Abstract
Aims
Our aims were to study the histologic changes in non-neoplastic pancreas and the effects on pancreatic intraepithelial neoplasia (PanIN) after neoadjuvant chemoradiation therapy (NCRT) for pancreatic ductal adenocarcinoma (PDAC).
Methods and results
We reviewed the archival H & E slides from 218 patients with PDAC who completed NCRT and pancreaticoduodenectomy. Sixty-five patients who underwent pancreaticoduodenectomy for PDAC without NCRT were used as control. Various histologic features were reviewed and correlated with NCRT and survival. The NCRT group compared to control group had lower density of PanIN2 (p=0.004) and PanIN3 (p=0.02). The extent of fibrosis, prevalence of neuroma-like nerve proliferation and islet cell aggregation were significantly higher in the NCRT group than control group (P<0.05). The intensity of inflammation was less in NCRT group than control group (p=0.02). Patents with moderate to severe fibrosis or grade 2 inflammation had poor survival than those with mild fibrosis (p=0.04) or those with grade 0 or grade 1 inflammation in NCRT group (p=0.003).
Conclusions
Patients who received NCRT had reduced density of high-grade PanIN lesions, more pancreatic fibrosis, higher frequencies of neuroma-like nerve proliferation and islet cell aggregation, but less inflammation in the non-neoplastic pancreas than those who did not receive NCRT.
Keywords: pancreatic cancer, neoadjuvant therapy, pancreatic intraepithelial neoplasia (PanIN), fibrosis, neuroma-like nerve proliferation, inflammation, islet cell aggregation
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a deadly disease with approximately 5% five-year survival. Traditional approach for patients with potentially resectable PDAC is to perform upfront pancreatectomy followed by post-operative adjuvant chemotherapy with or without radiation therapy. Adjuvant chemoradiation therapies have been shown to improve patient survival1–4. However, despite significant improvement in post-operative mortality rates and advancement in surgical and medical oncology, the overall survival for patients with PDAC shows marginal improvement in the last four decades5.
Recently, several tertiary referral centers worldwide, including our institution, have demonstrated the safety and feasibility of neoadjuvant chemoradiation therapy (NCRT) for patients with potentially resectable PDAC6–10. In some studies, survival of patients with potentially resectable PDAC treated with NCRT has been shown to be better than historical controls6, 10–13. In our previous studies, we demonstrated that systematic pathologic evaluation of the post-therapy pancreatectomy specimens plays a critical role in predicting the prognosis in patients with PDAC who completed NCRT and underwent pancreatectomy14–19. In this group of patients, the post-therapy American Joint Committee on Cancer (AJCC) stage, histologic grading of residual PDAC, tumor invasion into the muscular vessel(s) and perineural invasion are important prognostic factors for patients survival14–17, 19. Our previous study also showed that tumor invasion into the wall or lumen of the resected segment or portion of superior mesenteric vein/portal vein is associated with poor survival in patients with stage II PDAC who completed NCRT and underwent pancreatectomy18. However, the changes in non-neoplastic pancreatic tissue associated with NCRT have not been examined in detail. In this study, we reviewed the histologic features in non-neoplastic pancreatic tissue, including the frequency and density of pancreatic intraepithelial neoplasia (PanIN) of different histologic grades; neuroma-like nerve proliferation; islet cell aggregation; pancreatic fibrosis and inflammation from 218 patients with PDAC who completed NCRT and underwent pancreaticoduodenectomy at our institution. The findings were compared to the control group, which included 65 patients who underwent pancreaticoduodenectomy for PDAC without NCRT during the same time period. Our findings suggest that multiple histologic changes in non-neoplastic pancreatic tissue are commonly associated with pre-operative NCRT. By comparing the frequency and density of PanIN lesions of different histologic grades between these two groups, our study also provides new insights into the effects of NCRT on PanIN lesions.
Materials and Methods
Patient population
This study was approved by the Institutional Review Board at the University of Texas MD Anderson Cancer Center. The study population consisted of 218 patients (89 females and 129 males) with biopsy-proven PDAC who had completed NCRT and undergone pancreaticoduodenectomy from January of 1999 to December of 2007 (NCRT group) at the University of Texas MD Anderson Cancer Center. Sixty-five patients (29 females and 36 males) with PDAC who had undergone pancreaticoduodenectomy, but did not receive NCRT during the same time period were used as the control group. Patients who underwent distal pancreatectomy for PDAC or pancreaticoduodenectomy for other types of pancreatic neoplasms were excluded from this study. The clinical classifications for resectable disease are determined using multi-detector, contrast-enhanced computed tomography (CT). Patients had resectable tumor if they met all following criteria: (1) absence of metastases; (2) patent SMV-PV confluence; (3) Fat plane between the primary tumor and SMA (no involvement of SMA); and (4) no involvement of celiac axis. Our approach is heavily biased towards the NCRT treatment. Only the following patients underwent up front surgery: (1) inability to obtain a tissue diagnosis for NCRT; (2) contraindication to chemoradiation; (3) inability to obtain durable biliary decompression to start chemotherapy or chemoradiation; or (4) patient choice.20 For the patients who received NCRT, restaging evaluation after completion of NCRT was performed in all patients and the pancreaticoduodenectomy was performed only in patients with resectable disease who had no disease progression or metastasis and had no contraindications to major abdominal surgery. Patients' clinical and follow-up information was extracted from a prospectively maintained database. All clinical and follow-up data were verified by an independent review of patient medical records and the U.S. Social Security Index, when necessary.
