Abstract
Importance
Resting state functional connectivity magnetic resonance imaging (rs-fcMRI) has great potential for characterizing pathophysiological changes during the preclinical phase of Alzheimer’s disease (AD).
Objective
To assess the relationship between default mode network (DMN) integrity and cerebrospinal fluid (CSF) biomarkers of AD pathology in cognitively normal older individuals
Design
Cross-sectional cohort study
Setting
Knight Alzheimer’s Disease Research Center at Washington University in St Louis, Missouri.
Participants
207 older adults with normal cognition (Clinical Dementia Rating of 0).
Main Outcome measures
rs-fcMRI measures of DMN integrity.
Results
Decreased CSF Aβ42 or increased CSF phosphorylated tau181 (ptau181) were independently associated with reduced DMN integrity, with the most prominent decreases in functional connectivity observed between the posterior cingulate and medial temporal regions. Observed reductions in functional connectivity were not attributable to age or structural atrophy in the posterior cingulate and medial temporal areas. Similar rs-fcMRI findings in relation to CSF biomarkers were obtained using region-of-interest analyses and voxel-wise correlation mapping.
Conclusions
Both Aβ and tau pathology affect DMN integrity prior to clinical onset of AD.
Introduction
Accumulation of amyloid β (Aβ) and tau proteins, the pathologic hallmarks of Alzheimer’s disease (AD), starts years before clinical onset1–4. Pathophysiological abnormalities in the preclinical phase of AD may be detected using cerebrospinal fluid (CSF) and/or neuroimaging biomarkers. CSF biomarkers have been recognized as key elements of research criteria for the preclinical phases of AD5, 6. Resting state functional connectivity magnetic resonance imaging (rs-fcMRI)7, a non-invasive measure of brain integrity, has considerable potential in studies of preclinical AD5, 8,9.
CSF Aβ42 and amyloid imaging tracers such as Pittsburgh Compound B (PiB) measure amyloid burden in the brain10, 11. Both CSF tau and phosphorylated forms of tau (ptau) are hypothesized to reflect neurodegeneration and/or tau pathology12, 13. Symptomatic AD patients typically have a characteristic biomarker profile consisting of reduced CSF Aβ42, increased PiB binding in the brain, and elevated CSF tau and ptau11, 14. Cognitively normal individuals can also exhibit biomarker evidence of AD pathology, with Aβ abnormalities more prevalent than alterations in tau or ptau15–17.
rs-fcMRI abnormalities have been consistently observed in the default mode network (DMN) in symptomatic AD patients (for review see Greicius18). Aβ preferentially deposits in cortical association areas that prominently include nodes of the DMN19. Tau accumulation initially occurs in the limbic system20, a sub-component of the DMN21. More recent rs-fcMRI investigations have detected DMN changes in asymptomatic individuals with increased amyloid deposition using PiB22–24. However, the association between functional connectivity in the DMN and CSF biomarker abnormalities requires further study25, 26.
We investigated the relationship between CSF biomarkers (e.g., Aβ42 and ptau181) and rs-fcMRI in a large sample of cognitively normal individuals (N=207). We hypothesized that decreased CSF Aβ42 and increased ptau181levels would be associated with reduced DMN functional connectivity.
Materials and Methods
Participants
Participants were community-dwelling volunteers enrolled in aging and memory studies at the Charles F. and Joanne Knight Alzheimer’s Disease Research Center at Washington University in Saint Louis. Detailed information regarding recruitment has been previously published27. Inclusion criteria were: 1) completion of MRI scans and CSF collection within 12 months of clinical assessment, 2) normal cognition, determined by a Clinical Dementia Rating (CDR) of 028, at the assessments closest to the time of MRI scanning and CSF collection. Individuals were excluded if they had a medical or psychiatric illness that could affect longitudinal follow-up or adversely affect cognitive performance. All studies were approved by the Human Research Protection Office with written informed consent obtained from all participants.
Clinical assessment
An experienced clinician conducted separate semi-structured interviews with the participant and an informant, and determined the presence or absence of dementia based on the principle of intra-individual cognitive decline relative to prior functional level29. Only CDR 0 subjects (i.e., cognitively normal) were included in the primary analysis. 207 cognitively normal participants had both MRI scans and CSF collection within 12 months of clinical assessment. Demographic information and CSF biomarker profiles are provided in Table 1.
Table 1.
