Google Scholar]
- 8. Olgun Y, Kırkım G, Kolatan E, Kıray M, Bağrıyanık A et al. (2013) Otoprotective effect of recombinant erythropoietin in a model of newborn hypoxic-ischemic encephalopathy. Int J Pediatr Otorhinolaryngol 77: 739-746. doi: 10.1016/j.ijporl.2013.01.029. PubMed: 23433994. [DOI] [PubMed] [Google Scholar]
- 9. Amarjargal N, Andreeva N, Gross J, Haupt H, Fuchs J et al. (2009) Differential vulnerability of outer and inner hair cells during and after oxygen-glucose deprivation in organotypic cultures of newborn rats. Physiol Res 58: 895-902. PubMed: 19093732. [DOI] [PubMed] [Google Scholar]
- 10. Mazurek B, Winter E, Fuchs J, Haupt H, Gross J (2003) Susceptibility of the hair cells of the newborn rat cochlea to hypoxia and ischemia. Hear Res 182: 2-8. doi: 10.1016/S0378-5955(03)00134-5. PubMed: 12948595. [DOI] [PubMed] [Google Scholar]
- 11. Wang L, Wang P, Du B (2008) [Effect of the spiral ganglion cell and nerve fiber of rat cochlea in vitro to hypoxia]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi 22: 1040-1042 [PubMed]
- 12. Ding DL (1993) Functional and morphological changes of the cochlea in guinea pigs during anoxia. Zhonghua Er Bi Yan Hou Ke Za Zhi 28: 265-267, 8192926. [PubMed] [Google Scholar]
- 13. Amarjargal N, Mazurek B, Haupt H, Andreeva N, Fuchs J et al. (2008) Effects of SERCA and PMCA inhibitors on the survival of rat cochlear hair cells during ischemia in vitro. Physiol Res 57: 631-638. PubMed: 17705670. [DOI] [PubMed] [Google Scholar]
- 14. Khan M, Szczepek AJ, Haupt H, Olze H, Mazurek B (2010) Expression of the proinflammatory cytokines in cochlear explant cultures: influence of normoxia and hypoxia. Neurosci Lett 479: 249-252. doi: 10.1016/j.neulet.2010.05.072. PubMed: 20561939. [DOI] [PubMed] [Google Scholar]
- 15. Wareski P, Vaarmann A, Choubey V, Safiulina D, Liiv J et al. (2009) PGC-1{alpha} and PGC-1{beta} regulate mitochondrial density in neurons. J Biol Chem 284: 21379-21385. doi: 10.1074/jbc.M109.018911. PubMed: 19542216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL et al. (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011-2015. doi: 10.1126/science.1094637. PubMed: 14976264. [DOI] [PubMed] [Google Scholar]
- 17. Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S et al. (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191-196. doi: 10.1038/nature01960. PubMed: 12939617. [DOI] [PubMed] [Google Scholar]
- 18. Chen LF, Mu Y, Greene WC (2002) Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-kappaB. EMBO J 21: 6539-6548. doi: 10.1093/emboj/cdf660. PubMed: 12456660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR et al. (2004) Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J 23: 2369-2380. doi: 10.1038/sj.emboj.7600244. PubMed: 15152190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Segawa A, Loffredo F, Puxeddu R, Yamashina S, Testa Riva F et al. (1998) Exocytosis in human salivary glands visualized by high-resolution scanning electron microscopy. Cell Tissue Res 291: 325-336. doi: 10.1007/s004410051002. PubMed: 9426319. [DOI] [PubMed] [Google Scholar]
- 21. Mazurek B, Amarjargal N, Haupt H, Fuchs J, Olze H et al. (2011) Expression of genes implicated in oxidative stress in the cochlea of newborn rats. Hear Res 277: 54-60. doi: 10.1016/j.heares.2011.03.011. PubMed: 21447374. [DOI] [PubMed] [Google Scholar]
- 22. Naves T, Jawhari S, Jauberteau MO, Ratinaud MH, Verdier M (2013) Autophagy takes place in mutated p53 neuroblastoma cells in response to hypoxia mimetic CoCl (2). Biochem Pharmacol 85: 1153-1161 [DOI] [PubMed]
- 23. Fang D, Li Z, Zhong-ming Q, Mei WX, Ho YW et al. (2008) Expression of bystin in reactive astrocytes induced by ischemia/reperfusion and chemical hypoxia in vitro. Biochim Biophys Acta 1782: 658-663. doi: 10.1016/j.bbadis.2008.09.007. PubMed: 18929647. [DOI] [PubMed] [Google Scholar]
- 24. Tan XL, Huang XY, Gao WX, Zai Y, Huang QY et al. (2008) CoCl2-induced expression of p300 promotes neuronal-like PC12 cell damage. Neurosci Lett 441: 272-276. doi: 10.1016/j.neulet.2008.06.050. PubMed: 18586071. [DOI] [PubMed] [Google Scholar]