Pathologic examination
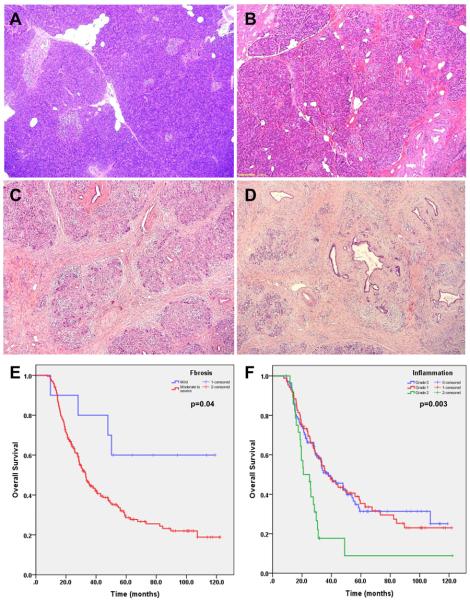
The archival hematoxylin-and-eosin (H & E) stained slides of the non-neoplastic pancreatic tissue from the pancreaticoduodenectomy specimens of all patients were reviewed by a pathologist (D.C.) who was blinded to all clinical, treatment-related, and follow-up information. The number of slides of non-neoplastic pancreatic tissue reviewed ranged from 1 to 45 with the mean of 8.9±6.6 in the study group and 1 to 22 with the mean of 7.7±5.5 in the control group (P>0.05). Pancreatic intraepithelial neoplasia (PanIN) were classified into PanIN1, PanIN2 and PanIN3 (Figure 1) using the criteria described previously21. The total number of PanIN lesions of different histologic grades in all non-neoplastic pancreatic tissue slides in each case was counted. When PanIN lesions of different grades were present in the same pancreatic duct, they were counted as separate PanIN lesions. Abrupt transition of highly atypical epithelial cells to normal ductal epithelium was considered as cancerization of the ducts, not PanIN3. PanIN3 lesions were counted in the benign pancreatic tissue greater than one high power field away from the invasive carcinoma. The areas of non-neoplastic pancreatic tissue were measured in cm2 by light microscopy (Olympus BX41). The density of PanIN lesions was calculated as a ratio of the total number of PanIN lesions of each grade divided by the total area of non-neoplastic pancreatic sections in each case. The average amount of pancreatic atrophy, and intralobular and interlobular fibrosis was scored using a four-tiered system based on the criteria proposed by van Geenen et al.22 as follows: No fibrosis (0–5% pancreatic fibrosis); mild (>5%, but ≤25% pancreatic fibrosis), moderate (>25%, but ≤50% pancreatic fibrosis) or severe (>50% pancreatic fibrosis, Figure 2A–2D). Islet cell aggregation was arbitrarily defined as the presence of enlarged islets (≥2.5 mm), aggregation of islets or budding of endocrine cells from the ductal epithelium (ductuloinsular complexes) with islet cells accounting for 10% or more of total cellularity of the non-neoplastic pancreatic parenchyma (Figure 3A, 3B). The degree of inflammation in the non-neoplastic pancreatic tissue was scored based on the number of inflammatory cells in the non-neoplastic pancreatic tissue using the modified grading system proposed by Wang et al23 as 0 (no significant or minimal inflammatory cell infiltrate, ≤50 inflammatory cells/10 high-power fields); 1 (50–500 inflammatory cells/10 high-power fields); and 2 (>500 inflammatory cells/10 high-power fields, Figure 3C–3D). The neuroma-like nerve proliferation was defined as haphazardly distributed, hypertrophic nerve bundles within fibrous stroma in the peripancreatic tissue, unrelated to perineural invasion (Figure 3F).
Figure 1.
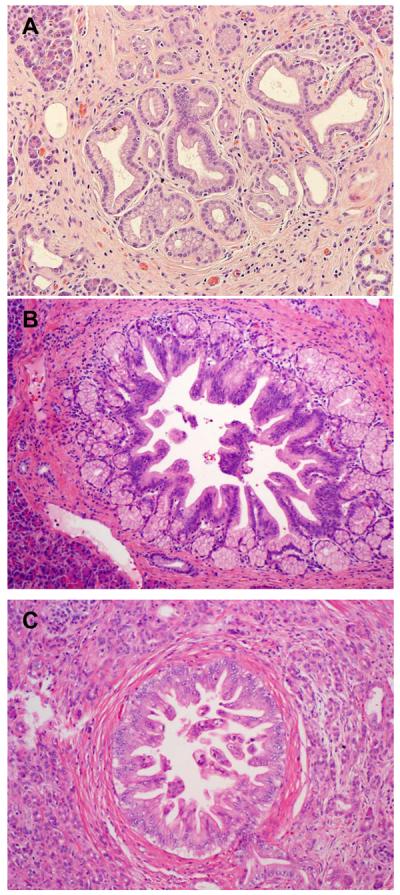
Representative micrographs show pancreatic intraepithelial neoplasia 1 (PanIN1, A), PanIN2 (B) and PanIN3 (C). Hematoxylin & eosin stain, original magnifications: 200×.
