Abstract
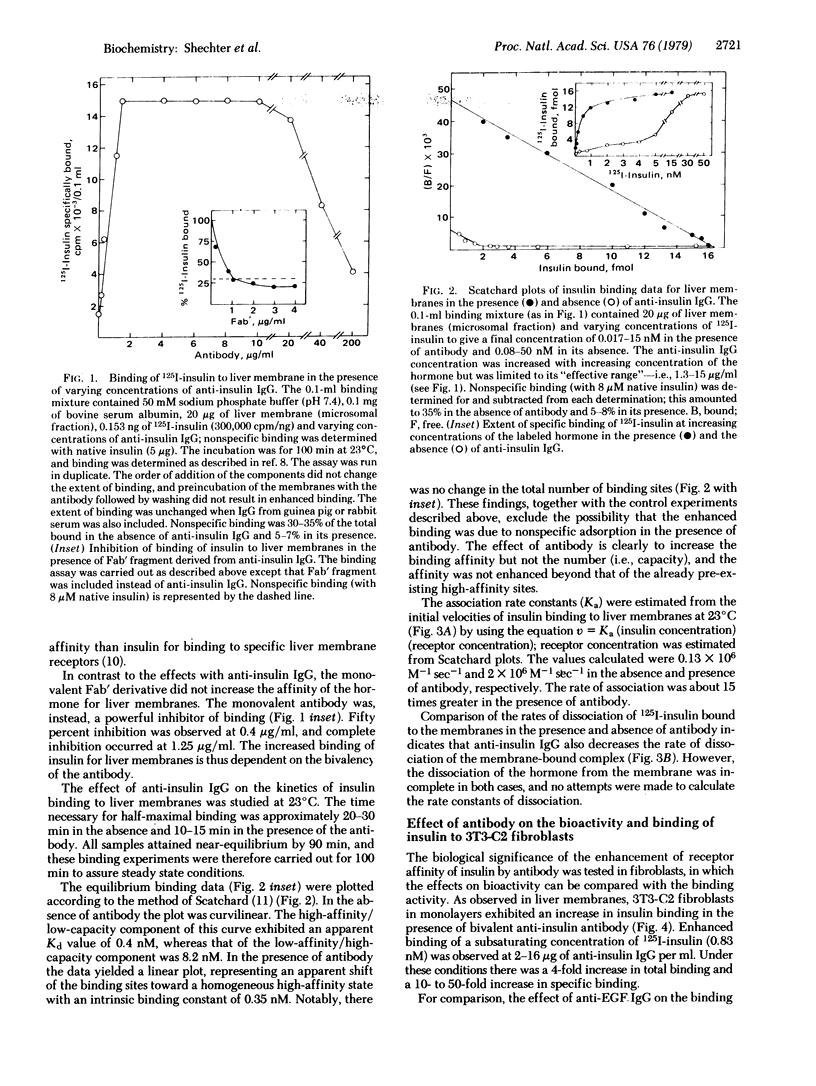
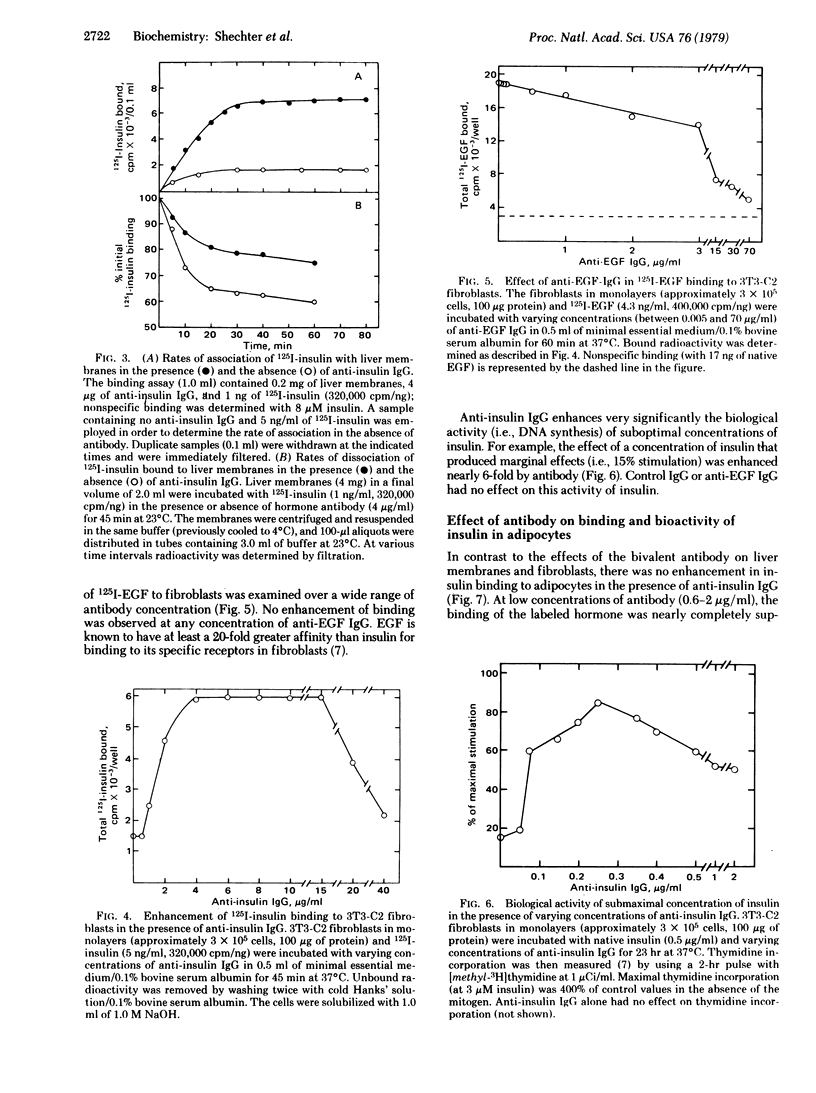
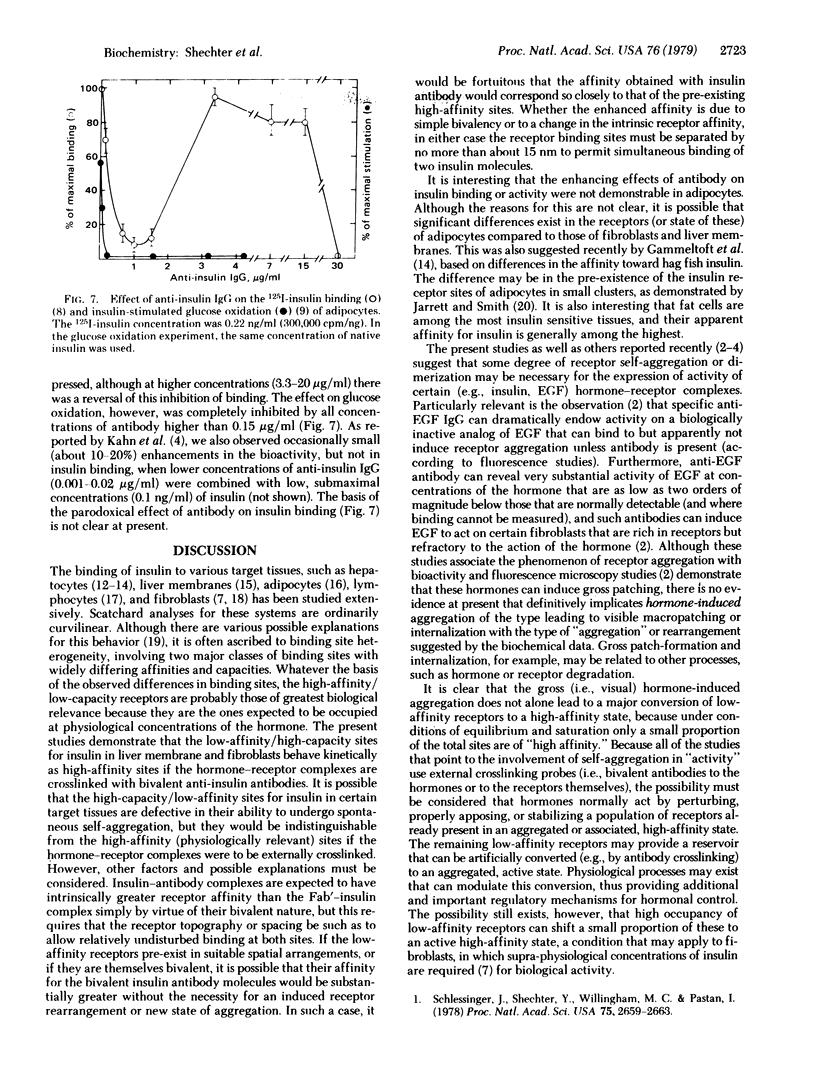
Incubation of physiological concentrations of 125I-labeled insulin with liver membranes in the presence of anti-insulin IgG results in a 7- to 15-fold increase in the specific binding of the hormone. The low-affinity/high-capacity binding sites are replaced by an apparently homogeneous class of high-affinity sites, and the nonlinear Scatchard plots are converted to linear plots without a change in the maximum number of binding sites. Similarly, the binding of insulin to receptors in 3T3 fibroblasts is increased substantially in the presence of anti-insulin antibody, and the biological activity of subactive concentrations of insulin is enhanced by antibody in these cells. However, the affinity of 125I-labeled epidermal growth factors (EGF) in fibroblasts is not affected by anti-EGF IgG. In adipocytes anti-insulin IgG in the same concentration range only inhibits the binding of insulin and suppresses insulin-mediated glucose oxidation. Monovalent Fab' fragments from anti-insulin IgG inhibit the binding of the hormone, indicating that the enhancement of binding in liver membranes and fibroblasts requires the bivalency of the antibody.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cuatrecasas P., Hollenberg M. D. Membrane receptors and hormone action. Adv Protein Chem. 1976;30:251–451. doi: 10.1016/s0065-3233(08)60481-7. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Properties of the insulin receptor isolated from liver and fat cell membranes. J Biol Chem. 1972 Apr 10;247(7):1980–1991. [PubMed] [Google Scholar]
