Abstract
Innate immune recognition is the first line of host defense against invading microorganisms. It is a based on the detection, by pattern recognition receptors (PRRs), of invariant molecular signatures that are unique to microorganisms. TLR2 is a PRR that plays a major role in the detection of Gram-positive bacteria by recognizing cell envelope lipid-linked polymers, also called macroamphiphiles, such as lipoproteins, lipoteichoic acids and mycobacterial lipoglycans. These microbe-associated molecular patterns (MAMPs) display a structure based on a lipid anchor, being either an acylated cysteine, a glycosylated diacylglycerol or a mannosyl-phosphatidylinositol respectively, and having in common a diacylglyceryl moiety. A fourth class of macroamphiphile, namely lipoglycans, whose lipid anchor is made, as for lipoteichoic acids, of a glycosylated diacylglycerol unit rather than a mannosyl-phosphatidylinositol, is found in Gram-positive bacteria and produced by certain Actinobacteria, including Micrococcus luteus, Stomatococcus mucilaginosus and Corynebacterium glutamicum. We report here that these alternative lipoglycans are also recognized by TLR2 and that they stimulate TLR2-dependant cytokine production, including IL-8, TNF-α and IL-6, and cell surface co-stimulatory molecule CD40 expression by a human macrophage cell line. However, they differ by their co-receptor requirement and the magnitude of the innate immune response they elicit. M. luteus and S. mucilaginosus lipoglycans require TLR1 for recognition by TLR2 and induce stronger responses than C. glutamicum lipoglycan, sensing of which by TLR2 is dependent on TLR6. These results expand the repertoire of MAMPs recognized by TLR2 to lipoglycans based on a glycosylated diacylglycerol lipid anchor and reinforce the paradigm that macroamphiphiles based on such an anchor, including lipoteichoic acids and alternative lipoglycans, induce TLR2-dependant innate immune responses.
Introduction
The innate immune system is genetically programmed to detect molecular signatures of microbes via a limited number of germline-encoded pattern recognition receptors (PRRs) [1], [2], [3], [4], [5], [6]. The signatures seen as foreign are structural motifs, referred to as pathogen/microbe-associated molecular patterns (PAMPs/MAMPs), which are unique to microorganisms and relatively invariant in most microorganisms of a given class. The molecular pattern recognized by PRRs is usually a small but conserved part of a macromolecule of microbial origin, such as the lipid anchor in bacterial macroamphiphiles, and which might be repeated such as monomers in peptidoglycan or in nucleic acids [7], [8].
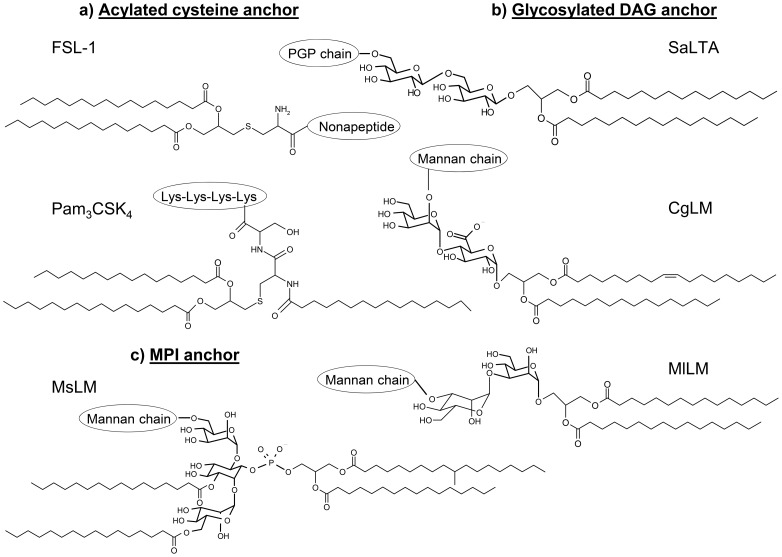
Bacterial macroamphiphiles, i.e. cell envelope lipid-linked polymers [9], [10], namely Gram-negative bacterial lipopolysaccharide (LPS), Gram-positive bacteria lipoteichoic acid (LTA), lipoproteins and mycobacterial lipoglycans, evidently meet PAMP/MAMP criteria and are well suited to innate immune recognition. They are mostly recognized via their lipid anchor by a family of PRRs, named Toll-like receptors (TLRs). TLR extracellular domains, which have leucine-rich repeat modules, adopt a horseshoe-like shape structure and are responsible for PAMP/MAMP binding [11], [12]. Their intracellular signalling domains trigger innate immune responses through NF-κB-dependent and IFN regulatory factor-dependant signalling pathways [1], [2], [3], [4]. Lipoproteins, LTA and mycobacterial lipoglycans, based on a lipid anchor being either an acylated cysteine (a), a glycosylated diacylglycerol (b) or a mannosyl-phosphatidylinositol (c) respectively, and having in common a diacylglyceryl moiety (Figure 1), are recognized by TLR2. The latter generally functions as a heterodimer with either TLR1 or TLR6, which is involved in discrimination of the acylation state of lipoproteins, its best characterized agonists [13], [14], [15]. Structure/function