Abstract
Differentiation therapy has emerged as a powerful way to target specific hematologic malignancies. One of the best examples is the use of All-trans-Retinoic acid (ATRA) in Acute Promyelocytic Leukemia (APL), which has significantly improved the outcome for patients with this specific form of Acute Myeloid Leukemia (AML). In considering how differentiation therapy could be used in other forms of AML, we predicted that compounds that induce terminal differentiation of megakaryocytes would be effective therapies for the megakaryocytic form of AML, named Acute Megakaryocytic Leukemia (AMKL). We also speculated that such agents would reduce the burden of abnormal hematopoietic cells in Primary Myelofibrosis (PMF) and alter the differentiation of megakaryocytes in Myelodysplastic Syndromes (MDS). Using a high-throughput chemical screening approach, we identified small molecules that promote many features of terminal megakaryocyte differentiation, including the induction of polyploidization, the process by which cells accumulate DNA to 32N or greater. Since the induction of polyploidization is an irreversible process, cells that enter this form of the cell cycle do not divide again. Thus, this would be an effective way to reduce the tumor burden. Clinical studies with polyploidy inducers, such as Aurora kinase A inhibitors, are underway for a wide variety of malignancies, while trials specifically for AMKL and PMF are in development. This novel form of differentiation therapy may be clinically available in the not too distant future.
Background
Polyploidization of megakaryocytes
A small number of cell types in humans undergo accumulation of multiple copies of their DNA (polyploidization) as they differentiate (Figure). There are 2 different mechanisms by which polyploidization can occur, cell fusion and DNA division without cytokinesis, a variant of the cell cycle termed endomitosis. Osteoclasts, for example, become polyploid by fusion of 2N cells to form large phagocytic cells with multiple separate nuclei. In contrast, megakaryocytes become polyploid by undergoing repeated rounds of DNA replication without completing cell division resulting in very large mature cells that usually contain a single multilobed nucleus with DNA contents up to 128N1. Polyploidization is essential for efficient platelet production, in part due to increased cytoplasmic volume and also due to upregulation of differentiation genes 2, 3. In acute megakaryoblastic leukemia (AMKL), low ploidy megakaryoblasts predominate. This lack of polyploid megakaryocytes is a consequence of a block in polyploidization and differentiation of the rapidly proliferating leukemic blasts.
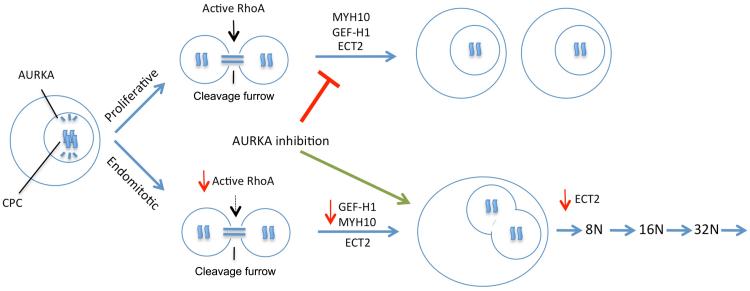
Figure.
Megakaryocyte progenitors must decide whether to divide to give rise to two daughter cells (top) or instead to commit to terminal differentiation and polyploidization (bottom). The switch from the proliferative cell cycle to the endomitotic one involves changes in activity and expression of several genes including RhoA, ECT2, GEF-H1, and MYH10. Most notably, the reduction in RhoA activity and MYH10 prevents the action of the contractile ring and the completion of cytokinesis. Inhibition of AURKA has been found to promote the endomitotic process over proliferation. Note that AURKA localizes to the bipolar spindles while active RhoA is associated with the cleavage furrow.