- Gammeltoft S., Kristensen L. O., Sestoft L. Insulin receptors in isolated rat hepatocytes. Reassessment of binding properties and observations of the inactivation of insulin at 37 degrees C. J Biol Chem. 1978 Dec 10;253(23):8406–8413. [PubMed] [Google Scholar]
- Gavin J. R., 3rd, Gorden P., Roth J., Archer J. A., Buell D. N. Characteristics of the human lymphocyte insulin receptor. J Biol Chem. 1973 Mar 25;248(6):2202–2207. [PubMed] [Google Scholar]
- Hollenberg M. D., Cuatrecasas P. Insulin and epidermal growth factor. Human fibroblast receptors related to deoxyribonucleic acid synthesis and amino acid uptake. J Biol Chem. 1975 May 25;250(10):3845–3853. [PubMed] [Google Scholar]
- Jacobs S., Chang K-J, Cuatrecasas P. Estimation of hormone receptor affinity by competitive displacement of labeled ligand: effect of concentration of receptor and of labeled ligand. Biochem Biophys Res Commun. 1975 Sep 16;66(2):687–692. doi: 10.1016/0006-291x(75)90564-1. [DOI] [PubMed] [Google Scholar]
- Jacobs S., Chang K. J., Cuatrecasas P. Antibodies to purified insulin receptor have insulin-like activity. Science. 1978 Jun 16;200(4347):1283–1284. doi: 10.1126/science.663609. [DOI] [PubMed] [Google Scholar]
- Jarett L., Smith R. M. The natural occurrence of insulin receptors in groups on adipocyte plasma membranes as demonstrated with monomeric ferritin-insulin. J Supramol Struct. 1977;6(1):45–59. doi: 10.1002/jss.400060104. [DOI] [PubMed] [Google Scholar]