Demographics and cerebrospinal fluid biomarkers in cognitively normal participants
| Mean age (SD), year | 70.8(6.3) |
| Age range, year | 60–88 |
| Sex, % Male | 37.2 |
| Mean Education (SD), year | 15.7 (2.8) |
| Mean MMSE score (SD) | 28.8 (1.3) |
| CDR sum of boxes [mean (SD)] | 0.03 (0.13) |
| APOE genotype, % at least one ε4 allele | 31.4 |
| Cerebrospinal fluid biomarkers | |
| Mean Aβ42 (SD), pg/ml | 624 (254) |
| Aβ42-negative vs. positive | 136:71 |
| Mean ptau181 (SD), pg/ml | 61 (31) |
| Ptau181-negative vs. positive | 161:46 |
SD: standard deviation, MMSE: mini-mental state examination, for which the range of scores is from 30 (“best”) to 0 (“worst”), CDR sum of boxes: Clinical Dementia Rating sum of boxes (the sum of individual CDR domain scores) range from 0 to 18, with lower scores indicating better performance.
APOE: Apolipoprotein E, Aβ: amyloid-β, ptau: phosphorylated tau.
Genotyping
DNA was extracted from peripheral blood samples. Genotyping for apolipoprotein E (APOE) was performed using procedures previously described30.
CSF collection and analysis
CSF (20–30 mL) was collected at 8:00 AM after overnight fasting as previously described10. CSF samples were analyzed for Aβ42, tau, and ptau181 by plate-based enzyme-linked immunosorbent assay (INNOTEST; Innogenetics, Ghent, Belgium). Since CSF tau was highly correlated with CSF ptau181 in the present cohort (Spearman rho = 0.836, p<0.001), we report only CSF ptau181 in relation to functional connectivity. In addition, neither CSF tau nor ptau181 was correlated with CSF Aβ42 (both p ≥ 0.469).
Image acquisition and pre-processing of rs-fcMRI data
Participants were scanned using a Siemens Trio 3T Trio scanner (Siemens Medical Systems, Erlangen, Germany). Two high-resolution structural scans were obtained with T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) sequence (echo time [TE] = 16 msec, repetition time [TR] = 2,400 msec, inversion time [TI] = 1,000 msec, flip angle = 8°, 256 × 256 acquisition matrix, 1 × 1 × 1 mm voxels). High-resolution T2-weighted fast spin echo (FSE) images were acquired (TE = 455 msec, TR = 3,200 msec, 256 × 256 acquisition matrix, 1 × 1 × 1 mm voxels). Two resting rs-fcMRI scans (164 volumes each) were acquired using a gradient echo sequence (TE = 27 msec, TR = 2.2 sec, 64 × 64 acquisition matrix, flip angle = 90°). Thirty-six axial slices with no gap parallel to the anterior–posterior commissure line with approximately 4.0 mm cubic voxels provided whole-brain coverage. Participants were instructed to fixate on a visual cross-hair, remain still, and not fall asleep during scanning. Details concerning rs-fcMRI preprocessing are provided in eMethod.
Quality assurance (QA) of rs-fcMRI data
The QA procedures for rs-fcMRI data have been previously described31, 32. Briefly, fMRI data quality was assessed by computing voxelwise root mean squared (rms) temporal variance (sd) averaged over the whole brain. Individuals with a mean preprocessed fMRI signal sd > 2.5% (before nuisance regression) or rms head motion > 1.25 mm were excluded. In addition, frames (volumes) with high variance were identified and removed33, 34. The number of frames excluded due to high variance did not correlate with any CSF variable (eMethod).
Definition of regions of interest
Generation of regions of interest (ROIs) within the DMN have been previously described32. Briefly, rs-fcMRI data were analyzed from a separate cohort of eight participants with mild AD dementia (CDR 1) and eight cognitively normal participants (CDR 0). A 6-mm-radius sphere centered on the PCC (MNI coordinates: −2, −54, 16) was used as a seed. Correlation maps using this PCC seed were obtained for each participant and averaged separately for the mild AD and cognitively normal groups. A group difference map was produced by subtracting the averaged map of mild AD participants from cognitively normal individuals. Participants with mild AD dementia showed reduced correlation between the PCC and other DMN nodes including the retrosplenial cortex extending to the precuneus, the left and right inferior parietal lobules (IPL), the left and right medial temporal lobe (MTL), and medial prefrontal cortex (MPFC) (see eTable 1). 6-mm-radius spheres centered on peak voxels from each region were subsequently used in the present ROI-based analyses.