The mechanisms that control endomitosis and the ways that it differs from the normal proliferative cell cycle have been investigated at the cellular and molecular levels. During a proliferative cell division, chromosomes are bound by the chromosome passenger complex, which is comprised of the proteins Survivin, INCENP, Aurora kinase B (AURKB), and Borealin. Chromosomes are tethered to bipolar spindles, sites that accumulate Aurora kinase A (AURKA), by microtubules and line up at a central region termed the midzone. As mitosis progresses, pairs of chromosomes are separated to opposite poles and a cleavage furrow, the region in which cells are separated that gradually closes like a purse string during cytokinesis, forms. During normal cytokinesis, RhoA activation at the site of initiation of cleavage furrow formation is orchestrated by the guanine exchange factor (GEF) ECT2 in coordination with proteins at the midzone to establish the actomyosin ring at the cleavage furrow. This ring generates the contraction required for ultimate cell separation (abcission) 4, 5. Activated RhoA and its functional effectors (e.g. ROCK, mDia) need to be localized to the cleavage furrow for cytokinesis to occur6–10.
Studies using time-lapse microscopy to observe megakaryocytes undergoing endomitosis suggest that the initial endomitotic cleavage event in which cells progress from 2N to 4N occurs due to failure very late in cytokinesis with normal cleavage furrow formation followed by, instead of abscission, furrow regression 11–14. These endomitotic megakaryocytes form an apparently intact midzone with normal localization of essential components including Survivin, AURKB, INCENP, PRC1 (protein regulating cytokinesis 1), MKLP1 and 2 (mitotic kinesin-like protein), MgcRacGAP and microtubules 12, 15. However, RhoA localization to the midzone and/or RhoA activation may be inhibited in endomitotic megakaryocytes. In contrast to normal cytokinesis, the contractile ring of megakaryocytes undergoing endomitosis contains decreased levels of RhoA at the 2N to 4N transition. In higher ploidy cells (greater than 4N), there is little to no cleavage furrow formation during anaphase, and RhoA is not detectable at sites where cleavage furrow would be expected (perpendicular to the midzone) 12, 15.
Rho family small GTPases (e.g. RhoA, Rac1, and Cdc42) are molecular switches that regulate many cellular processes including actin cytoskeleton reorganization, microtubule dynamics, cell cycle progression and cytokinesis 16. Activated RhoA facilitates actin polymerization via mDia and promotes contraction of the actin myosin ring by activating ROCK to phosphorylate and thus activate myosin. The myosins involved in cytokinesis are the nonmuscle myosins MYH9 and MYH10 (nonmuscle myosins IIa and IIb, respectively). Myosins generate the force needed to close the contractile ring, which leads to the final separation of the cells. In dividing 2N megakaryoblasts, MYH10 predominates in the cleavage furrow. However, in megakaryocytes undergoing endomitosis, neither MYH10 nor MYH9 is present. The lack of MYH10 at the 2N to 4N endomitotic stage may be due to repression of MYH10 mRNA transcription by Runx1, a transcription factor that is known to be required for both polyploidization and subsequent cytoplasmic maturation. But lack of MYH10 may not be the only reason for failure of complete closure of the cleavage furrow in endomitotic megakaryocytes.