- 25. Chen SL, Yang CT, Yang ZL, Guo RX, Meng JL et al. (2010) Hydrogen sulphide protects H9c2 cells against chemical hypoxia-induced injury. Clin Exp Pharmacol Physiol 37: 316-321. doi: 10.1111/j.1440-1681.2009.05289.x. PubMed: 19769612. [DOI] [PubMed] [Google Scholar]
- 26. Jung JY, Mo HC, Yang KH, Jeong YJ, Yoo HG et al. (2007) Inhibition by epigallocatechin gallate of CoCl2-induced apoptosis in rat PC12 cells. Life Sci 80: 1355-1363. doi: 10.1016/j.lfs.2006.11.033. PubMed: 17240404. [DOI] [PubMed] [Google Scholar]
- 27. Chen JX, Zhao T, Huang DX (2009) Protective effects of edaravone against cobalt chloride-induced apoptosis in PC12 cells. Neurosci Bull 25: 67-74. doi: 10.1007/s12264-009-1215-6. PubMed: 19290025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ji Q, Yang L, Zhou J, Lin R, Zhang J et al. (2012) Protective effects of paeoniflorin against cobalt chloride-induced apoptosis of endothelial cells via HIF-1alpha pathway. Toxicol in Vitro 26: 455-461. doi: 10.1016/j.tiv.2012.01.016. PubMed: 22269387. [DOI] [PubMed] [Google Scholar]
- 29. Lin CD, Kao MC, Tsai MH, Lai CH, Wei IH et al. (2011) Transient ischemia/hypoxia enhances gentamicin ototoxicity via caspase-dependent cell death pathway. Lab Invest 91: 1092-1106. doi: 10.1038/labinvest.2011.69. PubMed: 21519324. [DOI] [PubMed] [Google Scholar]
- 30. Zou W, Zeng J, Zhuo M, Xu W, Sun L et al. (2002) Involvement of caspase-3 and p38 mitogen-activated protein kinase in cobalt chloride-induced apoptosis in PC12 cells. J Neurosci Res 67: 837-843. doi: 10.1002/jnr.10168. PubMed: 11891799. [DOI] [PubMed] [Google Scholar]
- 31. Lee JH, Choi SH, Baek MW, Kim MH, Kim HJ et al. (2013) CoCl induces apoptosis through the mitochondria- and death receptor-mediated pathway in the mouse embryonic stem cells. Mol Cell Biochem. [DOI] [PubMed] [Google Scholar]
- 32. Lorito G, Giordano P, Petruccelli J, Martini A, Hatzopoulos S (2008) Different strategies in treating noiseinduced hearing loss with N-acetylcysteine. Med Sci Monit 14: BR159-164. [PubMed] [Google Scholar]
- 33. Lee M, Kang H, Jang SW (2013) CoCl2 induces PC12 cells apoptosis through p53 stability and regulating UNC5B. Brain. Res Bull 96: 19-27. doi: 10.1016/j.brainresbull.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 34. Alcendor RR, Gao S, Zhai P, Zablocki D, Holle E et al. (2007) Sirt1 regulates aging and resistance to oxidative stress in the heart. Circ Res 100: 1512-1521. doi: 10.1161/01.RES.0000267723.65696.4a. PubMed: 17446436. [DOI] [PubMed] [Google Scholar]
- 35. Erdem T, Bayindir T, Filiz A, Iraz M, Selimoglu E (2012) The effect of resveratrol on the prevention of cisplatin ototoxicity. Eur Arch Otorhinolaryngol 269: 2185-2188. doi: 10.1007/s00405-011-1883-5. PubMed: 22186767. [DOI] [PubMed] [Google Scholar]
- 36. Yumusakhuylu AC, Yazici M, Sari M, Binnetoglu A, Kosemihal E et al. (2012) Protective role of resveratrol against cisplatin induced ototoxicity in guinea pigs. Int J Pediatr Otorhinolaryngol 76: 404-408. doi: 10.1016/j.ijporl.2011.12.021. PubMed: 22261612. [DOI] [PubMed] [Google Scholar]
- 37. Pan WW, Li JD, Huang S, Papadimos TJ, Pan ZK et al. (2010) Synergistic activation of NF-{kappa}B by bacterial chemoattractant and TNF{alpha} is mediated by p38 MAPK-dependent RelA acetylation. J Biol Chem 285: 34348-34354. doi: 10.1074/jbc.M110.109165. PubMed: 20729202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. van der Kooij MA, Nijboer CH, Ohl F, Groenendaal F, Heijnen CJ et al. (2010) NF-kappaB inhibition after neonatal cerebral hypoxia-ischemia improves long-term motor and cognitive outcome in rats. Neurobiol Dis 38: 266-272. doi: 10.1016/j.nbd.2010.01.016. PubMed: 20132887. [DOI] [PubMed] [Google Scholar]
- 39. Chen LF, Williams SA, Mu Y, Nakano H, Duerr JM et al. (2005) NF-kappaB RelA phosphorylation regulates RelA acetylation. Mol Cell Biol 25: 7966-7975. doi: 10.1128/MCB.25.18.7966-7975.2005. PubMed: 16135789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhu X, Liu Q, Wang M, Liang M, Yang X et al. (2011) Activation of Sirt1 by resveratrol inhibits TNF-alpha induced inflammation in fibroblasts. PLOS ONE 6: e27081. doi: 10.1371/journal.pone.0027081. PubMed: 22069489. [DOI] [PMC free article] [PubMed] [Google Scholar]