Abstract
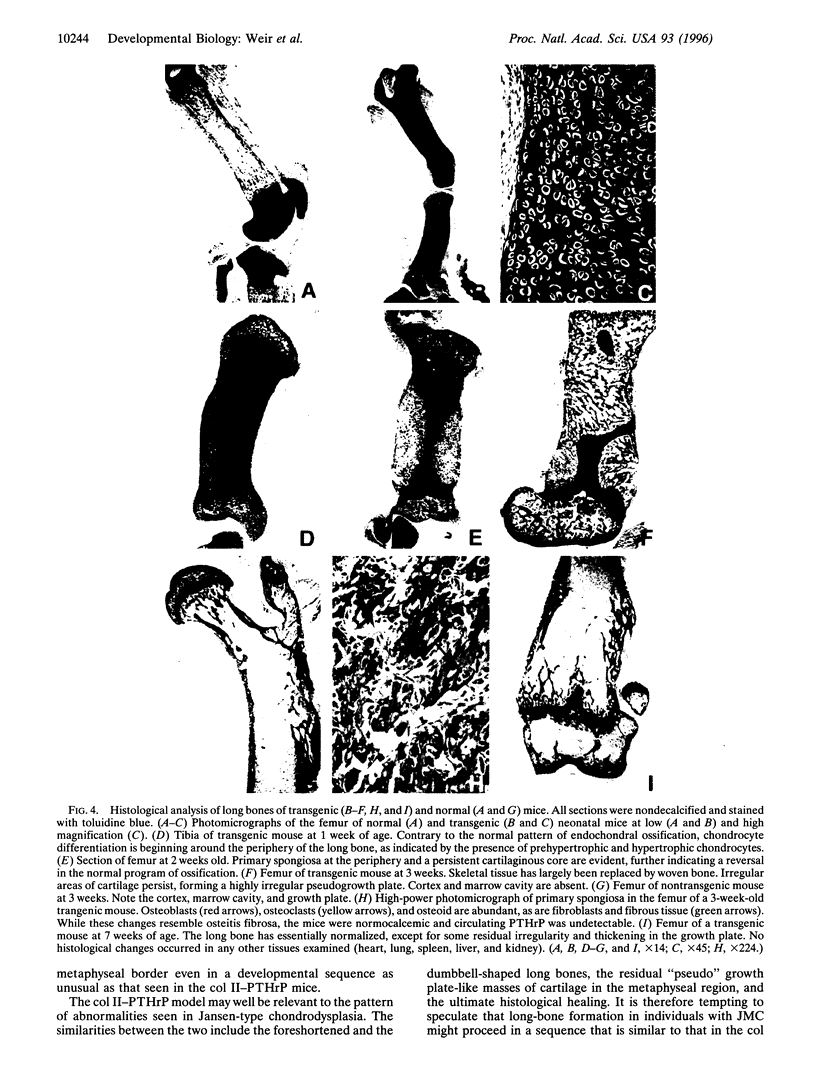
Parathyroid hormone-related peptide (PTHrP) was initially identified as a product of malignant tumors that mediates paraneoplastic hypercalcemia. It is now known that the parathyroid hormone (PTH) and PTHrP genes are evolutionarily related and that the products of these two genes share a common receptor, the PTH/PTHrP receptor. PTHrP and the PTH/PTHrP receptor are widely expressed in both adult and fetal tissues, and recent gene-targeting and disruption experiments have implicated PTHrP as a developmental regulatory molecule. Apparent PTHrP functions include the regulation of endochondral bone development, of hair follicle formation, and of branching morphogenesis in the breast. Herein, we report that overexpression of PTHrP in chondrocytes using the mouse type II collagen promoter induces a novel form of chondrodysplasia characterized by short-limbed dwarfism and a delay in endochondral ossification. This features a delay in chondrocyte differentiation and in bone collar formation and is sufficiently marked that the mice are born with a cartilaginous endochondral skeleton. In addition to the delay, chondrocytes in the transgenic mice initially become hypertrophic at the periphery of the developing long bones rather than in the middle, leading to a seeming reversal in the pattern of chondrocyte differentiation and ossification. By 7 weeks, the delays in chondrocyte differentiation and ossification have largely corrected, leaving foreshortened and misshapen but histologically near-normal bones. These findings confirm a role for PTHrP as an inhibitor of the program of chondrocyte differentiation. PTHrP may function in this regard to maintain the stepwise differentiation of chondrocytes that initiates endochondral ossification in the midsection of endochondral bones early in development and that also permits linear growth at the growth plate later in development.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amizuka N., Warshawsky H., Henderson J. E., Goltzman D., Karaplis A. C. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J Cell Biol. 1994 Sep;126(6):1611–1623. doi: 10.1083/jcb.126.6.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah K. S., Lau E. T., Au P. K., Tam P. P. Expression of the mouse alpha 1(II) collagen gene is not restricted to cartilage during development. Development. 1991 Apr;111(4):945–953. doi: 10.1242/dev.111.4.945. [DOI] [PubMed] [Google Scholar]
- Erlebacher A., Filvaroff E. H., Gitelman S. E., Derynck R. Toward a molecular understanding of skeletal development. Cell. 1995 Feb 10;80(3):371–378. doi: 10.1016/0092-8674(95)90487-5. [DOI] [PubMed] [Google Scholar]
- Gardner E. The embryology of the clavicle. Clin Orthop Relat Res. 1968 May-Jun;58:9–16. [PubMed] [Google Scholar]
- Henderson J. E., Amizuka N., Warshawsky H., Biasotto D., Lanske B. M., Goltzman D., Karaplis A. C. Nucleolar localization of parathyroid hormone-related peptide enhances survival of chondrocytes under conditions that promote apoptotic cell death. Mol Cell Biol. 1995 Aug;15(8):4064–4075. doi: 10.1128/mcb.15.8.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton W., Miyashita T., Kohno K., Hassell J. R., Yamada Y. Identification of a phenotype-specific enhancer in the first intron of the rat collagen II gene. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8864–8868. doi: 10.1073/pnas.84.24.8864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamoto M., Jikko A., Murakami H., Shimazu A., Nakashima K., Iwamoto M., Takigawa M., Baba H., Suzuki F., Kato Y. Changes in parathyroid hormone receptors during chondrocyte cytodifferentiation. J Biol Chem. 1994 Jun 24;269(25):17245–17251. [PubMed] [Google Scholar]
- Jüppner H., Abou-Samra A. B., Freeman M., Kong X. F., Schipani E., Richards J., Kolakowski L. F., Jr, Hock J., Potts J. T., Jr, Kronenberg H. M. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991 Nov 15;254(5034):1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- Karaplis A. C., Luz A., Glowacki J., Bronson R. T., Tybulewicz V. L., Kronenberg H. M., Mulligan R. C. Lethal skeletal dysplasia from targeted disruption of the parathyroid hormone-related peptide gene. Genes Dev. 1994 Feb 1;8(3):277–289. doi: 10.1101/gad.8.3.277. [DOI] [PubMed] [Google Scholar]