Figure 2.
Representative micrographs of non-neoplastic pancreas with no fibrosis in a patient who did not receive NCRT (A), and mild (B), moderate (C) and severe fibrosis (D) in patients who received NCRT. Hematoxylin & eosin stain, original magnifications: 20×; E. Kaplan-Meier survival curves of overall survival for patients with mild pancreatic fibrosis compared to those with moderate to severe fibrosis in NCRT group. The patients with mild pancreatic fibrosis had better survival than those patients who had moderate to severe pancreatic fibrosis in NCRT group (P=0.04). No difference in survival between the patients with moderate and severe pancreatic fibrosis was observed (P>0.05). F. Kaplan-Meier survival curves of overall survival for patients stratified by the grade of inflammatory infiltrate in non-neoplastic pancreatic tissue. Patients with grade 2 inflammation in non-neoplastic pancreas had poor survival than those with grade 0 and grade 1 inflammation in NCRT group (P=0.003).
Figure 3.
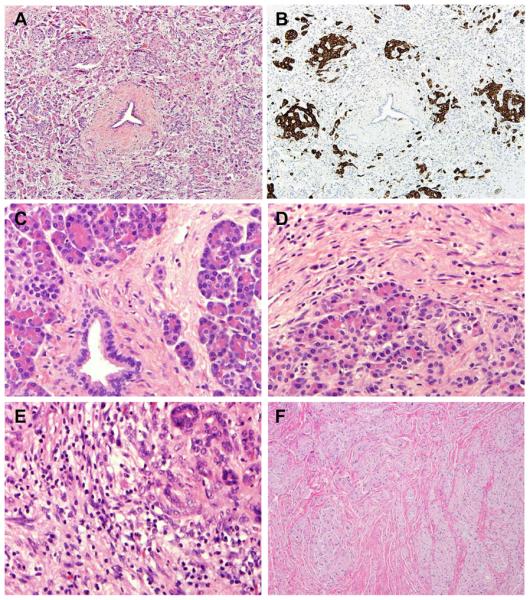
Representative micrographs show non-neoplastic pancreas tissue with islet cell aggregation in a patient who received NCRT (A and B), no inflammation (C), mild (D), and severe inflammation (E) and neuroma-like nerve proliferation in peripancreatic soft tissue in a patient who received NCRT (F). A, C–F, hematoxylin & eosin stain; B, immunohistochemical stain for synaptophysin; original magnifications: 40× for A, B, and F; 400× for C–E.
Statistical analysis
Chi-square analysis was used to compare categorical data and independent t tests were used to compare continuous variables, with two-sided p value of 0.05 or less considered as significant. Overall survivals were calculated as the time intervals between the date of diagnosis and date of death or the date of last follow up if death did not occur. The Kaplan-Meier method was used to construct the survival curves and the statistical significance of differences in survival was evaluated by the log-rank test. All statistical analyses were performed using IBM SPSS Statistics, Version 19 for Windows (IBM Corporation 2012, Somers, NY).
Results
Clinicopathologic Data
The clinical, pathologic and the NCRT information for the study and control groups are compared in Table 1. In the NCRT group, 37 (17.0%) received fluoropyrimidine-based chemoradiation (Group 1), 71 (32.6%) received gemcitabine-based chemoradiation (Group 2), 72 (33.0%) received systemic chemotherapy followed by gemcitabine-based chemoradiation (Group 3), 32 (14.7%) received systemic chemotherapy followed by fluoropyrimidine-based chemoradiation (Group 4) and the remaining six patients (2.7%) received neoadjuvant systemic chemotherapy alone (Group 5). The age of the patients ranged from 38 to 85 years with the median age of 63.0 years in the NCRT group and ranged from 24 to 80 years with the median age of 61.2 years in the control group. The NCRT group had smaller tumor sizes (p=0.01), lower frequency of lymph node metastasis (p=0.001) and lower AJCC stage than the control group (p<0.001). However, there were no significant differences in tumor differentiation, margin status, and pathologic tumor (pT) stage between these two groups (P>0.05).
Table 1.