relationship studies, corroborated by the reports of the structures of several TLR2-lipopeptide complexes determined by X-ray crystallography, have established that the acylated cysteinyl moiety is the structure recognized by the receptors and that triacylated lipoproteins are preferentially recognized by the TLR2/TLR1 complex, whereas diacylated lipoproteins are recognized by the TLR2/TLR6 complex [16], [17]. In the crystal structure of a TLR1-TLR2 heterodimer in complex with the model lipopeptide Pam3CSK4 [16] (Figure 1), the triacylated lipopeptide appears to form a bridge between TLR2 and TLR1 with the two ester-bound fatty acyl chains of the S-diacylglyceryl moiety inserted deep into a pocket in the hydrophobic core of TLR2, the third amide-linked acyl chain occupying a hydrophobic channel at the surface of TLR1 and the conserved polar head located at the region of contact between the two receptors. Crystal structure of a TLR2-TLR6-diacylated lipopeptide complex reveals that the lipid-binding channel of TLR6 is blocked by two phenylalanines that hamper the binding of any fatty acid chain [17]. Besides lipoproteins, the majority of low G+C Gram-positive bacteria (Firmicutes) produce LTA [10], [18], [19] that are composed of a lipid anchor, made of a diacylglycerol (DAG) unit substituted by a di- or tri-glycoside. In contrast, high G+C Gram-positive bacteria of the suborders Corynebacterineae, including mycobacteria, and Pseudonocardineae do not produce LTA but rather lipoglycans, with structures based on a multi-acylated mannosyl-phosphatidyl-myo-inositol (MPI) anchor [10], [19], [20], [21], [22], [23] (Figure 1). As described for diacylated lipopeptides, heterodimerization of TLR2 with TLR6 seems to be important for LTA recognition [24], [25]. A crystal structure of TLR2 in complex with Streptococcus pneumoniae LTA showed that the two fatty acyl chains of the DAG unit are inserted in the hydrophobic pocket of TLR2 [17]. Mycobacterial tri- and tetra-acylated lipoglycans are instead recognized by the TLR2-TLR1 heterodimer [26]. It is proposed, by analogy with lipopeptides, that in the case of tri-acylated lipoglycans, both fatty acids of the DAG unit are inserted in the hydrophobic pocket of TLR2 while the third acyl chain esterfying the mannosyl unit of the MPI anchor is inserted in the hydrophobic channel at the surface of TLR1 [8].
Figure 1. Structure of the lipid anchor of bacterial macroamphiphiles.
CgLM, Corynebacterium glutamicum lipomannan; DAG, diacylglycerol; FSL-1, synthetic N-terminal part of lipopoprotein LP44 of Mycoplasma salivarum; MlLM, Micrococcus luteus lipomannan; MPI, mannosyl-phosphatidyl-myo-inositol; MsLM, Mycobacterium smegmatis lipomannan; PGP, polyglycerolphosphate; SaLTA, Staphylococcus aureus lipoteichoic acid; The available evidence [29] suggests the structure of Stomatococcus mucilaginosus lipomannan (SmLM) is very similar to that of MlLM.
A fourth class of macroamphiphiles, namely lipoglycans whose lipid anchor is made, as for LTA, of a glycosylated DAG unit rather than a MPI, is found in some Gram-positive Actinobacteria, including Micrococcus luteus [27], [28], Stomatococcus mucilaginosus [29] and Corynebacterium glutamicum [30], [31] (Figure 1b). Here, we report that these alternative lipoglycans are also recognized by TLR2 and that they stimulate TLR2-dependant cytokine production and cell surface co-stimulatory molecule CD40 expression by a human macrophage cell line. Our results expand the repertoire of MAMPs recognized by TLR2 and reinforce the paradigm that macroamphiphiles based on a glycosylated DAG anchor, such as LTA and alternative lipoglycans, are ligands of TLR2.
Results
Lipoglycans based on a glycosylated DAG anchor (i.e. DAG-based lipoglycans) were purified from M. luteus [27], [28], C. glutamicum [30] and S. mucilaginosus [29]. These lipoglycans are lipomannans (LM) (Figure 1) and will be subsequently termed MlLM, CgLM and SmLM respectively.