ROI-based investigation of relationships between CSF biomarker levels and DMN integrity
Treating each of the CSF biomarkers as continuous variables, Spearman partial correlation was used to assess the relationships between CSF biomarkers and DMN integrity. ROI-based functional connectivity measures of the DMN (i.e., PCC-RSC, PCC-LIPL, PCC-RIPL, PCC-MPFC, PCC-LMTL and PCC-RMTL) were analyzed separately for each CSF biomarker controlling for potential confounding effects. Since prior work has suggested that APOE genotype (the presence or absence of ε4 allele) might impact CSF Aβ4235 and DMN functional connectivity36, 37, we first assessed whetherAPOE ε4 status modulated the relationship between CSF Aβ42 and DMN functional connectivity. The entire cohort of cognitively normal participants was divided into two sub-groups according to the presence or absence of at least one APOE ε4 allele. The correlations (Spearman’s rho) between CSF Aβ42 and functional connectivity were computed separately for the two sub-groups. For a given CSF Aβ42-functional connectivity relationship, we compared the correlations obtained from APOE ε4 non-carriers to APOE ε4 carriers, and reported these correlations if a significant difference was found. Otherwise, the relationship between CSF Aβ42 and functional connectivity was assessed within the entire cohort after adjusting for age, PCC and MTL volumes separately, and CSF tau/ptau181 levels. Details of the comparisons of correlation coefficients between APOE ε4 non-carriers and carriers are provided in eMethod. Moreover, the associations between CSF ptau181 and functional connectivity were examined after adjusting for age, PCC and MTL volumes separately, and CSF Aβ42 levels. Computation was implemented using R (Version 2.15.1)38 with a statistical threshold for significance of p < 0.05, uncorrected for multiple comparisons.
Voxel-wise whole-brain investigation of relationships between CSF biomarker levels and DMN integrity
Participants were classified as CSF Aβ42 negative (>500 pg/ml) or positive (≤500 pg/ml), and CSF ptau181 negative (<80 pg/ml) or positive (≥80 pg/ml)35. Correlation maps were generated for each participant using the PCC as a seed region. Fisher z-transformed subject-level correlation maps were submitted to second-level random effects analyses to identify voxels within a grey matter mask showing significant group contrast effects. These second-level analyses were conducted using two-sample t-tests implemented in Statistical Parametric Mapping 8 (SPM8) (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/); the resulting t-maps were thresholded at a cluster-level significance of p < 0.05.
Effects of PCC and MTL volumes on rs-fcMRI changes
To assess the likelihood that observed functional connectivity changes were attributed to PCC and MTL atrophy, high-resolution structural scans were processed using FreeSurfer (Version 5.10) (http://surfer.nmr.mgh.harvard.edu) to obtain regional volumes from the isthmus cingulate gyrus (i.e., PCC) and the hippocampus, entorhinal cortex and parahippocampal gyrus (combined to form MTL volume) (see eMethod). Each PCC and MTL volume was correlated separately for CSF Aβ42 and ptau181 using a Spearman correlation. Specifically, CSF Aβ42-volumetric relationships were assessed after adjustment for age and CSF ptau181, and CSF ptau181-volumetric relationships were evaluated after adjustment for age and CSF Aβ42.
Results
ROI-based measures of functional connectivity correlated with CSF biomarker levels
Functional connectivity between the PCC and the 6 independently defined DMN ROIs was computed using standard methodology31. Spearman partial correlations were computed between the functional connectivity measures and each CSF biomarker with adjustment for potential confounding factors (see Table 2 for results). Correlations (Spearman’s rho) between CSF Aβ42 and functional connectivity were not significantly different between APOE ε4 non-carriers and carriers (all p ≥ 0.154)(eTable 2). We therefore pooled APOE ε4 carriers and non-carriers in subsequent analyses. Decreased CSF Aβ42 was associated with reduced functional connectivity between the PCC:left MTL (rho = 0.155, p = 0.026) and the PCC:right MTL (rho = 0.231, p < 0.001) after adjusting for age, PCC and MTL volumes separately, and CSF ptau181. Increased CSF ptau181 levels were associated with reduced functional connectivity between the PCC:left MTL (rho = −0.182, p = 0.008), and a trend level decrease for the PCC:right MTL (rho = −0.122, p = 0.081), and the PCC-MPFC (rho = −0.115, p = 0.100) after adjusting for age, PCC and MTL volumes separately, and CSF Aβ42 levels. All other associations between functional connectivity and CSF Aβ42 or CSF ptau181 levels were not significant (all p ≥ 0.174).