Comparison of the Clinicopathologic Parameters Between the Treated and Control Groups
| Parameters | Treated group (n=218) | Control group (n=65) | P value |
|---|---|---|---|
| Median age at diagnosis (range, years) | 63.0 (38.9 – 85.4) | 61.2 (24.9 – 80.9) | 0.48 |
| Gender | 0.67 | ||
| Male | 129 (59.2%) | 36 (55.4%) | |
| Female | 89 (40.8%) | 29 (44.6%) | |
| Neoadjuvant Therapy | |||
| Group 1 | 37 (17.0%) | NA | |
| Group 2 | 71 (32.6%) | NA | |
| Group 3 | 72 (33.0%) | NA | |
| Group 4 | 32 (14.7%) | NA | |
| Group 5 | 6 (2.7%) | NA | |
| Median tumor size (range, cm) | 2.5 (0.1 – 8.5) | 3.0 (0.8–7.5) | 0.01 |
| Differentiation | 0.36 | ||
| Well | 1 (0.5%) | 2 (3.1%) | |
| Moderate | 135 (61.9%) | 42 (64.6%) | |
| Poor | 81 (37.2%) | 21 (32.3%) | |
| Margin | 0.50 | ||
| Negative | 195 (89.4%) | 56 (86.2%) | |
| Positive | 23 (10.6%) | 9 (13.8%) | |
| Lymph node status | 0.001 | ||
| Negative | 95 (43.6%) | 14 (21.5%) | |
| Positive | 123 (56.4%) | 51 (78.5%) | |
| Tumor stage (pT) | 0.21 | ||
| T0 | 1 (0.5%) | 0 (0%) | |
| T1 & T2 | 15 (6.9%) | 2 (3.1%) | |
| T3 | 202 (92.6%) | 62 (95.4%) | |
| T4 | 0 (0%) | 1 (1.5%) | |
| AJCC Stage | 0.000 | ||
| 0 | 1 (0.5%) | 0 (0%) | |
| IA & IB | 12 (5.5%) | 2 (3.1%) | |
| IIA | 82 (37.6%) | 12 (18.5%) | |
| IIB | 123 (56.4%) | 47 (72.3%) | |
| IV | 0 (0%) | 4 (6.2%) |
Frequency and density of PanIN Lesions
The frequencies of PanIN1, PanIN2, and PanIN3 were 84.4%, 72.5%, and 37.2%, respectively, in the NCRT groups compared to 87.7%, 80.0%, and 40%, respectively, in the control group (P>0.05, Table 2). The NCRT group had significantly lower mean densities for PanIN2 and PanIN3 lesions compared to the control group (0.82 ± 1.25 versus 1.42 ± 2.00, P=0.004; and 0.08 ± 0.21 versus 0.16 ± 0.31, P=0.02, respectively, Table 2). There was no significant difference in the mean density of PanIN1 between the NCRT and the control groups (P=0.20). Our data suggests that NCRT may reduce the density of high-grade PanIN lesions. When the densities of PanIN lesions among the patients who received different NCRT regimens were compared, the densities of PanIN1, PanIN2 and PanIN3 were lower in those who received systemic chemotherapy before chemoradiation (Groups 3, 4 and 5) compared to those who received chemoradiation therapies alone (Groups 1 and 2, P<0.05, Table 3). No correlations between the PanIN lesions and other clinicopathologic parameters or survival were identified.
Table 2.
The Frequency and Mean Density of Pancreatic Intraepithelial Neoplasia (PanIN) in Non-neoplastic Pancreas between the Treated and Control Groups
| Histologic parameter | Treated group (n=218) | Control group (n=65) | P value |
|---|---|---|---|
| Presence of PanIN | |||
| PanIN1 | 184 (84.4%) | 57 (87.7%) | >0.05 |
| PanIN2 | 158 (72.5%) | 52 (80.0%) | >0.05 |
| PanIN3 | 81 (37.2%) | 26 (40%) | >0.05 |
| PanIN Density (Mean ± SD, per cm2) | |||
| PanIN1 | 1.38 ± 1.56 | 1.69 ± 2.18 | 0.20 |
| PanIN2 | 0.82 ± 1.25 | 1.42 ± 2.00 | 0.004 |
| PanIN3 | 0.08 ± 0.21 | 0.16 ± 0.31 | 0.02 |
Table 3.
The Mean Density of Pancreatic Intraepithelial Neoplasia (PanIN) in Non-neoplastic Pancreas among Different NCRT Groups
| NCRT Groups | No of Patients | PanIN1 | PanIN2 | PanIN3 |
|---|---|---|---|---|
| PanIN Density (Mean ± SD, per cm2) | ||||
| Group 1 | 37 | 1.88 ± 2.72 | 1.21 ± 2.28 | 0.07 ± 0.15 |
| Group 2 | 71 | 1.58 ± 1.38 | 0.94 ± 1.09 | 0.13 ± 0.28 |
| Group 3 | 72 | 1.12 ± 0.95 | 0.61 ± 0.65 | 0.04 ± 0.15 |
| Group 4 | 32 | 0.99 ± 1.05 | 0.65 ± 0.89 | 0.04 ± 0.11 |
| Group 5 | 6 | 1.06 ± 0.85 | 0.56 ± 0.40 | 0.15 ± 0.37 |
| PanIN Density (Mean ± SD, per cm2) | ||||
| Group 1 and 2 | 108 | 1.69 ± 1.94 | 1.03 ± 1.60 | 0.11 ± 0.25 |
| Group 3, 4 and 5 | 110 | 1.08 ± 0.97 | 0.62 ± 0.72 | 0.05 ± 0.16 |
| P value | 0.04 | 0.02 | 0.02 |
Pancreatic atrophy, fibrosis and islet cell aggregation
No fibrosis, mild, moderate and severe fibrosis was observed in 18.5%, 44.6%, 27.7% and 9.2%, respectively, in the non-neoplastic pancreatic tissue in the control group. In contrast, all 218 cases (100%) in the NCRT group had mild to severe pancreatic atrophy and fibrosis (Table 4). Moderate to severe pancreatic fibrosis was observed in 95.4% and 36.9% in the NCRT and the control groups respectively. Compared to the control group, the non-neoplastic pancreas in the NCRT group had more pancreatic atrophy and fibrosis (p<0.001). In the NCRT group, patients who had mild fibrosis were associated with better survival than those with moderate to severe pancreatic fibrosis (p=0.04, Figure 2E). There was no significant difference in AJCC stage between the 10 NCRT patients with mild fibrosis (2 stage I, 1 stage IIA and 7 with stage IIB disease) and the NCRT patients with moderate to severe fibrosis (1 stage 0, 10 stage I, 81 stage IIA and 116 stage IIB) (P=0.24). Islet cell aggregation was present in 79 of 218 (36.2%) cases in the NCRT group compared to 5 of 65 (7.7%) in the control group (p<0.01, Table 4). However, there was no correlation between fibrosis and survival in the untreated group or between islet cell aggregation and survival in either NCRT group or control group (p>0.05).