Signaling via TLR2
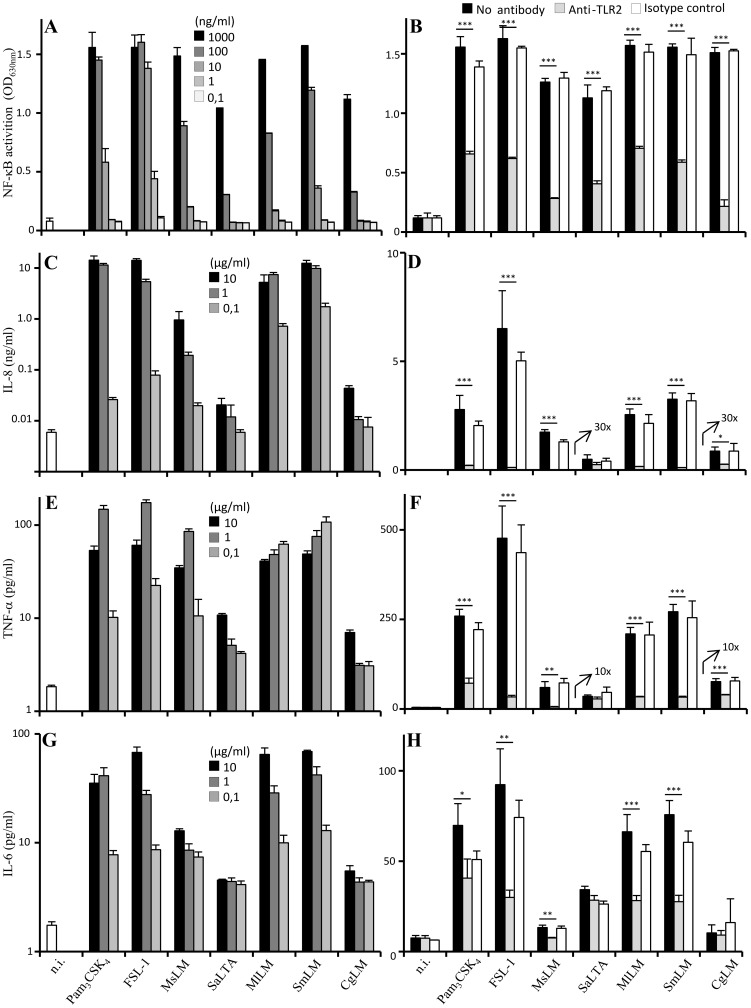
We first tested the ability of MlLM, CgLM and SmLM to stimulate HEK293 cells stably transfected with human TLR2 and CD14 genes and a NF-κB-inducible reporter system (HEK-TLR2 cells). The synthetic lipopeptides Pam3CSK4 and FSL-1, Mycobacterium smegmatis LM (MsLM) and Staphylococcus aureus LTA (SaLTA) were used as reference ligands of TLR2 based on a lipid anchor being either an acylated cysteine, a MPI or a glycosylated DAG respectively (Figure 1). Interestingly, MlLM, SmLM and CgLM each induced NF-κB activation in a dose-dependent fashion (Figure 2A) and their activity was inhibited by an anti-TLR2 antibody (Figure 2B). However, the magnitude of activation varied between the different ligands of TLR2. Synthetic lipopeptides were the strongest agonists with an EC50 in the range of 1 ng/ml, followed by MlLM and SmLM (EC50 ∼ 10 ng/ml), MsLM (EC50 ∼ 30 ng/ml) and finally SaLTA and CgLM (EC50 ∼ 100 ng/ml).
Figure 2. DAG-based lipoglycans are recognized by TLR2 in cooperation with TLR1 or TLR6.
A, C) HEK-TLR2 cells were incubated with the various stimuli at a concentration ranging from 0.1 to 1000 ng/ml. B) HEK-TLR2 cells were pre-incubated or not for 30 min at 37°C before stimuli addition with various monoclonal antibodies: anti-TLR2 (5 µg/ml), anti-TLR1 (10 µg/ml), anti-TLR6 (10 µg/ml) or IgG1 isotype control (10 µg/ml). FSL-1 was tested at a concentration of 1 ng/ml, Pam3CSK4 and MsLM at 10 ng/ml, SmLM at 50 ng/ml, MlLM and SaLTA at 100 ng/ml and CgLM at 300 ng/ml. In C, deacylated molecules (+) were prepared by treating native molecules (−) with 2M NH4OH for 2h at 110°C. NF-κB activation was determined by reading OD at 630 nm. The results are mean ± SD of triplicate wells and are representative of at least three separate experiments. ***, P<0.001. Iso ctrl, isotype control, n.i., not induced.
TLR2 activation by MlLM, CgLM and SmLM was dependent on the presence of a native acylated lipid anchor since they failed to activate NF-κB upon deacylation (Figure 2C). This result is in agreement with structure/function relationships studies and crystal structures that previously revealed that fatty acids are critical for lipopeptides, LTA and mycobacterial lipoglycans signaling via and binding to TLR2 [7], [8], [16], [17]. The requirement of TLR1 or TLR6 for recognition of MlLM, CgLM and SmLM by TLR2 was tested using blocking antibodies. As expected for the reference ligands, the activity of the triacylated Pam3CSK4 and MsLM was clearly dependent on TLR1, whereas that of the diacylated FSL-1 lipopeptide and SaLTA was dependent on TLR6 (Figure 2B). The TLR2-dependant activation of NF-κB by CgLM was strongly, although not completely, inhibited by the anti-TLR6 antibody and moderately inhibited by anti-TLR1 antibody. Surprisingly, activity of diacylated MlLM and SmLM was almost completely inhibited by anti-TLR1 but not anti-TLR6 antibodies (Figure 2B).