Table 2.
Associations between cerebrospinal fluid levels of Aβ42 or ptau181 and default mode network integrity
| CSF Aβ42 † | CSF ptau § | |||
|---|---|---|---|---|
| rho | p | rho | p | |
| PCC-RSC | −0.009 | 0.894 | −0.084 | 0.233 |
| PCC-Left IPL | −0.028 | 0.689 | −0.100 | 0.155 |
| PCC-Right IPL | 0.087 | 0.217 | −0.096 | 0.175 |
| PCC-MPFC | 0.096 | 0.174 | −0.115 | 0.100 |
| PCC-Left MTL | 0.155 | 0.026 | −0.182 | 0.008 |
| PCC-Right MTL | 0.231 | <0.001 | −0.122 | 0.081 |
Cerebrospinal fluid (CSF) biomarkers were treated as continuous variables. Default mode network (DMN) integrity was measured by inter-regional functional connectivity between independently defined DMN regions. The associations between CSF levels of Aβ42, tau, or ptau181 and DMN integrity were assessed using Spearman partial correlation (rho) with confounding effects being controlled.
rho values were adjusted for age, PCC and MTL volumes separately, and CSF ptau181 levels.
rho values were adjusted for age, PCC and MTL volumes separately, and CSF Aβ42 levels. Bold indicates the relationship is significant (p < 0.05), and italics indicate the relationship is at trend-level significance (0.05 < p < 0.10).
PCC: posterior cingulate cortex, RSC: retrosplenial cortex, IPL: inferior parietal lobule; MPFC: medial prefrontal cortex; MTL: medial temporal lobe.
Topographies of altered functional connectivity as a function of CSF biomarker
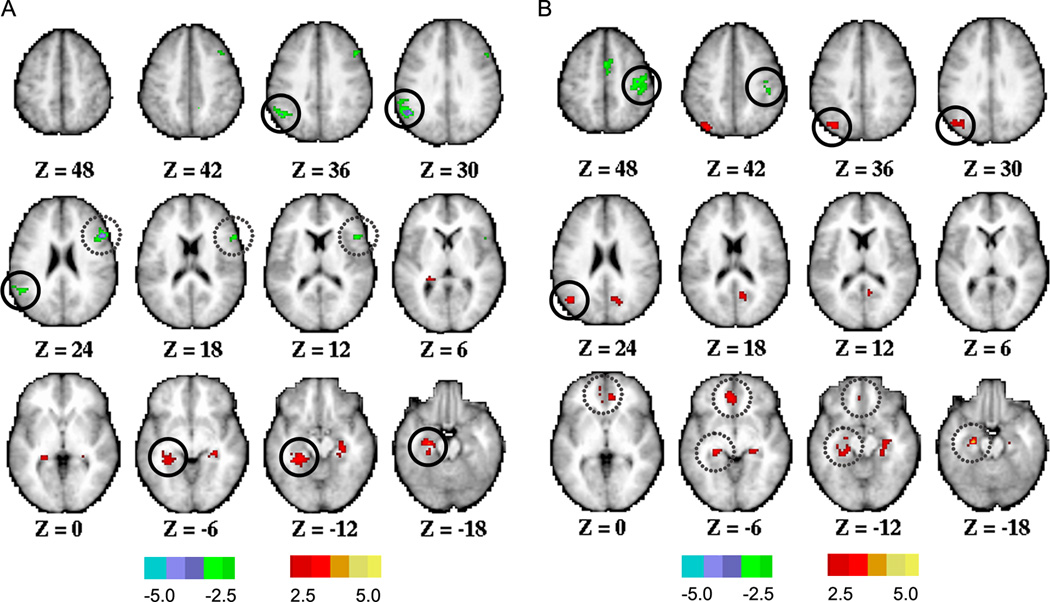
In the voxel-wise analyses, each CSF biomarker was treated as a dichotomous variable. Group-contrast correlation maps are shown in Figure 1. CSF Aβ42-positive pariticipants (≤500 pg/ml) exhibited lower positive correlations (reduced functional connectivity) between the PCC and left MTL compared to CSF Aβ42-negative participants (Figure 1A, and eTable 3). The same group contrast revealed a reduction in the magnitude of anticorrelations between the PCC and left supramarginal gyrus, and between the PCC and right inferior frontal gyrus (at a trend-level). Parallel effects of CSF biomarkers on positive correlations and anticorrelations are consistent with previous rs-fcMRI results obtained in symptomatic (CDR 0.5 and CDR 1) individuals31. Relative to CSF ptau181-negative participants, CSF ptau181-positive (≥80 pg/ml) participants exhibited lower positive correlations between the PCC and left angular gyrus, and between the PCC and left MTL (at a trend-level). A reduction in the magnitude of anticorrelations was also observed between the PCC and right postcentral gyrus (Figure 1B, and eTable 3). Other functional connectivity changes that did not reach cluster-level significance (p < 0.05) are listed in eTable 3.