- Lee K., Deeds J. D., Segre G. V. Expression of parathyroid hormone-related peptide and its receptor messenger ribonucleic acids during fetal development of rats. Endocrinology. 1995 Feb;136(2):453–463. doi: 10.1210/endo.136.2.7835276. [DOI] [PubMed] [Google Scholar]
- Martin T. J. Properties of parathyroid hormone-related protein and its role in malignant hypercalcaemia. Q J Med. 1990 Aug;76(280):771–786. [PubMed] [Google Scholar]
- McLeod M. J. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology. 1980 Dec;22(3):299–301. doi: 10.1002/tera.1420220306. [DOI] [PubMed] [Google Scholar]
- Metsäranta M., Garofalo S., Smith C., Niederreither K., de Crombrugghe B., Vuorio E. Developmental expression of a type II collagen/beta-galactosidase fusion gene in transgenic mice. Dev Dyn. 1995 Oct;204(2):202–210. doi: 10.1002/aja.1002040211. [DOI] [PubMed] [Google Scholar]
- Metsäranta M., Toman D., de Crombrugghe B., Vuorio E. Mouse type II collagen gene. Complete nucleotide sequence, exon structure, and alternative splicing. J Biol Chem. 1991 Sep 5;266(25):16862–16869. [PubMed] [Google Scholar]
- Sandberg M., Vuorio E. Localization of types I, II, and III collagen mRNAs in developing human skeletal tissues by in situ hybridization. J Cell Biol. 1987 Apr;104(4):1077–1084. doi: 10.1083/jcb.104.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savagner P., Miyashita T., Yamada Y. Two silencers regulate the tissue-specific expression of the collagen II gene. J Biol Chem. 1990 Apr 25;265(12):6669–6674. [PubMed] [Google Scholar]
- Schipani E., Kruse K., Jüppner H. A constitutively active mutant PTH-PTHrP receptor in Jansen-type metaphyseal chondrodysplasia. Science. 1995 Apr 7;268(5207):98–100. doi: 10.1126/science.7701349. [DOI] [PubMed] [Google Scholar]
- Strewler G. J., Nissenson R. A. Hypercalcemia in malignancy. West J Med. 1990 Dec;153(6):635–640. [PMC free article] [PubMed] [Google Scholar]
- Vikkula M., Metsäranta M., Syvänen A. C., Ala-Kokko L., Vuorio E., Peltonen L. Structural analysis of the regulatory elements of the type-II procollagen gene. Conservation of promoter and first intron sequences between human and mouse. Biochem J. 1992 Jul 1;285(Pt 1):287–294. doi: 10.1042/bj2850287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. Q., Balakir R., Horton W. E., Jr Identification of a cis-acting sequence in the collagen II enhancer required for chondrocyte expression and the binding of a chondrocyte nuclear factor. J Biol Chem. 1991 Oct 25;266(30):19878–19881. [PubMed] [Google Scholar]
- Weir E. C., Horowitz M. C., Baron R., Centrella M., Kacinski B. M., Insogna K. L. Macrophage colony-stimulating factor release and receptor expression in bone cells. J Bone Miner Res. 1993 Dec;8(12):1507–1518. doi: 10.1002/jbmr.5650081214. [DOI] [PubMed] [Google Scholar]
- Wood A., Ashhurst D. E., Corbett A., Thorogood P. The transient expression of type II collagen at tissue interfaces during mammalian craniofacial development. Development. 1991 Apr;111(4):955–968. doi: 10.1242/dev.111.4.955. [DOI] [PubMed] [Google Scholar]
- Wysolmerski J. J., Broadus A. E., Zhou J., Fuchs E., Milstone L. M., Philbrick W. M. Overexpression of parathyroid hormone-related protein in the skin of transgenic mice interferes with hair follicle development. Proc Natl Acad Sci U S A. 1994 Feb 1;91(3):1133–1137. doi: 10.1073/pnas.91.3.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski J. J., McCaughern-Carucci J. F., Daifotis A. G., Broadus A. E., Philbrick W. M. Overexpression of parathyroid hormone-related protein or parathyroid hormone in transgenic mice impairs branching morphogenesis during mammary gland development. Development. 1995 Nov;121(11):3539–3547. doi: 10.1242/dev.121.11.3539. [DOI] [PubMed] [Google Scholar]