Mitosis and endomitosis are also controlled by different guanine exchange factors, which facilitate Rho GTPase switching from the inactive GDP-bound state to the active GTP-bound state. The Dbl family guanine nucleotide-exchange factors (GEFs) have a tandem Dbl homology (DH) - Pleckstrin homology (PH) domain, in which the DH domain contains GDP/GTP exchange activity 17. GEFs are involved in RhoA localization and activation during different stages of cytokinesis. Two such GEFs, ECT2 and GEFH1 are involved in different stages of cytokinesis. The GEF ECT2 (Epithelial Cell Transforming Sequence 2) is required for initiation of cleavage furrow formation and GEFH1 is required for the final stages of cleavage furrow closure and cell division. To initiate cleavage furrow formation perpendicular to the midzone after breakdown of the nuclear envelope during mitosis, ECT2 is recruited to the central spindle by the central spindlin complex during late anaphase, where it then promotes cleavage furrow formation 18, 19. ECT2 is an oncogene that resides on chromosome 3q26, a region frequently targeted for chromosomal alterations in human tumors and overexpressed in many primary human tumors 20, 21. RNAi knock-down of ECT2 results in mitotic failure and binucleate cells due to the lack of cleavage furrow ingression 22. We have found that downregulation of ECT2 expression occurs during megakaryocyte maturation and is required for later stages of endomitosis (starting when 4N cells become 8N) in which little to no cleavage furrow is apparent. In contrast, at the 2N to 4N stage of endomitosis, the cleavage furrow forms and anaphase is almost complete before the furrow regresses and the 2 separated nuclei rejoin. For abortion of cytokinesis at this late stage of anaphase, in addition to a lack of MYH10, we have found that GEFH1 is entirely absent from the central spindle and the cleavage furrow. The molecular regulation of GEFH1 requires expression of MKL1, a transcriptional cofactor that is mutated as part of the t(1;22) translocation that is occurs in non-Down Syndrome associated AMKL (DS-AMKL). In AMKL cells with the t(1;22), GEFH1 levels are extremely high and the cells do not undergo polyploidization most likely due to the presence of GEFH1, which localizes and activates RhoA to promote closure of the cleavage furrow and complete abscission.
Malignant megakaryocytes are defective in polyploidization
In the neoplastic process, tumor cells acquire alterations that result in excessive proliferation and impaired differentiation. Similar to APL, in which leukemic blasts continue to divide instead of differentiate 23, immature megakaryocytes in AMKL continue to progress through mitosis and fail to enter the endomitotic cell cycle. In myelodysplastic syndrome (MDS), a disease that involves both abnormal hematopoietic stem cell self-renewal and a deficiency in terminal differentiation of mature myeloid cells, and in PMF, a clonal disorder characterized by abnormal hematopoiesis and myelofibrosis, megakaryocytes also show impaired polyploidization. Based on this observed block in polyploidization, two labs envisioned the development of “polyploidy inducing agents” as potential therapies for MDS, PMF or AMKL. In the first instance, Drachman and colleagues discovered that the small molecule SU6656 could potently induce polyploidization of a variety of cell lines and megakaryocytes collected from the bone marrows of individuals with MDS 24. SU6656 was found to inhibit multiple kinases, including members of the Src as well as the Aurora kinase families. Such a compound was proposed as a new therapy for MDS.
In the second study, we screened for small molecules that could drive polyploidization and induce features of terminal differentiation of malignant megakaryocytes25. Using the CMK cell line, which was derived from an individual with DS-AMKL, we identified 205 small molecules that induced statistically significant polyploidization of the cells. Of these, the compound with the strongest polyploidy and differentiation activity was the kinase inhibitor dimethylfasudil (diMF). Using a multi-pronged target identification strategy, AURKA was discovered as a major target of diMF in megakaryocytes. We demonstrated that diMF and the highly selective AURKA inhibitor, MLN8237 (Alisertib)26, showed potent anti-AMKL activity both in vitro and in vivo. For example, diMF significantly increased survival of mice engrafted with an AMKL cell line, with a 40% long-term survival in the group treated with 66 mg/kg (p=0.003) and 30% with 33 mg/kg (p=0.004)25. Furthermore, MLN8237 led to significant reductions in tumor burden of NSG immunocompromised mice engrafted with primary human non-DS blasts25, 27. Based upon these results, we propose that polyploidy inducers are a novel therapeutic strategy for AMKL. Furthermore, induction of polyploidization may also be effective in the treatment of other diseases of megakaryocytes. For example, since it is believed that immature megakaryocytes directly contribute to fibrosis in PMF, induction of differentiation and polyploidization is likely to benefit these patients as well.