Table 4.
Comparison of the Histologic Changes in Non-neoplastic Pancreatic Tissue between the Treated and Control Groups
| Histologic parameter | Treated group (n=218) | Control group (n=65) | P value |
|---|---|---|---|
| Fibrosis and atrophy | <0.001 | ||
| Absent | 0 (0%) | 12 (18.5%) | |
| Mild | 10 (4.6%) | 29 (44.6%) | |
| Moderate | 149 (68.3%) | 18 (27.7%) | |
| Severe | 59 (27.1%) | 6 (9.2%) | |
| Islet cell aggregation | <0.001 | ||
| Present | 79 (36.2%) | 5 (7.7%) | |
| Absent | 139 (63.8%) | 60 (92.3%) | |
| Inflammation | 0.02 | ||
| Grade 0 | 103 (47.3%) | 23 (35.4%) | |
| Grade 1 | 86 (39.4%) | 21 (32.3%) | |
| Grade 2 | 29 (13.3%) | 21 (32.3%) | |
| Neuroma-like nerve proliferation | 0.005 | ||
| Present | 34 (15.6%) | 2 (3.1%) | |
| Absent | 184 (84.4%) | 63 (96.9%) |
Inflammation and Neuroma-like nerve proliferation
In the NCRT group, 103 of 218 (47.3%) patients had grade 0 (no significant inflammation), 86 (39.4%) had grade 1 (mild to moderate inflammation), and 29 (13.3%) had grade 2 inflammation (severe inflammation). In comparison, grade 0, grade 1 and grade 2 inflammation were observed in 35.5%, 32.3%, and 32.3% respectively in the control group (p=0.02, Table 4). The inflammatory cells were predominantly lymphocytic. Neutrophilic infiltrate, plasma cells or other types of inflammatory cells were rare. Patients with grade 2 inflammation in non-neoplastic pancreas had poor survival (median survival: 20.1 ± 4.0 months) than those with grade 0 and grade 1 inflammation (median: 38.0± 6.3 months and 38.0 ± 4.7 months respectively) in NCRT group (p=0.003, Figure 2F). Neuroma-like nerve proliferation was present in 15.6% (34/218) in the NCRT group compared to 3.1% (2/65) in the control group (p=0.005, Table 4). In all cases that were positive for neuroma-like nerve proliferation, this was identified in the peripancreatic soft tissue and was not related to perineural invasion. No correlation between the grade of inflammation and survival in the untreated group and no correlation between neuroma-like nerve proliferation and survival were observed in either NCRT group or control group (p>0.05).
Discussion
In this study, we compared the incidence and density of PanIN lesions and other histologic features in non-neoplastic pancreatic tissue from 218 patients who completed NCRT and underwent pancreaticoduodenectomy for PDAC to 65 patients who received upfront pancreaticoduodenectomy, but no NCRT. We found that patients who received NCRT had lower mean density of PanIN lesions and less inflammation, but more severe pancreatic atrophy and fibrosis, higher incidences of neuroma-like nerve proliferation and islet aggregation than those who did not receive NCRT. Patents with moderate to severe fibrosis or grade 2 inflammation had poor survival than those with mild fibrosis (p=0.04) or those with grade 0 or grade 1 inflammation in NCRT group (p=0.003). Our results not only provide new insight into the effect of NCRT on PanIN lesions, but also identify other histologic changes in non-neoplastic pancreas after being treated with NCRT.
PanINs are microscopic precursor lesions for PDAC that are commonly identified in the pancreas resected for PDAC or other neoplastic and non-neoplastic diseases24. PanINs typically arise in small pancreatic ducts, but may involve large ducts and are graded as PanIN1, PanIN2, and PanIN3 based on the degree of cytologic atypia of the involved pancreatic ducts25. Molecular studies have demonstrated that many of the genetic changes seen in PDAC, such as activating KRAS mutations and inactivation of p16, TP53, and SMAD4 tumor suppressor genes, are present in PanIN lesions, particularly PanIN326, 27. Since PanIN lesions are microscopic and are usually not detectable by routine radiologic imaging, the clinical significance and management for PanIN lesions are unknown. The effect of chemotherapy and/or radiation therapy on PanIN lesions has not been examined previously. In this study, we systemically examined the frequency and density of PanIN lesions in the pancreaticoduodenectomy specimens from a large cohort of patients with PDAC who completed NCRT. The incidences of PanIN1, PanIN2 and PanIN3 in both the NCRT and the control groups in our study are comparable to the previously reported incidence of 82%, 52%, and 38% for PanIN1, PanIN2 and PanIN3, respectively, in patients with PDAC28. Although we did not observe significant differences in the incidence of PanIN lesions of any histologic grades between the NCRT group and the control group, we observed significantly lower mean densities of high-grade PanIN (PanIN2 and PanIN3) lesions in the NCRT group compared to the control group. Compared to the control group, 18%, 42% and 50% reduction in the densities of PanIN1, PanIN2 and PanIN3 respectively was observed in the NCRT group. In addition, our data also showed that patients who received neoadjuvant systemic chemotherapy prior to chemoradiation had lower density of PanIN lesions of all histologic grades than those who received pre-operative chemoradiation alone in our NCRT group. The mechanisms of neoadjuvant systemic chemotherapy on PanIN lesions are unknown. Since PanIN lesions share molecular changes similar to invasive PDAC, it is possible that neoadjuvant systemic chemotherapy may have direct killing effect on PanIN cells and/or prime the PanIN cells to make them more sensitive to subsequent NCRT therapy.
