Abstract
BACKGROUND
Previous studies have shown that late sodium channel current (INa) blockers such as ranolazine can exert antiarrhythmic effects by suppressing early and delayed after-depolarization (EAD and DAD)-induced triggered activity.
OBJECTIVE
To evaluate the electrophysiological properties of GS-458967 (GS967), a potent and highly selective late INa blocker, in canine Purkinje fibers (PFs) and pulmonary vein (PV) and superior vena cava (SVC) sleeve preparations.
METHODS
Transmembrane action potentials were recorded from canine PFs and PV and SVC sleeve preparations by using standard microelectrode techniques. The rapidly activating delayed rectifier potassium channel current blocker E-4031 (2.5–5 μM) and the late INa agonist ATX-II (10 nM) were used to induce EADs in PFs. Isoproterenol (1 μM), high calcium ([Ca2+]o = 5.4 mM), or their combination was used to induce DADs and triggered activity.
RESULTS
In PFs, GS967 (10–300 nM) caused a significant concentration-dependent reduction in action potential duration without altering the maximum rate of rise of the action potential upstroke, action potential amplitude, or resting membrane potential at any rate studied (basic cycle lengths of 1000, 500, and 300 ms) or concentration evaluated (n = 5; P < .05). GS967 (30–100 nM) abolished EADs and EAD-induced triggered activity elicited in PFs by exposure to E-4031 (n = 4) or ATX-II (n = 4). In addition, GS967 reduced or abolished DADs and suppressed DAD-induced triggered activity elicited in PFs (n = 4) and PV (n = 4) and SVC (n = 3) sleeve preparations by exposure to isoproterenol, high calcium, or their combination.
CONCLUSIONS
Our data suggest that the selective inhibition of late INa with GS967 can exert antiarrhythmic effects by suppressing EAD- and DAD-mediated extrasystolic activity in PFs and PV and SVC sleeve preparations.
Keywords: Antiarrhythmic drugs, Electrophysiology, Pharmacology, Late sodium current, Ranolazine
Introduction
Purkinje fibers (PFs) have been shown to develop marked action potential prolongation as well as early afterdepolarization (EAD)-induced triggered activity following exposure to agents that slow ventricular repolarization1,2 or promote delayed after-depolarization (DAD)-induced triggered activity under conditions of calcium overload or beta-adrenergic stimulation.3,4
Pulmonary veins (PVs) have been shown to be the most common ectopic sites capable of initiating atrial fibrillation (AF).5 The superior vena cava (SVC) is the main non-PV site of origin of extrasystoles capable of triggering AF.6 Previous studies have shown that late phase 3 EAD- and DAD-induced triggered activity can be easily elicited in PV and SVC sleeve preparations by parasympathetic and/or sympathetic stimulation.7,8 In addition to PFs, PV, and SVC, epicardial ventricular muscle cells have been reported to develop EADs and triggered activity in aged rat hearts exposed to oxidative stress.9 In the last several years, a number of studies have shown that the inhibition of late sodium channel current (INa) with drugs such as ranolazine, flecainide, and mexiletine exerts antiarrhythmic effects by suppressing EAD- and DAD-induced triggered activity in experimental models (see Reference10 for review). However, none of these drugs are sufficiently selective inhibitors of late INa to provide definitive evidence of the role of this current in the genesis of arrhythmias in various cardiac tissues.
A recent study showed that the late INa blocker GS-458967 (GS967) can exert antiarrhythmic effects in rabbit ventricular myocytes, rabbit isolated hearts, and anesthetized rabbits.11 EADs and EAD-induced triggered activity were observed in isolated myocytes, and EAD-dependent extra-systoles were observed in isolated rabbit hearts.
The present study was designed to evaluate the electro-physiological and antiarrhythmic properties of GS967 previously described in the isolated rabbit heart in diverse cardiac preparations such as canine PFs and PV and SVC sleeve preparations known to be involved in the genesis of extrasystoles responsible for the development of major cardiac arrhythmias including AF and ventricular tachycardia/ ventricular fibrillation. Preliminary data have been reported in abstract form.12
Methods
This investigation conforms to the Guide for Care and Use of Laboratory Animals published by the National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Animal Care and Use Committee of the Masonic Medical Research Laboratory.
Adult mongrel dogs weighing 20–35 kg were anticoagulated with heparin (180 IU/kg) and anesthetized with sodium pentobarbital (35 mg/kg, intravenous). The chest was opened via a left thoracotomy, and the heart was excised and placed in a cold cardioplegic solution ([K+]0 = 12 mM; 4°C).
PFs and PV and SVC sleeve preparations
PFs (false tendons) were isolated from left or right canine ventricles; PV and SVC sleeve preparations (approximately 2.0 × 1.5 cm) were isolated from the left and right canine atria, respectively. The thickness of the PV and SVC preparations was approximately 2 mm. The preparations were placed in a small tissue bath and superfused with Tyrode’s solution of the following composition: 129 mM NaCl, 4 mM KCl, 0.9 mM NaH2PO4, 20 mM NaHCO3, 1.8 mM CaCl2, 0.5 mM MgSO4, 5.5 mM glucose, buffered with 95% O2/5% CO2 (35 ± 0.5°C). PFs and PV and SVC sleeve preparations were stimulated at a basic cycle length (BCL) of 1000 ms during the equilibration period (1 hour) using electrical stimuli (1–3 ms duration; 2.5 times diastolic threshold intensity) delivered through silver bipolar electrodes insulated except at the tips. Transmembrane potentials were recorded by using glass microelectrodes filled with 3 M KCl (10–20 MΩ direct current resistance) connected to a high input-impedance amplification system (Model KS-700, World Precision Instruments, New Haven, CT). In PFs, action potential duration at 90% repolarization (APD90), maximum rate of rise of the action potential upstroke (Vmax), action potential amplitude (APA), and MDP were evaluated before and after exposure to GS967. Transmembrane action potentials (APs) were recorded at a sampling rate of 41 kHz.