Given that megakaryocytes are poised to become polyploid, we predicted that polyploidy inducers would not affect other cell types, at least at the doses that promote megakaryocyte polyploidization. Indeed, treatment of both human and mouse primary cell cultures with diMF led to increased polyploidy of the CD41 positive megakaryocytes, but no change in the CD41-negative fraction which includes all other bone marrow cells25. Furthermore, we did not observe and defects in other hematopoietic cells in healthy mice treated with either diMF or MLN823725. However, in many cases, once a cell becomes 4N, it cannot undergo DNA synthesis due to cell cycle checkpoints. Thus, if highly proliferative leukemia cells of other lineages could be induced to undergo polyploidization with these inhibitors, they may also be susceptible to such treatments.
Clinical Translational Advances
AMKL is a rare form of acute myeloid leukemia that affects both children and adults. In pediatric cases, the leukemia is most often seen in children with Down syndrome (DS), who are several hundred fold increased risk for AMKL compared to children without DS28, 29. DS-AMKL is characterized by trisomy 21 and mutations in the essential transcription factor gene GATA130. AMKL is also observed in young children without DS, but in these cases, there are no mutations in GATA1. Instead, the tumors tend to acquire chromosomal translocations, such a t(1;22)31, which results in expression of the RBM15-MKL1 fusion protein32, 33 or Inv(16)(p13.3q24.3), which leads to expression of the CBFA2T3-GLIS2 fusion27, 34. Recurrent translocations involving Jarid1A (KDM5A) on chromosome 12 have also been reported for non-DS-AMKL27, 35. Finally, AMKL is also seen in elderly patients. The genetic basis for this leukemia is largely undefined, although mutations in JAK2, JAK3, and FLT3 have been detected in rare cases36–38.
Among these subtypes, children with DS have the best prognosis. Studies by the Children's Oncology Group found a 5 year event free survival (EFS) rate of 79%, and an overall survival rate of 84%39. Similar results were seen in the POG9241 trial40. Despite the excellent outcome, there is room for improvement in this subtype. Pediatric non-DS AMKL is an aggressive malignancy with inferior outcome relative to DS-AMKL. The POG9241 study found that non-DS AMKL patients had a 36.3% 5-year EFS despite intensive chemotherapy41. Of note, patients with the t(1;22) showed an excellent outcome, with all 5 patients achieving complete remission. In contrast, the Inv(16)(p13.3q24.3) cases have an inferior outcome compared to other non-DS AMKL cases34. The COG study 2891 found that children with AMKL had significantly inferior overall survival compared to children with M0-M5 subtypes of AML42. Similarly poor outcomes for non-DS AMKL were seen in a St. Jude study43. Finally, AMKL in adults has a poor prognosis. The vast majority of patients undergoes remission, but relapse. Two separate studies reported an extremely poor outcome, with a median survival of 40 weeks44, 45. Clearly, new therapeutic options are needed for AMKL patients.
Based upon work with polyploidy inducers, we propose that these agents, alone or more likely in combination with conventional chemotherapy, would provide sustained remission. MLN8237 is under investigation for a wide variety of tumors, including multiple hematologic malignancies such as myeloma, diffuse large B cell lymphoma, non-Hodgkin lymphoma, and other advanced hematologic malignancies and solid tumors. In a phase 2 study of MLN8237 in advanced AML or intermediate-2/high risk MDS, use of MLN9237 as a single agent showed a 13% overall response rate46. Based on the rarity of AMKL, however, it is likely that few if any patients with this subtype were included in this or other clinical studies of MLN8237.
In addition to single agent studies, MLN8237 has been tested in combination with a variety of conventional chemotherapies. For example, in pre-clinical studies of multiple myeloma, combining MLN8237 with dexamethasone, doxorubicin or bortezomib induced a synergistic/additive response47. In pre-clinical studies of MLN8237 in esophageal adenocarcinoma, the addition of cisplatin enhanced the antitumor effect48. With respect to AMKL, the addition of conventional chemotherapies, such as cytarabine and anthracyclines, to polyploidy agents may not be required, as the MLN8237 and diMF efficiently target leukemia blasts. In contrast, combining polyploidy inducers with new therapies that eradicate leukemia stem cells, may improve efficacy.
