Abstract
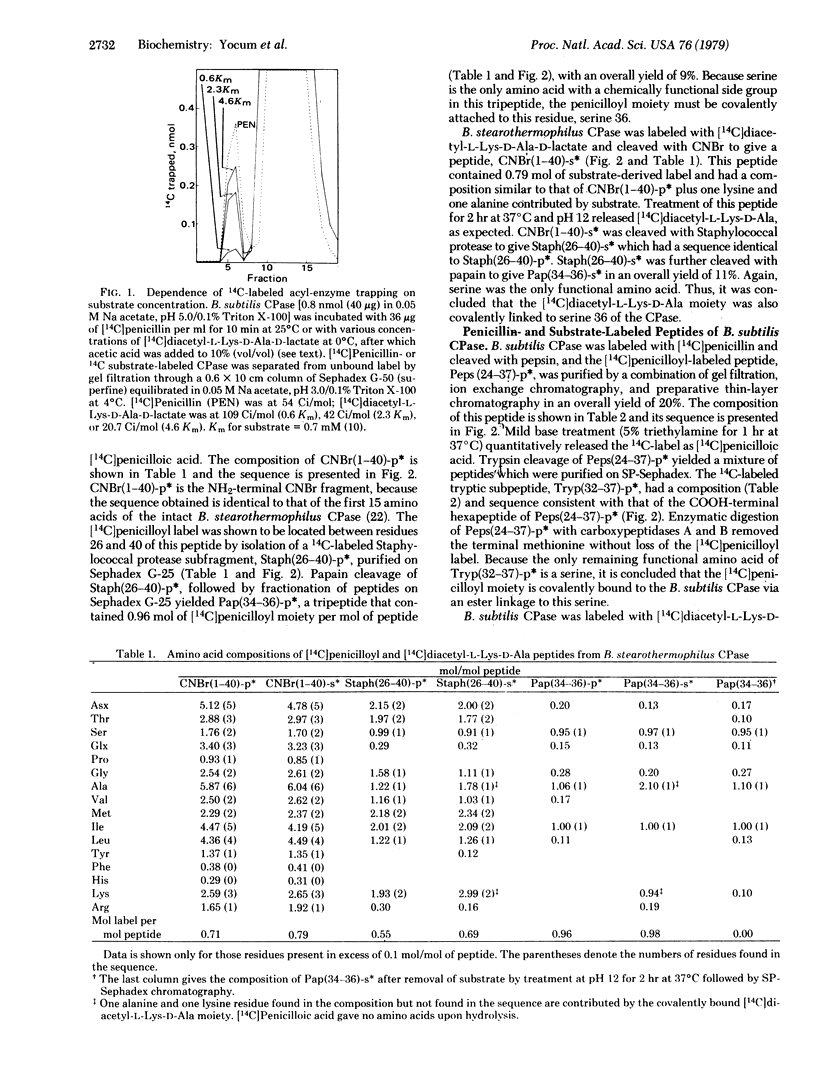
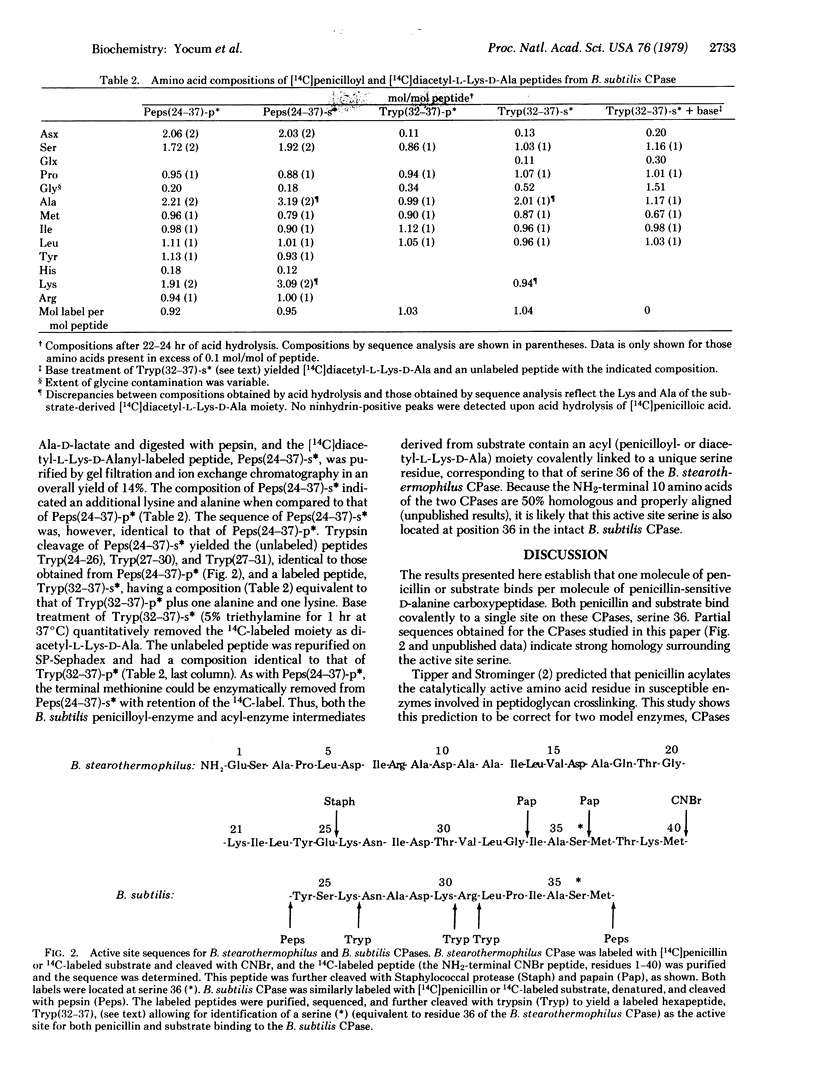
It has been hypothesized that penicillin acts as a structural analog of the acyl-D-alanyl-D-alanine terminus of nascent bacterial cell wall and that it consequently binds to and acylates the active site of the enzyme(s) that crosslinks the cell wall to form an inactive penicilloyl enzyme [Tipper, D.J. & Strominger, J.L. (1965) Proc. Natl. Acad. Sci. USA 64, 1133-1138]. This study directly proves that penicillin acylates the active site of two penicillin-sensitive enzymes, D-alanine carboxypeptidases from Bacillus stearothermophilus and Bacillus subtilis. Active site peptides were generated by chemical or enzymatic cleavage of these carboxypeptidases after covalently labeling with [14C]penicillin G or after trapping an acyl-enzyme intermediate derived from the depsipeptide substrate. [14C]diacetyl-L-lysyl-D-alanyl-D-lactate. The amino acid sequences of the penicillin- and substrate-labeled peptides were identical. Both penicillin and substrate were covalently bound via an ester linkage to the same active site residue, a serine at position 36 of the B. stearothermophilus carboxypeptidase and the corresponding serine in the B. subtilis carboxypeptidase. The two D-alanine carboxypeptidases showed significant homology around the active site. Moreover, homology between these two enzymes and four beta-lactamases of known sequence suggests that these two groups of enzymes are evolutionally related.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg P. M., Strominger J. L. Interaction of penicillin with the bacterial cell: penicillin-binding proteins and penicillin-sensitive enzymes. Bacteriol Rev. 1974 Sep;38(3):291–335. doi: 10.1128/br.38.3.291-335.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Yocum R. R., Willoughby E., Strominger J. L. Binding of (14C)penicillin G to the membrane-bound and the purified D-alanine carboxypeptidases from Bacillus stearothermophilus and Bacillus subtilis and its release. J Biol Chem. 1974 Nov 10;249(21):6828–6835. [PubMed] [Google Scholar]
- Boyd D. B. Transition state structures of a dipeptide related to the mode of action of beta-lactam antibiotics. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5239–5243. doi: 10.1073/pnas.74.12.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis S. J., Strominger J. L. Effects of sulfhydryl reagents on the binding and release of penicillin G by D-alanine carboxypeptidase IA of Escherichia coli. J Biol Chem. 1978 Apr 25;253(8):2584–2588. [PubMed] [Google Scholar]