- Kahn C. R., Baird K. L., Jarrett D. B., Flier J. S. Direct demonstration that receptor crosslinking or aggregation is important in insulin action. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4209–4213. doi: 10.1073/pnas.75.9.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn C. R., Freychet P., Roth J., Neville D. M., Jr Quantitative aspects of the insulin-receptor interaction in liver plasma membranes. J Biol Chem. 1974 Apr 10;249(7):2249–2257. [PubMed] [Google Scholar]
- Olefsky J., Johnson J., Liu F., Edwards P., Baur S. Comparison of 125-I-insulin binding and degradation to isolated rat hepatocytes and liver membranes. Diabetes. 1975 Sep;24(9):801–810. doi: 10.2337/diab.24.9.801. [DOI] [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Rechler M. M., Podskalny J. M. Insulin receptors in cultured human fibroblasts. Diabetes. 1976 Apr;25(4):250–255. doi: 10.2337/diab.25.4.250. [DOI] [PubMed] [Google Scholar]
- Savage C. R., Jr, Cohen S. Epidermal growth factor and a new derivative. Rapid isolation procedures and biological and chemical characterization. J Biol Chem. 1972 Dec 10;247(23):7609–7611. [PubMed] [Google Scholar]
- Schechter Y., Hernaez L., Schlessinger J., Cuatrecasas P. Local aggregation of hormone-receptor complexes is required for activation by epidermal growth factor. Nature. 1979 Apr 26;278(5707):835–838. doi: 10.1038/278835a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Willingham M. C., Pastan I. Direct visualization of binding, aggregation, and internalization of insulin and epidermal growth factor on living fibroblastic cells. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2659–2663. doi: 10.1073/pnas.75.6.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]