Conclusion
Acute megakaryoblastic leukemia remains an intractable disease with poor outcome. Similarly, MDS and PMF are disorders that require new therapeutic strategies. Novel therapies that specifically target the immature megakaryocytes and override the blocks to polyploidization provide a new strategy to induce sustained remission and increased overall survival. Moreover, continued advances in our understanding of the process of polyploidization and how it differs from the proliferative cell cycle will provide additional targets for therapeutic intervention.
Acknowledgements
This review was supported in part by grants from the Samuel Waxman Cancer Research Foundation and the NCI (R01 CA101774) to JC, and from the NIH (DK086267) and state of CT (10SCB03) to DSK.
Footnotes
The authors have no conflicts of interest.
References
- 1.Battinelli EM, Hartwig JH, Italiano JE., Jr. Delivering new insight into the biology of megakaryopoiesis and thrombopoiesis. Current Opinion in Hematology. 2007;14:419–26. doi: 10.1097/MOH.0b013e3282bad151. [DOI] [PubMed] [Google Scholar]
- 2.Raslova H, Roy L, Vourc'h C, Le Couedic JP, Brison O, Metivier D, et al. Megakaryocyte polyploidization is associated with a functional gene amplification. Blood. 2003;101:541–4. doi: 10.1182/blood-2002-05-1553. [DOI] [PubMed] [Google Scholar]
- 3.Raslova H, Kauffmann A, Sekkai D, Ripoche H, Larbret F, Robert T, et al. Interrelation between polyploidization and megakaryocyte differentiation: a gene profiling approach. Blood. 2007;109:3225–34. doi: 10.1182/blood-2006-07-037838. [DOI] [PubMed] [Google Scholar]
- 4.Narumiya S, Yasuda S. Rho GTPases in animal cell mitosis. Current Opinion in Cell Biology. 2006;18:199–205. doi: 10.1016/j.ceb.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Bement WM, Benink HA, von Dassow G. A microtubule-dependent zone of active RhoA during cleavage plane specification. Journal of Cell Biology. 2005;170:91–101. doi: 10.1083/jcb.200501131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madaule P, Eda M, Watanabe N, Fujisawa K, Matsuoka T, Bito H, et al. Role of citron kinase as a target of the small GTPase Rho in cytokinesis. Nature. 1998;394:491–4. doi: 10.1038/28873. [DOI] [PubMed] [Google Scholar]
- 7.Yasui Y, Amano M, Nagata K, Inagaki N, Nakamura H, Saya H, et al. Roles of Rho-associated kinase in cytokinesis; mutations in Rho-associated kinase phosphorylation sites impair cytokinetic segregation of glial filaments. Journal of Cell Biology. 1998;143:1249–58. doi: 10.1083/jcb.143.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kosako H, Goto H, Yanagida M, Matsuzawa K, Fujita M, Tomono Y, et al. Specific accumulation of Rho-associated kinase at the cleavage furrow during cytokinesis: cleavage furrow-specific phosphorylation of intermediate filaments. Oncogene. 1999;18:2783–8. doi: 10.1038/sj.onc.1202633. [DOI] [PubMed] [Google Scholar]
- 9.Kosako H, Yoshida T, Matsumura F, Ishizaki T, Narumiya S, Inagaki M. Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene. 2000;19:6059–64. doi: 10.1038/sj.onc.1203987. [DOI] [PubMed] [Google Scholar]
- 10.Tolliday N, VerPlank L, Li R. Rho1 directs formin-mediated actin ring assembly during budding yeast cytokinesis. Current Biology. 2002;12:1864–70. doi: 10.1016/s0960-9822(02)01238-1. [DOI] [PubMed] [Google Scholar]