The effect of NCRT on the adjacent non-neoplastic pancreatic parenchyma in patients who received NCRT has not been previously reported. The histologic changes in the non-neoplastic pancreas after NCRT observed in this study are similar to the findings commonly seen in chronic pancreatitis, including pancreatic exocrine atrophy, fibrosis, islet cell aggregation and inflammation. However, there are no standardized grading schemes for each of the above-mentioned histologic parameters for chronic pancreatitis. Although Armann et al's grading system of pancreatic fibrosis (score 0–12), which is based on the intralobular fibrosis, perilobular fibrosis, and the distribution of fibrosis (focal or diffuse), have been shown to correlate with sonographic findings in patients with chronic pancreatitis29–31, we found that this grading system was difficult to apply in our study. Therefore we used the grading scheme for pancreatic fibrosis proposed by van Geenen et al22. This scoring system emphasizes a subdivision between intralobular, extralobular, and total pancreatic fibrosis based on routine H & E stained slides. Our study showed that the score of pancreatic fibrosis was higher in the NCRT group than the untreated control group, which is consistent with those observed in other organs treated with NCRT. It is interesting that patients with mild pancreatic fibrosis had better prognosis than those with moderate to severe pancreatic fibrosis in our NCRT group. However, the total number of patients with mild fibrosis (10 patients) is too small. Further studies are needed to confirm this finding.
In this study, we observed a significantly higher incidence of islet cell aggregation in NCRT group than the untreated control group, particularly in the non-neoplastic pancreatic parenchyma immediately adjacent to the tumor. In this study, we observed solid cords or nests of islet cells closely associated with pancreatic ducts or extending from pancreatic ducts more often in the NCRT group than the control group. This is most likely due to more severe atrophy and loss of pancreatic exocrine component and fibrosis secondary to NCRT-induced pancreatic injury in the NCRT group.
Inflammation plays an important role in the initiation and progression of PDAC32, 33. In this study, we found that the presence of grade 2 inflammation in non-neoplastic pancreatic tissue was associated with poor survival compared to those with grade 0 or grade 1 inflammation in our NCRT patient population, but not in untreated group. This finding was contrast to the previous study by Shia et al. in patients with rectal adenocarcinoma who received NCRT and resection. They found that fibrotic-type stromal response with minimal amount of tumor-associated inflammatory infiltrates is associated with a reduced recurrence free survival34. The difference between their study and ours may be due to the difference in tumor biology between rectal adenocarcinoma and PDAC. In addition, our study is focused on the inflammatory infiltrates in non-neoplastic pancreas and we did not examine the intra-tumoral inflammatory infiltrates. It is interesting that the inflammatory cell infiltrates was significantly lower in the NCRT group than in the control group. This may be due in part to the recovery of inflammation associated with NCRT since there is typically a 5–6 weeks resting interval between the completion of NCRT and surgery. It may also be due to the direct killing effect of NCRT on the inflammatory cells.
The pancreas is richly innervated by the vagus nerve and the sympathetic splanchnic nerves via the celiac and superior mesenteric plexuses. In this study, we found higher incidence of neuroma-like nerve proliferation in the NCRT group than the untreated control group. Chronic pancreatitis has been associated with increased neural proliferation irrespective of the type of initiating event35 and also with increased mean nerve diameters36. Ceyhan et al37 found increased neural density and hypertrophy in chronic pancreatitis and PDAC, which was strongly associated with GAP-43 overexpression and abdominal pain. Previous post-mortem study by Matsukuma et al showed that pancreatic neuroma-like lesions are associated with previous upper abdominal surgery (gastrectomy, esophagectomy, or esophageal transection), but were not correlated with degree of pancreatic fibrosis or abdominal pain38. However, the vast majority of patients with neuroma-like nerve proliferation in this study had no prior history of abdominal surgery. Although the mechanism leading to neuroma-like nerve proliferation is not clear in our study, it is possible that the NCRT-induced neural damage may induce the local production of excessive artemin and other neurotrophic factors by the ganglia or Schwann cells and stimulate neural proliferation and regeneration, which in turn leads to the neuroma-like nerve proliferation39.
In summary, our study identified multiple histologic changes that were associated with NCRT in the non-neoplastic pancreas in patients with PDAC who received NCRT and subsequent pancreaticoduodenectomy. This is the first demonstration that NCRT may reduce the density of PanIN lesions in patients with PDAC. The findings from this study will not only provide guidance for systematic histologic examination of non-neoplastic pancreas in patients who received NCRT, but also suggest a possible role for chemoprevention in the precursor lesions of pancreatic cancer.