Activation of THP-1 human macrophage cell line
We then investigated the capacity of DAG-based lipoglycans to activate the human THP-1 monocyte/macrophage cells, using a cell line derivative that stably expresses a NF-κB-inducible reporter system. MlLM, SmLM and CgLM induced NF-κB activation in a dose-dependent fashion (Figure 3A) and their activity was strongly inhibited by an anti-TLR2 antibody (Figure 3B). The magnitude of activation of the different macroamphiphiles followed the hierarchy observed with HEK-TLR2 cells (Figure 2A). Lipopeptides were the most stimulatory with an EC50 below 10 ng/ml, followed by the TLR2-TLR1 signaling lipoglycans SmLM, MsLM and MlLM (EC50 below 100 ng/ml), and finally the TLR2-TLR6 signaling SaLTA and CgLM (EC50 above 300 ng/ml).
Figure 3. DAG-based lipoglycans stimulate TLR2-dependant NF-κB activation and IL-8, TNF-α and IL-6 production by human THP-1 monocyte/macrophage cell line.
A) THP-1 cells were incubated with the various stimuli at a concentration ranging from 0.1 to 1000 ng/ml. C, E, G) THP-1 cells were incubated with the various stimuli at a concentration ranging from 0.1 to 10 µg/ml. B, D, F, H) THP-1 cells were pre-incubated or not for 30 min at 37°C with 10 µg/ml of anti-TLR2 or IgG1 isotype control antibodies and stimulated overnight with the different TLR2 ligands. FSL-1, Pam3CSK4 FSL-1, MsLM, MlLM, SmLM were tested at a concentration of 1 µg/ml, SaLTA and CgLM at 10 µg/ml. In A and B, NF-κB activation was determined by reading OD at 630 nm. In C to H, cytokines were assayed in the culture supernatant by sandwich ELISA. The results are mean ± SD of triplicate wells and are representative of at least three separate experiments. 10x and 30x mean that the concentration of cytokine detected is amplified 10 and 30 fold respectively. *, P<0.05; **, P<0.01; ***, P<0.001. n.i., not induced.
Activation of innate immunity ultimately regulates adaptive immunity, most particularly via the production of cytokines and the expression of co-stimulatory molecules by antigen-presenting cells [1], [2], [3], [4]. THP-1 cells being strong producers of IL-8 [32], this cytokine was analyzed first. All the macroamphiphiles induced a dose-dependent production of IL-8 (Figure 3C) that was almost completely abrogated by an anti-TLR2 antibody (Figure 3D). However, MlLM and SmLM were the most potent inducers, except for synthetic lipopeptides, a plateau of around 10 ng/ml of IL-8 being reached at a concentration of DAG-based lipoglycans of 1 µg/ml. SaLTA and CgLM were much weaker inducers, whereas MsLM showed an intermediate potency (Figure 3C). Similar data were obtained with TNF-α (Figure 3E) and IL-6 (Figure 3G), although these cytokines were produced by THP-1 cells in lower amount. It is worth noting that a TNF-α production plateau of around 100 pg/ml was reached for an MlLM or SmLM concentration of 100 ng/ml only (Figure 3E). An 1 log increase in concentration was required for MsLM to reach the same effect. A 2 log increase in concentration of SaLTA and CgLM led to a production of TNF-α of around 10 pg/ml only. TNF-α (Figure 3F) and IL-6 (Figure 3H) production elicited by the different stimuli was strongly and significantly inhibited by an anti-TLR2 antibody, except for SaLTA and CgLM (which induced insufficient amount of cytokines for accurate determination). These data confirm the earlier observation that CgLM stimulated TNF-α production by the human macrophage cell line THP-1 [31].
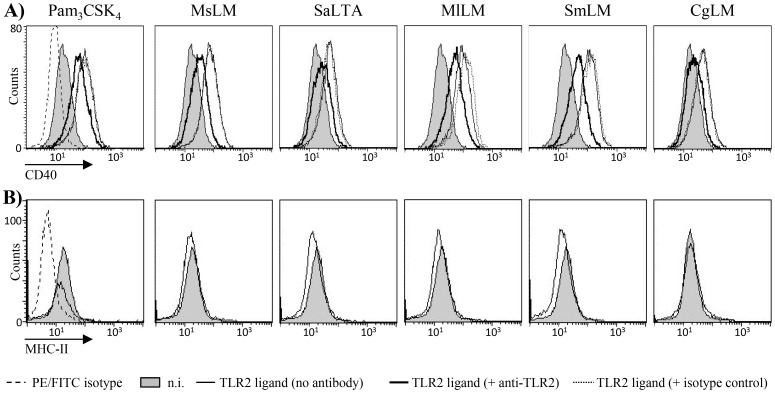
Finally, all the TLR2 ligands tested were able, at a concentration of 1 µg/ml, to induce the expression of the cell surface co-stimulatory molecule CD40 but failed to induce MHCII (Figure 4). Again, DAG-based MlLM and SmLM were the most stimulatory lipoglycans. SaLTA and CgLM were the least stimulatory ligands whereas MsLM showed an intermediate potency. Induction of CD40 expression by the different stimuli was partially inhibited by an anti-TLR2 antibody (Figure 4).