- Frere J., Ghuysen J., Degelaen J., Loffet A., Perkins H. R. Fragmentation of benzylpenicillin after interaction with the exocellular DD-carboxypeptidase-transpeptidases of Streptomyces R61 and R39. Nature. 1975 Nov 13;258(5531):168–170. doi: 10.1038/258168a0. [DOI] [PubMed] [Google Scholar]
- Frère J. M., Duez C., Ghuysen J. M., Vandekerkhove J. Occurrence of a serine residue in the penicillin-binding site of the exocellular DD-carboxy-peptidase-transpeptidase from Streptomyces R61. FEBS Lett. 1976 Nov;70(1):257–260. doi: 10.1016/0014-5793(76)80770-3. [DOI] [PubMed] [Google Scholar]
- Georgopapadakou N., Hammarström S., Strominger J. L. Isolation of the penicillin-binding peptide from D-alanine carboxypeptidase of Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1009–1012. doi: 10.1073/pnas.74.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghuysen J. M., Leyh-Bouille M., Frère J. M., Dusart J., Marquet A. The penicillin receptor in Streptomyces. Ann N Y Acad Sci. 1974 May 10;235(0):236–268. doi: 10.1111/j.1749-6632.1974.tb43269.x. [DOI] [PubMed] [Google Scholar]
- Hammarström S., Strominger J. L. Degradation of penicillin G to phenylacetylglycine by D-alanine carboxypeptidase from Bacillus stearothermophilus. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3463–3467. doi: 10.1073/pnas.72.9.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Knott-Hunziker V., Waley S. G., Orlek B. S., Sammes P. G. Penicillinase active sites: labelling of serine-44 in beta-lactamase I by 6beta-bromopenicillanic acid. FEBS Lett. 1979 Mar 1;99(1):59–61. doi: 10.1016/0014-5793(79)80248-3. [DOI] [PubMed] [Google Scholar]
- Kozarich J. W., Nishino T., Willoughby E., Strominger J. L. Hydroxylaminolysis of penicillin binding componenets is enzymatically catalyzed. J Biol Chem. 1977 Nov 10;252(21):7525–7529. [PubMed] [Google Scholar]
- Kozarich J. W., Strominger J. L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978 Feb 25;253(4):1272–1278. [PubMed] [Google Scholar]
- Lawrence P. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XV. The binding of radioactive penicillin to the particulate enzyme preparation of Bacillus subtilis and its reversal with hydroxylamine or thiols. J Biol Chem. 1970 Jul 25;245(14):3653–3659. [PubMed] [Google Scholar]