Figure 1. Voxel-wise analyses assessing functional connectivity of the posterior cingulate cortex (PCC) in cognitively normal individuals with abnormal levels of cerebrospinal fluid (CSF) Aβ42 or ptau181.
Two-sample t-test assessed decreases (hot color) or increases (cold color) in functional connectivity of the PCC in CSF Aβ42-positive individuals (≤500 pg/ml) compared to CSF Aβ42-negative individuals (>500 pg/ml) (A), and in CSF ptau181-positive individuals (≥80 pg/ml) compared to CSF ptau181-negative individuals (<80 pg/ml) (B). Maps were displayed at a voxel-level |t| > 2.5 and cluster size > 35 voxels. Solid and dashed circles represent regions reaching a significance level of p < 0.05 and 0.05 < p < 0.1 respectively, corrected at cluster-level. Detailed information about anatomic location and statistics of observed functional connectivity differences are listed in eTable 3.
Relationship between CSF biomarkers and PCC and MTL volumetrics
We computed PCC and MTL volumes using FreeSurfer-defined ROIs. CSF Aβ42 was correlated with MTL (Spearman rho = 0.172, p = 0.012) but not PCC volume (Spearman rho = −0.044, p = 0.530) after adjustment for age and CSF ptau181. CSF ptau181 was not correlated with MTL or PCC volumes (all p ≥ 0.100).
Comment
We assessed the relationship between neuroimaging indices of brain functional network integrity and well-validated CSF biomarkers of AD pathology in cognitively normal old individuals. We demonstrated that decreased CSF Aβ42 and increased CSF ptau181 were associated with reduced magnitude of correlations in the DMN and within areas normally anti-correlated to the DMN. The most prominent decreases in functional connectivity were seen between the PCC and MTL regions. The effects of CSF Aβ42, CSF tau and ptau181, each of which independently affected DMN functional connectivity, were not attributable to age or structural atrophy in the PCC and MTL.
Amyloid plaques preferentially form within DMN regions including the PCC and precuneus, anterior prefrontal, lateral parietal and temporal regions11, 19. Reduced functional connectivity among these regions22–24, 39 is well documented in cognitively normal elderly with high amyloid burden22–24, 39. However, the MTL is not an early site of plaque formation. Therefore, the link between reduced PCC-MTL functional connectivity and lower CSF Aβ42 may be related to other mechanims. One possibility is that functional connectivity changes are more related to soluble than fibrillary forms of Aβ. Animal studies have demonstrated that oligomeric Aβ directly impairs synaptic function or causes synaptic loss, particularly within the MTL40, 41. We observed that reduced CSF Aβ42 was associated with volume loss in the MTL but not PCC, which is consistent with the oligomere toxicity hypothesis. Further work examining CSF Aβ42 in relation to soluble forms of Aβ are warranted. Another possibility is that the preferential involvement of PCC-MTL functional connectivity is related to the experimental findings demonstrating that regional Aβ deposition causes aberrant electrophysiological activity within spatially distributed functional networks42,43. Given that Aβ preferentially accumulates in the PCC, we speculate that reduced PCC-MTL functional connectivity could be a consequence of aberrant activity in extensive anatomical connections between the PCC and MTL44.
The association between CSF ptau and functional connectivity may be related to the progression of tangle pathology in the brain. Neurofibrillary tangles composed of ptau initially form in the trans-entorhinal cortex and spread in a topographically stereotypical manner, possibly via anatomic connections20. In our data, increased CSF ptau levels were associated with reduced functional connectivity within the anatomic pathways through which neurofibrillary tangles spread45. We suspect that the inverse relationship of CSF ptau and functional connectivity may be driven by the progression of tangle pathology. In cognitively normal individuals, tangles are largely confined to the MTL1 and these tangles are associated with little or no neuronal loss46. In contrast, patients with very mild AD dementia (CDR0.5) have substantial neuronal loss (30–50%) in the entorhinal cortex46, 47. It is possible that elevated CSF ptau may be associated with neuronal and synaptic loss in the MTL that is not yet sufficent to produce overt clinical symptoms and not detectable by the present volumetric measure but is detectable at the group level using rs-fcMRI.