Figure 4. DAG-based lipoglycans stimulate TLR2-dependant cell surface co-stimulatory molecule CD40 but not MHCII expression by human THP-1 monocyte/macrophage cell line.
THP-1 cells were pre-incubated or not for 30 min at 37°C with 10 µg/ml of anti-TLR2 or IgG1 isotype control antibodies and stimulated overnight with the different TLR2 ligands at a concentration of 1 µg/ml. CD40 (A) and MHC-II (B) expression was monitored by flow cytometry. One representative experiment (from three repeats) is shown. n.i., not induced.
Discussion
Bacterial cell wall macroamphiphiles are detected by the innate immune system as foreign molecular signatures [8]. LPS is recognized by TLR4-MD2 whereas lipoproteins, LTA, and mycobacterial lipoglycans are recognized by TLR2-TLR1/TLR6. Structure/function relationships studies and crystal structures have established that macroamphiphiles are mostly recognized via their lipid anchor, most particularly through binding of fatty acids into hydrophobic pockets of the receptors [12]. Lipoproteins, LTA, and mycobacterial lipoglycans display a structure based on a lipid anchor consisting in an acylated cysteine, a glycosylated DAG or a MPI respectively. Besides these last three classes of macroamphiphiles, a fourth one is found in Gram-positive bacteria, namely lipoglycans whose lipid anchor is made of a glycosylated DAG unit.
We report here that representatives of these alternative DAG-based lipoglycans, MlLM, SmLM and CgLM, are also recognized by TLR2 and that they stimulate TLR2-dependant cytokine production, including IL-8, TNF-α and IL-6, and cell surface co-stimulatory molecule CD40 expression by a human macrophage cell line. However, they differ in their co-receptor requirement and the magnitude of the innate immune response they elicit.
As expected for a diacylated molecule, recognition of CgLM by TLR2 was mostly dependent on TLR6, although it was partially, but significantly, inhibited by an anti-TLR1 antibody. More surprisingly, MlLM and SmLM recognition was almost completely dependent on TLR1. These results are in sharp contrast with the paradigm of receptor usage as a function of the acylation pattern since triacylated lipoproteins are preferentially recognized by the TLR2/TLR1 complex, whereas diacylated lipoproteins are recognized by the TLR2/TLR6 complex [13], [14], [15]. However, it has been also previously described that in addition to the acylation pattern, the nature of the amino acids of the peptidic chain of lipopeptides can modulate the specificity of the recognition by TLR2 heterodimers [33], [34]. A similar phenomenon seems to apply with DAG-based lipoglycans, where the acylation pattern is not sufficient to dictate the TLR2 heterodimer usage. The underlying molecular bases remain to be uncovered, however these results further demonstrate that the pattern recognized by the receptors is not strictly restricted to the lipid moiety of lipopeptides or lipoglycans, but also involves in part the hydrophilic moiety of these TLR2 agonists. We previously established that the (α1→6)-mannopyranosyl backbone, which is a highly conserved structural feature of MPI-based lipoglycans, is an integral part of the pattern recognized by TLR2 [35]. Similarly, the mannan chain is required for DAG-based lipoglycan activity since DAG alone (dipalmitin) did not activate TLR2 (not shown).
Interestingly, SmLM and MlLM that induce signalling via TLR2-TLR1 were much stronger activators than CgLM that activates via TLR2-TLR6. Activity of SlLM and MlLM was even higher than that of the MPI-based lipoglycan MsLM, which contributes to the innate immune detection of M. smegmatis by TLR2-TLR1 [32]. In contrast, CgLM activity was weaker and similar to that of the TLR2-TLR6 agonist SaLTA. The weaker activity of CgLM and SaLTA was not a result of a low expression of TLR6 in THP-1 cells. Indeed, TLR6 expression was confirmed by flow cytometry analysis (not shown). Moreover, the TLR2-TLR6 ligand FSL-1 lipopeptide was highly stimulatory in these cells. An interesting correlate of the difference in response to CgLM compared to MlLM and SmLM is that the former has a distinctive charged α-glucopyranosyluronic residue proximal to the DAG anchor [30] rather than an uncharged mannosyl residue. Kang et al. [17] reported a crystal structure of TLR2 in complex with S. pneumoniae LTA, where the two fatty acyl chains of the DAG unit are inserted in the hydrophobic pocket of TLR2. Interestingly, although S. pneumoniae LTA was found by the authors to bind TLR2 with high affinity, in contrast to lipopeptides, it did not induce heterodimerization of the extracellular domains of TLR2 and TLR6. Although requiring TLR6 for activity, CgLM, similarly to S. pneumoniae LTA, may bind TLR6 with a weak affinity, and this might be correlated to the lower pro-inflammatory activity observed for LTA molecules [7] and CgLM as compared to lipopeptides. Nevertheless, the low affinity for TLR6 is most probably partly compensated in physiological conditions by the usage of soluble or membrane-anchored accessory receptors, such as CD14, LBP or CD36, that increase the bioavailability of the ligands [36], [37], [38]. As such, it is worth noting that cytokine production elicited by the different stimuli was investigated in the present study in non-differentiated THP-1 cells; in PMA-differentiated THP-1 cells, SaLTA was found to induce much higher concentrations of pro-inflammatory cytokines, although still less than the Pam3CSK4 lipopeptide [39].