- 11.Papadantonakis N, Makitalo M, McCrann DJ, Liu K, Nguyen HG, Martin G, et al. Direct visualization of the endomitotic cell cycle in living megakaryocytes: differential patterns in low and high ploidy cells. Cell Cycle. 2008;7:2352–6. doi: 10.4161/cc.6325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lordier L, Jalil A, Aurade F, Larbret F, Larghero J, Debili N, et al. Megakaryocyte endomitosis is a failure of late cytokinesis related to defects in the contractile ring and Rho/Rock signaling. Blood. 2008;112:3164–74. doi: 10.1182/blood-2008-03-144956. [DOI] [PubMed] [Google Scholar]
- 13.Geddis AE, Fox NE, Tkachenko E, Kaushansky K. Endomitotic megakaryocytes that form a bipolar spindle exhibit cleavage furrow ingression followed by furrow regression. Cell Cycle. 2007;6:455–60. doi: 10.4161/cc.6.4.3836. [DOI] [PubMed] [Google Scholar]
- 14.Leysi-Derilou Y, Robert A, Duchesne C, Garnier A, Boyer L, Pineault N. Polyploid megakaryocytes can complete cytokinesis. Cell Cycle. 2010;9:2589–99. doi: 10.4161/cc.9.13.12078. [DOI] [PubMed] [Google Scholar]
- 15.Geddis AE, Kaushansky K. Endomitotic megakaryocytes form a midzone in anaphase but have a deficiency in cleavage furrow formation. Cell Cycle. 2006;5:538–45. doi: 10.4161/cc.5.5.2537. [DOI] [PubMed] [Google Scholar]
- 16.Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–35. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- 17.Rossman KL, Der CJ, Sondek J. GEF means go: turning on RHO GTPases with guanine nucleotide-exchange factors. Nature Reviews Molecular Cell Biology. 2005;6:167–80. doi: 10.1038/nrm1587. [DOI] [PubMed] [Google Scholar]
- 18.Petronczki M, Glotzer M, Kraut N, Peters JM. Polo-like kinase 1 triggers the initiation of cytokinesis in human cells by promoting recruitment of the RhoGEF Ect2 to the central spindle. Developmental Cell. 2007;12:713–25. doi: 10.1016/j.devcel.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 19.Yuce O, Piekny A, Glotzer M. An ECT2-centralspindlin complex regulates the localization and function of RhoA. Journal of Cell Biology. 2005;170:571–82. doi: 10.1083/jcb.200501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fields AP, Justilien V. The guanine nucleotide exchange factor (GEF) Ect2 is an oncogene in human cancer. Advances in Enzyme Regulation. 2010;50:190–200. doi: 10.1016/j.advenzreg.2009.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyoda M, Kasamatsu A, Ishigami T, Nakashima D, Endo-Sakamoto Y, Ogawara K, et al. Epithelial cell transforming sequence 2 in human oral cancer. PLoS ONE [Electronic Resource] 2010;5:e14082. doi: 10.1371/journal.pone.0014082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Birkenfeld J, Nalbant P, Bohl BP, Pertz O, Hahn KM, Bokoch GM. GEF-H1 modulates localized RhoA activation during cytokinesis under the control of mitotic kinases. Dev Cell. 2007;12:699–712. doi: 10.1016/j.devcel.2007.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Melnick A, Licht JD. Deconstructing a disease: RARalpha, its fusion partners, and their roles in the pathogenesis of acute promyelocytic leukemia. Blood. 1999;93:3167–215. [PubMed] [Google Scholar]