- Lee B. Conformation of penicillin as a transition-state analog of the substrate of peptidoglycan transpeptidase. J Mol Biol. 1971 Oct 28;61(2):463–469. doi: 10.1016/0022-2836(71)90393-7. [DOI] [PubMed] [Google Scholar]
- Marquet A., Frère J. M., Ghuysen J. M., Loffet A. Effects of nucleophiles on the breakdown of the benzylpenicilloyl-enzyme complex EI formed between benzylpenicillin and the exocellular DD-carboxypeptidase--transpeptiase of Streptomyces strain R61. Biochem J. 1979 Mar 1;177(3):909–916. doi: 10.1042/bj1770909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino T., Kozarich J. W., Strominger J. L. Kinetic evidence for an acyl-enzyme intermediate in D-alanine carboxypeptidases of Bacillus subtilis and Bacillus stearothermophilus. J Biol Chem. 1977 May 10;252(9):2934–2939. [PubMed] [Google Scholar]
- Pratt R. F., Loosemore M. J. 6-beta-bromopenicillanic acid, a potent beta-lactamase inhibitor. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4145–4149. doi: 10.1073/pnas.75.9.4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen J. R., Strominger J. L. Utilization of a depsipeptide substrate for trapping acyl-enzyme intermediates of penicillin-sensitive D-alanine carboxypeptidases. Proc Natl Acad Sci U S A. 1978 Jan;75(1):84–88. doi: 10.1073/pnas.75.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHEPARTZ S. A., JOHNSON M. J. The nature of the binding of penicillin by bacterial cells. J Bacteriol. 1956 Jan;71(1):84–90. doi: 10.1002/path.1700710112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer R. T., Anderegg R. Primary structure of the lambda repressor. Biochemistry. 1978 Mar 21;17(6):1092–1100. doi: 10.1021/bi00599a024. [DOI] [PubMed] [Google Scholar]
- Tamura T., Imae Y., Strominger J. L. Purification to homogeneity and properties of two D-alanine carboxypeptidases I From Escherichia coli. J Biol Chem. 1976 Jan 25;251(2):414–423. [PubMed] [Google Scholar]
- Tipper D. J., Strominger J. L. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1133–1141. doi: 10.1073/pnas.54.4.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise E. M., Jr, Park J. T. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proc Natl Acad Sci U S A. 1965 Jul;54(1):75–81. doi: 10.1073/pnas.54.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum R. R., Blumberg P. M., Strominger J. L. Purification and characterization of the thermophilic D-alanine carboxypeptidase from membranes of Bacillus stearothermophilus. J Biol Chem. 1974 Aug 10;249(15):4863–4871. [PubMed] [Google Scholar]