Acknowledgments
Supported by the National Institutes of Health grant (1R21CA149544-01A1) and G. S. Hogan Gastrointestinal Cancer Research Fund at The University of Texas M.D. Anderson Cancer Center
References
- 1.Alberts SR, Gores GJ, Kim GP, et al. Treatment options for hepatobiliary and pancreatic cancer. Mayo Clin Proc. 2007;82:628–637. doi: 10.4065/82.5.628. [DOI] [PubMed] [Google Scholar]
- 2.Desai S, Ben-Josef E, Griffith KA, et al. Gemcitabine-based combination chemotherapy followed by radiation with capecitabine as adjuvant therapy for resected pancreas cancer. Int J Radiat Oncol Biol Phys. 2009;75:1450–1455. doi: 10.1016/j.ijrobp.2009.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Picozzi VJ, Kozarek RA, Traverso LW. Interferon-based adjuvant chemoradiation therapy after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am J Surg. 2003;185:476–480. doi: 10.1016/s0002-9610(03)00051-5. [DOI] [PubMed] [Google Scholar]
- 4.Regine WF, Winter KA, Abrams RA, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: A randomized controlled trial. JAMA. 2008;299:1019–1026. doi: 10.1001/jama.299.9.1019. [DOI] [PubMed] [Google Scholar]
- 5.Garcea G, Dennison AR, Pattenden CJ, Neal CP, Sutton CD, Berry DP. Survival following curative resection for pancreatic ductal adenocarcinoma. A systematic review of the literature. JOP. 2008;9:99–132. [PubMed] [Google Scholar]
- 6.Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3496–3502. doi: 10.1200/JCO.2007.15.8634. [DOI] [PubMed] [Google Scholar]
- 7.Le Scodan R, Mornex F, Girard N, et al. Preoperative chemoradiation in potentially resectable pancreatic adenocarcinoma: Feasibility, treatment effect evaluation and prognostic factors, analysis of the sfro-ffcd 9704 trial and literature review. Ann Oncol. 2009;20:1387–1396. doi: 10.1093/annonc/mdp015. [DOI] [PubMed] [Google Scholar]
- 8.Meszoely IM, Wang H, Hoffman JP. Preoperative chemoradiation therapy for adenocarcinoma of the pancreas: The fox chase cancer center experience, 1986–2003. Surg Oncol Clin N Am. 2004;13:685–696. x. doi: 10.1016/j.soc.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 9.Turrini O, Viret F, Moureau-Zabotto L, et al. Neoadjuvant 5 fluorouracil-cisplatin chemoradiation effect on survival in patients with resectable pancreatic head adenocarcinoma: A ten-year single institution experience. Oncology. 2009;76:413–419. doi: 10.1159/000215928. [DOI] [PubMed] [Google Scholar]
- 10.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol. 2008;26:3487–3495. doi: 10.1200/JCO.2007.15.8642. [DOI] [PubMed] [Google Scholar]
- 11.Moutardier V, Magnin V, Turrini O, et al. Assessment of pathologic response after preoperative chemoradiotherapy and surgery in pancreatic adenocarcinoma. Int J Radiat Oncol Biol Phys. 2004;60:437–443. doi: 10.1016/j.ijrobp.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Roy R, Maraveyas A. Chemoradiation in pancreatic adenocarcinoma: A literature review. Oncologist. 15:259–269. doi: 10.1634/theoncologist.2009-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Snady H, Bruckner H, Cooperman A, Paradiso J, Kiefer L. Survival advantage of combined chemoradiotherapy compared with resection as the initial treatment of patients with regional pancreatic carcinoma. An outcomes trial. Cancer. 2000;89:314–327. doi: 10.1002/1097-0142(20000715)89:2<314::aid-cncr16>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 14.Chatterjee D, Katz MH, Rashid A, et al. Histologic grading of the extent of residual carcinoma following neoadjuvant chemoradiation in pancreatic ductal adenocarcinoma: A predictor for patient outcome. Cancer. 2012;118:3182–3190. doi: 10.1002/cncr.26651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chatterjee D, Katz MH, Rashid A, et al. Perineural and intraneural invasion in posttherapy pancreaticoduodenectomy specimens predicts poor prognosis in patients with pancreatic ductal adenocarcinoma. Am J Surg Pathol. 2012;36:409–417. doi: 10.1097/PAS.0b013e31824104c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chatterjee D, Rashid A, Wang H, et al. Tumor invasion of muscular vessels predicts poor prognosis in patients with pancreatic ductal adenocarcinoma who have received neoadjuvant therapy and pancreaticoduodenectomy. Am J Surg Pathol. 2012;36:552–559. doi: 10.1097/PAS.0b013e318240c1c0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Estrella JS, Rashid A, Fleming JB, et al. Post-therapy pathologic stage and survival in patients with pancreatic ductal adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:268–277. doi: 10.1002/cncr.26243. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Estrella JS, Peng L, et al. Histologic tumor involvement of superior mesenteric vein/portal vein predicts poor prognosis in patients with stage ii pancreatic adenocarcinoma treated with neoadjuvant chemoradiation. Cancer. 