In conclusion, our results expand the repertoire of MAMPs recognized by TLR2 to DAG-based lipoglycans and confirm the major role played by this receptor in the detection of Gram-positive bacteria [40]. Moreover, they reinforce the paradigm that macroamphiphiles based on a glycosylated DAG lipid anchor, such as LTA and alternative lipoglycans, induce TLR2-dependant innate immune responses. Although the diacylglyceryl unit, which is common to all the macroamphiphiles recognized by TLR2, is also found in lipids of higher eukaryotes, its substitution by a glycosylated polyglycerolphosphate chain in LTA, a (α1→6)-mannopyranosyl chain in alternative lipoglycans, a (α1→6)-mannopyranosyl chain linked to an acylated mannosyl-phosphoinositol in mycobacterial lipoglycans or a cysteine in lipopeptides/lipoproteins make the resulting molecules unique signatures of bacteria.
Materials and Methods
Lipoglycan purification – TLR2 ligands
DAG-based lipoglycans were purified as previously described from M. luteus (MlLM) [27], [28], C. glutamicum (CgLM) [30] and S. mucilaginosus (SmLM) [29]. MPI-based lipomannan was purified from Mycobacterium smegmatis (MsLM) [26]. Pam3CSK4, FSL-1 and SaLTA were purchased from InvivoGen. Lipoglycans, lipopeptides and LTA were deacylated by incubating with 2M NH4OH for 2h at 110°C.
HEK-TLR2 experiments
The HEK-Blue™-2 cell line (InvivoGen), a derivative of HEK293 cells that stably expresses the human TLR2 and CD14 genes along with a NF-κB-inducible reporter system (secreted alkaline phosphatase) was used according to the manufacturer’s instruction. The different stimuli were added, at concentrations indicated in the figure legends, in 96-wells plates and cells were then distributed at 5×104 cells per well in 200 µl DMEM culture medium (Lonza). Alkaline phosphatase activity was measured after 18 h by mixing 20 µl of the culture supernatant and 180 µl of Quanti-Blue™, and reading O.D. at 630 nm. To investigate the TLR dependence of stimuli activity, HEK-TLR2 cells were pre-incubated for 30 min at 37°C, before stimuli addition, with various antibodies: 5 µg/ml of monoclonal anti-TLR2 (clone T2.5, InvivoGen) or 10 µg/ml monoclonal anti-TLR1 (Clone H2G2, InvivoGen), monoclonal anti-TLR6 (Clone C5C8, InvivoGen) or an IgG1 isotype control (eBioscience).
THP-1 experiments
THP-1-Dual™ cells (Invivogen), derivatives of THP-1 monocyte/macrophage human cells that stably express a NF-κB-inducible reporter system (secreted alkaline phosphatase), were used according to the manufacturer’s instruction. The different stimuli were added, at concentrations indicated in the figure legends, in 96-wells plates and cells were then distributed at 105 cells per well in 200 µl RPMI 1640 culture medium (Lonza). After 18 h, NF-κB activation was measured as described above and cytokines were assayed in the culture supernatant by sandwich ELISA using commercially available kits (eBioscience). To investigate TLR2 dependence, cells were pre-incubated for 30 min at 37°C, before stimuli addition, with 10 µg/ml of anti-TLR2 monoclonal antibody (clone T2.5, InvivoGen) or isotype control (IgG1, eBioscience). For monitoring CD40 and MHC-II expression, cells were harvested and resuspended in Dubelco’s PBS, 0.5% BSA and labelled with CD40-PE or MHC-II-FITC conjugated antibody (Beckman Coulter). Cells were subjected to flow cytometry analysis by using the CellQuest software on a flow cytometer (FACSCalibur; Becton Dickinson).
Statistical analysis
Results are expressed as a mean ± SD and were analyzed using One-way analysis of variance followed by Tukey test to determine significant differences between samples.
Acknowledgments
We gratefully acknowledge Miss Fanny Pineau for helpful technical assistance.