- 24.Lannutti BJ, Blake N, Gandhi MJ, Reems JA, Drachman JG. Induction of polyploidization in leukemic cell lines and primary bone marrow by Src kinase inhibitor SU6656. Blood. 2005;105:3875–8. doi: 10.1182/blood-2004-10-3934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wen Q, Goldenson B, Silver SJ, Schenone M, Dancik V, Huang Z, et al. Identification of Regulators of Polyploidization Presents Therapeutic Targets for Treatment of AMKL. Cell. 2012;150:575–89. doi: 10.1016/j.cell.2012.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manfredi MG, Ecsedy JA, Chakravarty A, Silverman L, Zhang M, Hoar KM, et al. Characterization of Alisertib (MLN8237), an investigational small-molecule inhibitor of aurora A kinase using novel in vivo pharmacodynamic assays. Clinical Cancer Research. 2011;17:7614–24. doi: 10.1158/1078-0432.CCR-11-1536. [DOI] [PubMed] [Google Scholar]
- 27.Thiollier C, Lopez CK, Gerby B, Ignacimouttou C, Poglio S, Duffourd Y, et al. Characterization of novel genomic alterations and therapeutic approaches using acute megakaryoblastic leukemia xenograft models. Journal of Experimental Medicine. 2012;209:2017–31. doi: 10.1084/jem.20121343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lange B. The management of neoplastic disorders of haematopoiesis in children with Down's syndrome. British journal of Haematology. 2000;110:512–24. doi: 10.1046/j.1365-2141.2000.02027.x. [DOI] [PubMed] [Google Scholar]
- 29.Zipursky A, Peeters M, Poon A. Megakaryoblastic leukemia and Down's syndrome: a review. Pediatric Hematology & Oncology. 1987;4:211–30. doi: 10.3109/08880018709141272. [DOI] [PubMed] [Google Scholar]
- 30.Wechsler J, Greene M, McDevitt MA, Anastasi J, Karp JE, Le Beau MM, et al. Acquired mutations in GATA1 in the megakaryoblastic leukemia of Down syndrome. Nat Genet. 2002;32:148–52. doi: 10.1038/ng955. [DOI] [PubMed] [Google Scholar]
- 31.Lion T, Haas OA, Harbott J, Bannier E, Ritterbach J, Jankovic M, et al. The translocation t(1;22)(p13;q13) is a nonrandom marker specifically associated with acute megakaryocytic leukemia in young children. Blood. 1992;79:3325–30. [PubMed] [Google Scholar]
- 32.Mercher T, Coniat MB, Monni R, Mauchauffe M, Nguyen Khac F, Gressin L, et al. Involvement of a human gene related to the Drosophila spen gene in the recurrent t(1;22) translocation of acute megakaryocytic leukemia. Proc Natl Acad Sci U S A. 2001;98:5776–9. doi: 10.1073/pnas.101001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma Z, Morris SW, Valentine V, Li M, Herbrick JA, Cui X, et al. Fusion of two novel genes, RBM15 and MKL1, in the t(1;22)(p13;q13) of acute megakaryoblastic leukemia. Nat Genet. 2001;28:220–1. doi: 10.1038/90054. [DOI] [PubMed] [Google Scholar]
- 34.Gruber TA, Larson Gedman A, Zhang J, Koss CS, Marada S, Ta HQ, et al. An Inv(16)(p13.3q24.3)-encoded CBFA2T3-GLIS2 fusion protein defines an aggressive subtype of pediatric acute megakaryoblastic leukemia. Cancer Cell. 2012;22:683–97. doi: 10.1016/j.ccr.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Rooij JD, Hollink IH, Arentsen-Peters ST, van Galen JF, Berna Beverloo H, Baruchel A, et al. NUP98/JARID1A is a novel recurrent abnormality in pediatric acute megakaryoblastic leukemia with a distinct HOX gene expression pattern. Leukemia. 2013 Mar 27; doi: 10.1038/leu.2013.87. doi: 10.1038/leu.2013.87. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 36.Walters DK, Mercher T, Gu TL, O'Hare T, Tyner JW, Loriaux M, et al. Activating alleles of JAK3 in acute megakaryoblastic leukemia. Cancer Cell. 2006;10:65–75. doi: 10.1016/j.ccr.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 37.Malinge S, Ragu C, Della-Valle V, Pisani D, Constantinescu SN, Perez C, et al. Activating mutations in human acute megakaryoblastic leukemia. Blood. 2008;112:4220–6. doi: 10.1182/blood-2008-01-136366. [DOI] [PubMed] [Google Scholar]