2012;118:3801–3811. doi: 10.1002/cncr.26717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao Q, Rashid A, Gong Y, et al. Pathologic complete response to neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma is associated with a better prognosis. Annals of diagnostic pathology. 2012;16:29–37. doi: 10.1016/j.anndiagpath.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varadhachary GR, Tamm EP, Abbruzzese JL, et al. Borderline resectable pancreatic cancer: Definitions, management, and role of preoperative therapy. Annals of surgical oncology. 2006;13:1035–1046. doi: 10.1245/ASO.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 21.Haugk B. Pancreatic intraepithelial neoplasia-can we detect early pancreatic cancer? Histopathology. 2010;57:503–514. doi: 10.1111/j.1365-2559.2010.03610.x. [DOI] [PubMed] [Google Scholar]
- 22.van Geenen EJ, Smits MM, Schreuder TC, van der Peet DL, Bloemena E, Mulder CJ. Smoking is related to pancreatic fibrosis in humans. Am J Gastroenterol. 2011;106:1161–1166. doi: 10.1038/ajg.2011.43. quiz 1167. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Sternfeld L, Yang F, et al. Enhanced susceptibility to pancreatitis in severe hypertriglyceridaemic lipoprotein lipase-deficient mice and agonist-like function of pancreatic lipase in pancreatic cells. Gut. 2009;58:422–430. doi: 10.1136/gut.2007.146258. [DOI] [PubMed] [Google Scholar]
- 24.Andea A, Sarkar F, Adsay VN. Clinicopathological correlates of pancreatic intraepithelial neoplasia: A comparative analysis of 82 cases with and 152 cases without pancreatic ductal adenocarcinoma. Mod Pathol. 2003;16:996–1006. doi: 10.1097/01.MP.0000087422.24733.62. [DOI] [PubMed] [Google Scholar]
- 25.Hruban RH, Takaori K, Klimstra DS, et al. An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol. 2004;28:977–987. doi: 10.1097/01.pas.0000126675.59108.80. [DOI] [PubMed] [Google Scholar]
- 26.Hruban RH, Maitra A, Goggins M. Update on pancreatic intraepithelial neoplasia. Int J Clin Exp Pathol. 2008;1:306–316. [PMC free article] [PubMed] [Google Scholar]
- 27.Delpu Y, Hanoun N, Lulka H, et al. Genetic and epigenetic alterations in pancreatic carcinogenesis. Curr Genomics. 2011;12:15–24. doi: 10.2174/138920211794520132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Recavarren C, Labow DM, Liang J, et al. Histologic characteristics of pancreatic intraepithelial neoplasia associated with different pancreatic lesions. Human pathology. 2011;42:18–24. doi: 10.1016/j.humpath.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 29.Ammann RW, Heitz PU, Kloppel G. Course of alcoholic chronic pancreatitis: A prospective clinicomorphological long-term study. Gastroenterology. 1996;111:224–231. doi: 10.1053/gast.1996.v111.pm8698203. [DOI] [PubMed] [Google Scholar]
- 30.Chong AK, Hawes RH, Hoffman BJ, Adams DB, Lewin DN, Romagnuolo J. Diagnostic performance of eus for chronic pancreatitis: A comparison with histopathology. Gastrointest Endosc. 2007;65:808–814. doi: 10.1016/j.gie.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Albashir S, Bronner MP, Parsi MA, Walsh RM, Stevens T. Endoscopic ultrasound, secretin endoscopic pancreatic function test, and histology: Correlation in chronic pancreatitis. Am J Gastroenterol. 2010;105:2498–2503. doi: 10.1038/ajg.2010.274. [DOI] [PubMed] [Google Scholar]
- 32.Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- 33.Ling J, Kang Y, Zhao R, et al. Krasg12d-induced ikk2/beta/nf-kappab activation by il-1alpha and p62 feedforward loops is required for development of pancreatic ductal adenocarcinoma. Cancer cell. 2012;21:105–120. doi: 10.1016/j.ccr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shia J, Guillem JG, Moore HG, et al. Patterns of morphologic alteration in residual rectal carcinoma following preoperative chemoradiation and their association with long-term outcome. Am J Surg Pathol. 2004;28:215–223. doi: 10.1097/00000478-200402000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Friess H, Shrikhande S, Shrikhande M, et al. Neural alterations in surgical stage chronic pancreatitis are independent of the underlying aetiology. Gut. 2002;50:682–686. doi: 10.1136/gut.50.5.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bockman DE, Buchler M, Malfertheiner P, Beger HG. Analysis of nerves in chronic pancreatitis. Gastroenterology. 1988;94:1459–1469. doi: 10.1016/0016-5085(88)90687-7. [DOI] [PubMed] [Google Scholar]
- 37.Ceyhan GO, Bergmann F, Kadihasanoglu M, et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–186. e171. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 38.Matsukuma S, Sato K. Pancreatic neuroma-like lesions after upper abdominal surgery: A clinicopathological postmortem study. Virchows Arch. 2010;457:651–657. doi: 10.1007/s00428-010-0999-0. [DOI] [PubMed] [Google Scholar]
- 39.Ceyhan GO, Demir IE, Maak M, Friess H. Fate of nerves in chronic pancreatitis: Neural remodeling and pancreatic neuropathy. Best Pract Res Clin Gastroenterol. 2010;24:311–322. doi: 10.1016/j.bpg.2010.03.001. [DOI] [PubMed] [Google Scholar]