Funding Statement
This work was supported by CNRS and Université de Toulouse. RC and AR are recipients of PhD fellowships from the French Ministère de l’Enseignement Supérieur et de la Recherche. AR was awarded a “ Fin de Thèse ” fellowship from Fondation pour la Recherche Médicale (FRM). LB PhD fellowship was sponsored by Cayla-Invivogen (Toulouse) and the Région Midi-Pyrénées. AKM acknowledges the support and sponsorship from Prof Douglas Young and MRC Grant MRC U117581288. GSB acknowledges support in the form of a Personal Research Chair from Mr James Bardrick, Royal Society Wolfson Research Merit Award, as a former Lister Institute-Jenner Research Fellow, the Medical Research Council and the Wellcome Trust (081569/Z/06/Z). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Medzhitov R (2007) Recognition of microorganisms and activation of the immune response. Nature 449: 819–826. [DOI] [PubMed] [Google Scholar]
- 2. Iwasaki A, Medzhitov R (2010) Regulation of adaptive immunity by the innate immune system. Science 327: 291–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akira S, Uematsu S, Takeuchi O (2006) Pathogen recognition and innate immunity. Cell 124: 783–801. [DOI] [PubMed] [Google Scholar]
- 4. Moresco EM, LaVine D, Beutler B (2011) Toll-like receptors. Curr Biol 21: R488–493. [DOI] [PubMed] [Google Scholar]
- 5. Thomas CJ, Schroder K (2013) Pattern recognition receptor function in neutrophils. Trends Immunol 34: 317–328. [DOI] [PubMed] [Google Scholar]
- 6. O'Neill LA, Golenbock D, Bowie AG (2013) The history of Toll-like receptors - redefining innate immunity. Nat Rev Immunol 13: 453–460. [DOI] [PubMed] [Google Scholar]
- 7. Zahringer U, Lindner B, Inamura S, Heine H, Alexander C (2008) TLR2 - promiscuous or specific? A critical re-evaluation of a receptor expressing apparent broad specificity. Immunobiology 213: 205–224. [DOI] [PubMed] [Google Scholar]
- 8. Ray A, Cot M, Puzo G, Gilleron M, Nigou J (2013) Bacterial cell wall macroamphiphiles: pathogen-/microbe-associated molecular patterns detected by mammalian innate immune system. Biochimie 95: 33–42. [DOI] [PubMed] [Google Scholar]
- 9. Rahman O, Dover LG, Sutcliffe IC (2009) Lipoteichoic acid biosynthesis: two steps forwards, one step sideways? Trends Microbiol 17: 219–225. [DOI] [PubMed] [Google Scholar]
- 10.Fischer W (1994) Lipoteichoic acids and lipoglycans. In: , editors. Bacterial cell wall. Amsterdam, The Netherlands.: Elsevier Science B.V. pp. 199–215.
- 11. Jin MS, Lee JO (2008) Structures of the toll-like receptor family and its ligand complexes. Immunity 29: 182–191. [DOI] [PubMed] [Google Scholar]
- 12. Kang JY, Lee JO (2011) Structural biology of the Toll-like receptor family. Annu Rev Biochem 80: 917–941. [DOI] [PubMed] [Google Scholar]
- 13. Ozinsky A, Underhill DM, Fontenot JD, Hajjar AM, Smith KD, et al. (2000) The repertoire for pattern recognition of pathogens by the innate immune system is defined by cooperation between toll-like receptors. Proc Natl Acad Sci U S A 97: 13766–13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Takeuchi O, Kawai T, Muhlradt PF, Morr M, Radolf JD, et al. (2001) Discrimination of bacterial lipoproteins by Toll-like receptor 6. Int Immunol 13: 933–940. [DOI] [PubMed] [Google Scholar]
- 15. Takeuchi O, Sato S, Horiuchi T, Hoshino K, Takeda K, et al. (2002) Cutting edge: role of Toll-like receptor 1 in mediating immune response to microbial lipoproteins. J Immunol 169: 10–14. [DOI] [PubMed] [Google Scholar]
- 16. Jin MS, Kim SE, Heo JY, Lee ME, Kim HM, et al. (2007) Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 130: 1071–1082. [DOI] [PubMed] [Google Scholar]
- 17. Kang JY, Nan X, Jin MS, Youn SJ, Ryu YH, et al. (2009) Recognition of lipopeptide patterns by Toll-like receptor 2-Toll-like receptor 6 heterodimer. Immunity 31: 873–884. [DOI] [PubMed] [Google Scholar]
- 18. Sutcliffe IC, Shaw N (1991) Atypical lipoteichoic acids of gram-positive bacteria. J Bacteriol 173: 7065–7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sutcliffe IC (1994) The lipoteichoic acids and lipoglycans of Gram-positive Bacteria: A chemotaxonomic perspective. System Appl Microbiol 17: 467–480. [Google Scholar]
- 20. Chatterjee D, Khoo KH (1998) Mycobacterial lipoarabinomannan: an extraordinary lipoheteroglycan with profound physiological effects. Glycobiology 8: 113–120. [DOI] [PubMed] [Google Scholar]
- 21. Nigou J, Gilleron M, Puzo G (2003) Lipoarabinomannans: from structure to biosynthesis. Biochimie 85: 153–166. [DOI] [PubMed] [Google Scholar]