- 38.Hussein K, Bock O, Theophile K, Schulz-Bischof K, Porwit A, Schlue J, et al. MPLW515L mutation in acute megakaryoblastic leukaemia. Leukemia. 2009;23:852–5. doi: 10.1038/leu.2008.371. [DOI] [PubMed] [Google Scholar]
- 39.Sorrell AD, Alonzo TA, Hilden JM, Gerbing RB, Loew TW, Hathaway L, et al. Favorable survival maintained in children who have myeloid leukemia associated with Down syndrome using reduced-dose chemotherapy on Children's Oncology Group trial A2971: a report from the Children's Oncology Group. Cancer. 2012;118:4806–14. doi: 10.1002/cncr.27484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Brien MM, Taub JW, Chang MN, Massey GV, Stine KC, Raimondi SC, et al. Cardiomyopathy in children with Down syndrome treated for acute myeloid leukemia: a report from the Children's Oncology Group Study POG 9421. Journal of Clinical Oncology. 2008;26:414–20. doi: 10.1200/JCO.2007.13.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O'Brien MM, Cao X, Pounds S, Dahl GV, Raimondi SC, Lacayo NJ, et al. Prognostic features in acute megakaryoblastic leukemia in children without Down syndrome: a report from the AML02 multicenter trial and the Children's Oncology Group Study POG 9421. Leukemia. 2013;27:731–4. doi: 10.1038/leu.2012.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barnard DR, Alonzo TA, Gerbing RB, Lange B, Woods WG. Comparison of childhood myelodysplastic syndrome, AML FAB M6 or M7, CCG 2891: Report from the Children's Oncology Group. Pediatr Blood Cancer. 2007;49:17–22. doi: 10.1002/pbc.20951. [DOI] [PubMed] [Google Scholar]
- 43.Athale UH, Razzouk BI, Raimondi SC, Tong X, Behm FG, Head DR, et al. Biology and outcome of childhood acute megakaryoblastic leukemia: a single institution's experience. Blood. 2001;97:3727–32. doi: 10.1182/blood.v97.12.3727. [DOI] [PubMed] [Google Scholar]
- 44.Tallman MS, Neuberg D, Bennett JM, Francois CJ, Paietta E, Wiernik PH, et al. Acute megakaryocytic leukemia: the Eastern Cooperative Oncology Group experience. Blood. 2000;96:2405–11. [PubMed] [Google Scholar]
- 45.Pagano L, Pulsoni A, Vignetti M, Mele L, Fianchi L, Petti MC, et al. Acute megakaryoblastic leukemia: experience of GIMEMA trials. Leukemia. 2002;16:1622–6. doi: 10.1038/sj.leu.2402618. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg SL, Fenaux P, Craig MD, Gyan E, Lister J, Kassis J, et al. Phase 2 Study of MLN8237, An Investigational Aurora A Kinase (AAK) Inhibitor In Patients with Acute Myelogenous Leukemia (AML) or Myelodysplastic Syndromes (MDS) Blood ASH Annual Meeting Abstracts. 2010;116:3273. [Google Scholar]
- 47.Gorgun G, Calabrese E, Hideshima T, Ecsedy J, Perrone G, Mani M, et al. A novel Aurora-A kinase inhibitor MLN8237 induces cytotoxicity and cell-cycle arrest in multiple myeloma. Blood. 2010;115:5202–13. doi: 10.1182/blood-2009-12-259523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sehdev V, Peng D, Soutto M, Washington MK, Revetta F, Ecsedy J, et al. The aurora kinase A inhibitor MLN8237 enhances cisplatin-induced cell death in esophageal adenocarcinoma cells. Molecular Cancer Therapeutics. 2012;11:763–74. doi: 10.1158/1535-7163.MCT-11-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]