The convergent effects of decreased CSF Aβ42 and increased CSF ptau181 on the DMN provides insights into the early pathophysiology of AD. The PCC and MTL are two critical nodes of a larger network supporting episodic memory48. Stronger PCC-MTL functional connectivity is associated with better performance on memory tasks49. Structural atrophy of the PCC-MTL pathway is consistent with commonly recognized memory impairments in AD50. Thus, the available data suggest that memory impairment in the early phases of AD may be attributable to the convergent effects of both amyloid and tau pathology.
The present study has several limitations. Using both hypothesis-driven ROI-based analysis and voxel-wise whole-brain exploration, we observed that abnormal levels of CSF Aβ42 and ptau181 were associated with reduced functional connectivity within nodes of the DMN, most prominently in PCC:MTL measures. However, the effect sizes were modest, most likely because we studied pre-symptomatic individuals. Replication of our findings is needed in additional independent samples. Although we found no evidence that rs-fcMRI changes were attributable to PCC or MTL atrophy, we note that volumetric measurements were derived from FreeSurfer-defined regions that did not completely overlap with ROIs used in the rs-fcMRI analysis. In addition, volumetric changes in other DMN regions (besides the PCC and MTL) were not included in our analysis. Further studies using more rigorous approaches to control for the effects of structural brain changes are warranted. Patients with the mild symptomatic AD exhibit changes in multiple resting state networks (RSNs)31. Further work is warranted to examine the effects of CSF biomarker abnormalities in RSNs other than the DMN.
Supplementary Material
Acknowledgements
Knight Alzheimer’s Disease Research Center (ADRC) Pilot Grant (3255 ADRC 26) (BMA), National Institute of Mental Health (NIMH) (K23MH081786) (BMA), National Institute of Nursing Research (NINR) (R01NR012907 and R01NR012657) (BMA), Alzheimer’s Association (BMA), National Institute of Aging (NIA) R01AG034119, R01AG029672, and P01AG50837 (CX), NIA P01AG026276, P01AG03991, P50 AG05681 and U19AG032438 (JCM), National Institute of Neurological Disorders and Stroke (NS06833) (AZS), NIMH P30NS048056 (AZS), and The American Roentgen Ray Society Foundation (TB).
We thank the Knight ADRC’s Clinical Core for participant assessments [participants were enrolled under NIA grants P01AG026276 (JC Morris, PI), P01AG03991 (JC Morris, PI), and P50AG05681 (JC Morris, PI)], Biomarker Core for cerebrospinal fluid assays, and Genetics Core for apolipoprotein E genotyping. We especially thank all of our research participants.
Dr. Ances has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Dr. Fagan consults for Roche and Lilly USA. Dr. Benzinger consults for Biomedical Systems, Inc. and ICON Medical Imaging and receives research support from Avid Radiopharmaceuticals. Dr. Holtzman reports consulting for Pfizer, Bristol-Myers Squibb, and Innogenetics and is on the scientific advisory boards of En Vivo, Satori, and C2N Diagnostics. Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company. Dr. Morris has participated or is currently participating in clinical trials of antidementia drugs sponsored by the following companies: Janssen Immunotherapy, and Pfizer. Dr. Morris has served as a consultant for the following companies: Eisai, Esteve, Janssen Alzheimer Immunotherapy Program, Glaxo-Smith-Kline, Novartis, and Pfizer. Dr. Morris receives research support from Eli Lilly/Avid Radiopharmaceuticals.
Footnotes
Financial disclosure
Drs. Wang, Xiong, Snyder, and Ances as well as Mr. Brier and Mr. Thomas report no disclosures.