- 22. Torrelles JB, Schlesinger LS (2010) Diversity in Mycobacterium tuberculosis mannosylated cell wall determinants impacts adaptation to the host. Tuberculosis (Edinb) 90: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Briken V, Porcelli SA, Besra GS, Kremer L (2004) Mycobacterial lipoarabinomannan and related lipoglycans: from biogenesis to modulation of the immune response. Mol Microbiol 53: 391–403. [DOI] [PubMed] [Google Scholar]
- 24. Henneke P, Morath S, Uematsu S, Weichert S, Pfitzenmaier M, et al. (2005) Role of lipoteichoic acid in the phagocyte response to group B streptococcus. J Immunol 174: 6449–6455. [DOI] [PubMed] [Google Scholar]
- 25. Bunk S, Sigel S, Metzdorf D, Sharif O, Triantafilou K, et al. (2010) Internalization and coreceptor expression are critical for TLR2-mediated recognition of lipoteichoic acid in human peripheral blood. J Immunol 185: 3708–3717. [DOI] [PubMed] [Google Scholar]
- 26. Gilleron M, Nigou J, Nicolle D, Quesniaux V, Puzo G (2006) The acylation state of mycobacterial lipomannans modulates innate immunity response through toll-like receptor 2. Chem Biol 13: 39–47. [DOI] [PubMed] [Google Scholar]
- 27. Pakkiri LS, Waechter CJ (2005) Dimannosyldiacylglycerol serves as a lipid anchor precursor in the assembly of the membrane-associated lipomannan in Micrococcus luteus . Glycobiology 15: 291–302. [DOI] [PubMed] [Google Scholar]
- 28. Pakkiri LS, Wolucka BA, Lubert EJ, Waechter CJ (2004) Structural and topological studies on the lipid-mediated assembly of a membrane-associated lipomannan in Micrococcus luteus . Glycobiology 14: 73–81. [DOI] [PubMed] [Google Scholar]
- 29. Sutcliffe IC, Old LA (1995) Stomatococcus mucilaginosus produces a mannose-containing lipoglycan rather than lipoteichoic acid. Arch Microbiol 163: 70–75. [DOI] [PubMed] [Google Scholar]
- 30. Tatituri RV, Illarionov PA, Dover LG, Nigou J, Gilleron M, et al. (2007) Inactivation of Corynebacterium glutamicum NCgl0452 and the role of MgtA in the biosynthesis of a novel mannosylated glycolipid involved in lipomannan biosynthesis. J Biol Chem 282: 4561–4572. [DOI] [PubMed] [Google Scholar]
- 31. Mishra AK, Klein C, Gurcha SS, Alderwick LJ, Babu P, et al. (2008) Structural characterization and functional properties of a novel lipomannan variant isolated from a Corynebacterium glutamicum pimB' mutant. Antonie Van Leeuwenhoek 94: 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Krishna S, Ray A, Dubey SK, Larrouy-Maumus G, Chalut C, et al. (2011) Lipoglycans contribute to innate immune detection of mycobacteria. PLoS One 6: e28476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, et al. (2005) Toll-like receptor 6-independent signaling by diacylated lipopeptides. Eur J Immunol 35: 282–289. [DOI] [PubMed] [Google Scholar]
- 34. Buwitt-Beckmann U, Heine H, Wiesmuller KH, Jung G, Brock R, et al. (2006) TLR1- and TLR6-independent recognition of bacterial lipopeptides. J Biol Chem 281: 9049–9057. [DOI] [PubMed] [Google Scholar]
- 35. Nigou J, Vasselon T, Ray A, Constant P, Gilleron M, et al. (2008) Mannan chain length controls lipoglycans signaling via and binding to TLR2. J Immunol 180: 6696–6702. [DOI] [PubMed] [Google Scholar]
- 36. Hermann C, Spreitzer I, Schroder NW, Morath S, Lehner MD, et al. (2002) Cytokine induction by purified lipoteichoic acids from various bacterial species—role of LBP, sCD14, CD14 and failure to induce IL-12 and subsequent IFN-gamma release. Eur J Immunol 32: 541–551. [DOI] [PubMed] [Google Scholar]
- 37. Schroder NW, Morath S, Alexander C, Hamann L, Hartung T, et al. (2003) Lipoteichoic acid (LTA) of Streptococcus pneumoniae and Staphylococcus aureus activates immune cells via Toll-like receptor (TLR)-2, lipopolysaccharide-binding protein (LBP), and CD14, whereas TLR-4 and MD-2 are not involved. J Biol Chem 278: 15587–15594. [DOI] [PubMed] [Google Scholar]
- 38. Hoebe K, Georgel P, Rutschmann S, Du X, Mudd S, et al. (2005) CD36 is a sensor of diacylglycerides. Nature 433: 523–527. [DOI] [PubMed] [Google Scholar]
- 39. Cot M, Ray A, Gilleron M, Vercellone A, Larrouy-Maumus G, et al. (2011) Lipoteichoic acid in Streptomyces hygroscopicus: structural model and immunomodulatory activities. PLoS One 6: e26316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Elson G, Dunn-Siegrist I, Daubeuf B, Pugin J (2007) Contribution of Toll-like receptors to the innate immune response to Gram-negative and Gram-positive bacteria. Blood 109: 1574–1583. [DOI] [PubMed] [Google Scholar]