References
- 1.Price JL, Morris JC. Tangles and plaques in nondemented aging and "preclinical" Alzheimer's disease. Annals of neurology. 1999 Mar;45(3):358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt FA, Davis DG, Wekstein DR, Smith CD, Ashford JW, Markesbery WR. "Preclinical" AD revisited: neuropathology of cognitively normal older adults. Neurology. 2000 Aug 8;55(3):370–376. doi: 10.1212/wnl.55.3.370. [DOI] [PubMed] [Google Scholar]
- 3.Bennett DA, Schneider JA, Arvanitakis Z, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006 Jun 27;66(12):1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- 4.Knopman DS, Parisi JE, Salviati A, et al. Neuropathology of cognitively normal elderly. J Neuropathol Exp Neurol. 2003 Nov;62(11):1087–1095. doi: 10.1093/jnen/62.11.1087. [DOI] [PubMed] [Google Scholar]
- 5.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging- Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 May;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dubois B, Feldman HH, Jacova C, et al. Revising the definition of Alzheimer's disease: a new lexicon. Lancet Neurol. 2010 Nov;9(11):1118–1127. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]
- 7.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995 Oct;34(4):537–541. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 8.Hampel H, Wilcock G, Andrieu S, et al. Biomarkers for Alzheimer's disease therapeutic trials. Prog Neurobiol. 2011 Dec;95(4):579–593. doi: 10.1016/j.pneurobio.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR, Jr, Albert MS, Knopman DS, et al. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia : the journal of the Alzheimer's Association. 2011 May;7(3):257–262. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fagan AM, Mintun MA, Mach RH, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Annals of neurology. 2006 Mar;59(3):512–519. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- 11.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh Compound-B. Annals of neurology. 2004 Mar;55(3):306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 12.Buerger K, Ewers M, Pirttila T, et al. CSF phosphorylated tau protein correlates with neocortical neurofibrillary pathology in Alzheimer's disease. Brain : a journal of neurology. 2006 Nov;129(Pt 11):3035–3041. doi: 10.1093/brain/awl269. [DOI] [PubMed] [Google Scholar]
- 13.Blennow K, Hampel H, Weiner M, Zetterberg H. Cerebrospinal fluid and plasma biomarkers in Alzheimer disease. Nature reviews. Neurology. 2010 Mar;6(3):131–144. doi: 10.1038/nrneurol.2010.4. [DOI] [PubMed] [Google Scholar]
- 14.Sunderland T, Linker G, Mirza N, et al. Decreased beta-amyloid1-42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA : the journal of the American Medical Association. 2003 Apr 23–30;289(16):2094–2103. doi: 10.1001/jama.289.16.2094. [DOI] [PubMed] [Google Scholar]
- 15.Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology. 2006 Aug 8;67(3):446–452. doi: 10.1212/01.wnl.0000228230.26044.a4. [DOI] [PubMed] [Google Scholar]
- 16.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Archives of neurology. 2007 Mar;64(3):343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 17.Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med. 2009 Nov;1(8–9):371–380. doi: 10.1002/emmm.200900048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greicius M. Resting-state functional connectivity in neuropsychiatric disorders. Curr Opin Neurol. 2008 Aug;21(4):424–430. doi: 10.1097/WCO.0b013e328306f2c5. [DOI] [PubMed] [Google Scholar]
- 19.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005 Aug 24;25(34):7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82(4):239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 21.Buckner RL, Andrews-Hanna JR, Schacter DL. The brain's default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences. 2008 Mar;1124:1–38. doi: 10.1196/annals.1440.011. [DOI] [PubMed] [Google Scholar]
- 22.Hedden T, Van Dijk KR, Becker JA, et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009 Oct 7;29(40):12686–12694. doi: 10.1523/JNEUROSCI.3189-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheline YI, Raichle ME, Snyder AZ, et al. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol Psychiatry. 2010 Mar 15;67(6):584–587. doi: 10.1016/j.biopsych.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mormino EC, Smiljic A, Hayenga AO, et al. Relationships between beta-amyloid and functional connectivity in different components of the default mode network in aging. Cerebral cortex. 2011 Oct;21(10):2399–2407. doi: 10.1093/cercor/bhr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Li TQ, Andreasen N, Wiberg MK, Westman E, Wahlund LO. Ratio of Abeta42/P-tau(181p) in CSF is associated with aberrant default mode network in AD. Sci Rep. 2013 Feb 26;3:1339. doi: 10.1038/srep01339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damoiseaux JS, Seeley WW, Zhou J, et al. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012 Jun 13;32(24):8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berg L, McKeel DW, Jr, Miller JP, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Archives of neurology. 1998 Mar;55(3):326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- 28.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993 Nov;43(11):2412–2414. doi: 10.1212/wnl.43.11.2412-a. [DOI] [PubMed] [Google Scholar]
- 29.Morris JC, Weintraub S, Chui HC, et al. The Uniform Data Set (UDS): clinical and cognitive variables and descriptive data from Alzheimer Disease Centers. Alzheimer disease and associated disorders. 2006 Oct-Dec;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- 30.Pastor P, Roe CM, Villegas A, et al. Apolipoprotein Eepsilon4 modifies Alzheimer's disease onset in an E280A PS1 kindred. Annals of neurology. 2003 Aug;54(2):163–169. doi: 10.1002/ana.10636. [DOI] [PubMed] [Google Scholar]
- 31.Brier MR, Thomas JB, Snyder AZ, et al. Loss of intranetwork and internetwork resting state functional connections with Alzheimer's disease progression. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012 Jun 27;32(26):8890–8899. doi: 10.1523/JNEUROSCI.5698-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang L, Roe CM, Snyder AZ, et al. Alzheimer disease family history impacts resting state functional connectivity. Annals of neurology. 2012 Oct;72(4):571–577. doi: 10.1002/ana.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 2011 Oct 14; doi: 10.1016/j.neuroimage.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smyser CD, Inder TE, Shimony JS, et al. Longitudinal analysis of neural network development in preterm infants. Cerebral cortex. 2010 Dec;20(12):2852–2862. doi: 10.1093/cercor/bhq035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Annals of neurology. 2010 Jan;67(1):122–131. doi: 10.1002/ana.21843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheline YI, Morris JC, Snyder AZ, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010 Dec 15;30(50):17035–17040. doi: 10.1523/JNEUROSCI.3987-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machulda MM, Jones DT, Vemuri P, et al. Effect of APOE epsilon4 status on intrinsic network connectivity in cognitively normal elderly subjects. Archives of neurology. 2011 Sep;68(9):1131–1136. doi: 10.1001/archneurol.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Team}} RDC. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 39.Drzezga A, Becker JA, Van Dijk KR, et al. Neuronal dysfunction and disconnection of cortical hubs in non-demented subjects with elevated amyloid burden. Brain : a journal of neurology. 2011 Jun;134(Pt 6):1635–1646. doi: 10.1093/brain/awr066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walsh DM, Selkoe DJ. A beta oligomers - a decade of discovery. J Neurochem. 2007 Jun;101(5):1172–1184. doi: 10.1111/j.1471-4159.2006.04426.x. [DOI] [PubMed] [Google Scholar]
- 41.Shankar GM, Li S, Mehta TH, et al. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat Med. 2008 Aug;14(8):837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palop JJ, Mucke L. Amyloid-beta-induced neuronal dysfunction in Alzheimer's disease: from synapses toward neural networks. Nat Neurosci. 2010 Jul;13(7):812–818. doi: 10.1038/nn.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harris JA, Devidze N, Verret L, et al. Transsynaptic progression of amyloid-betainduced neuronal dysfunction within the entorhinal-hippocampal network. Neuron. 2010 Nov 4;68(3):428–441. doi: 10.1016/j.neuron.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II Cortical afferents. Journal of Comparative Neurology. 2003 Nov 3;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- 45.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer's disease. Cereb Cortex. 1991 Jan-Feb;1(1):103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 46.Price JL, Ko AI, Wade MJ, Tsou SK, McKeel DW, Morris JC. Neuron number in the entorhinal cortex and CA1 in preclinical Alzheimer disease. Archives of neurology. 2001 Sep;58(9):1395–1402. doi: 10.1001/archneur.58.9.1395. [DOI] [PubMed] [Google Scholar]
- 47.Gomez-Isla T, Price JL, McKeel DW, Jr, Morris JC, Growdon JH, Hyman BT. Profound loss of layer II entorhinal cortex neurons occurs in very mild Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1996 Jul 15;16(14):4491–4500. doi: 10.1523/JNEUROSCI.16-14-04491.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sperling RA, Dickerson BC, Pihlajamaki M, et al. Functional alterations in memory networks in early Alzheimer's disease. Neuromolecular medicine. 2010 Mar;12(1):27–43. doi: 10.1007/s12017-009-8109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang L, Laviolette P, O'Keefe K, et al. Intrinsic connectivity between the hippocampus and posteromedial cortex predicts memory performance in cognitively intact older individuals. Neuroimage. 2010 Jun;51(2):910–917. doi: 10.1016/j.neuroimage.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stoub TR, deToledo-Morrell L, Stebbins GT, Leurgans S, Bennett DA, Shah RC. Hippocampal disconnection contributes to memory dysfunction in individuals at risk for Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2006 Jun 27;103(26):10041–10045. doi: 10.1073/pnas.0603414103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.