Induction of arrhythmias
The rapidly activating delayed rectifier potassium channel current (IKr) blocker E-4031 (2.5–5 μM) and the late INa agonist ATX-II (10 nM) were used to induce EADs and triggered activity in canine PFs. Isoproterenol (1 μM), high calcium ([Ca2+]o = 5.4 mM), or their combination was used to induce DADs and triggered activity in canine PFs and PV and SVC sleeve preparations. This arrhythmia model has previously been shown to generate DAD-induced triggered activity in 100% PV sleeve preparations.7
Drugs
GS967 (Gilead, Foster City, CA) was dissolved in distilled water to form a stock solution of 100 μM and used at a final concentration of 10–300 nM. Isoproterenol (Sigma-Aldrich, St Louis, MO) was dissolved in distilled water to form a stock solution of 5 mM and used at a final concentration of 1 μM. E-4031l (Sigma-Aldrich) was dissolved in distilled water to form a stock solution of 5 mM and used at a final concentration of 2.5–5 μM. ATX-II (Sigma-Aldrich) was dissolved in distilled water to form a stock solution of 200 nM and used at a final concentration of 10 nM.
Statistical analysis
The statistical analysis of the electrophysiological effects of GS967 in canine PFs was performed by using 1-way repeated measures analysis of variance followed by the Bonferroni test. The statistical analysis of incidence of EADs was assessed by using the Fisher exact test. Mean values were considered to be significantly different at P < .05. All data are reported as mean ± SD.
Results
We determined the viability and stability of superfused PFs and PV and SVC sleeve preparations by superfusing them with normal Tyrode’s solution and continuously recording the electrical activity for a period up to 120 minutes. No significant changes in AP morphology were observed over the 2-hour period.
Electrophysiological effects of GS967 in canine PFs
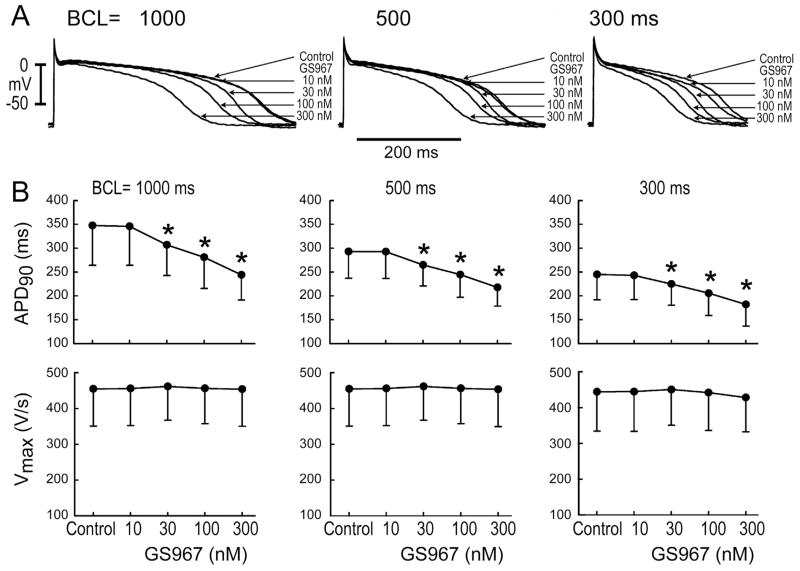
In one series of experiments, we determined the effect of GS967 on the electrophysiological characteristics of canine PFs isolated from both ventricles of normal dogs. As illustrated in Figure 1A, GS967 caused a marked concentration-dependent reduction of APD without altering APA or MDP. Data summarizing the effects of GS967 on APD90 and Vmax are shown in Figure 1B. APD90 was significantly reduced at BCLs of 1000, 500, and 300 ms and concentrations of 30, 100, and 300 nM. At a concentration of 100 nM, APD90 was reduced from 347 ± 83 to 281 ± 65 ms at a BCL of 1000 ms and from 245 ± 53 to 205 ± 46 ms at a BCL of 300 ms. No significant changes in Vmax, APA, or MDP were observed at any BCL or concentration studied. At a BCL of 1000 ms, APA and MDP were 122.4 ± 8 and −85.2 ± 5 mV, respectively, under control conditions and 121.9 ± 7 and −85.4 ± 5 following GS967 (100 nM). At a BCL of 300 ms, APA and MDP were 121.8 ± 7 and −85.1 ± 5 mV, respectively, under control conditions and 120.7 ± 7 and −84.8 ± 5 mV following GS967 (100 nM).
Figure 1.
Effect of GS-458967 (GS967; 10–300 nM) on action potentials (APs) recorded from canine Purkinje fibers. A: Effect of GS967 on AP morphology recorded at basic cycle lengths (BCLs) of 1000, 500, and 300 ms. B: Composite data of the effect of GS967 on the action potential duration measured at 90% repolarization (APD90) and on the maximal rate of rise of the action potential upstroke (Vmax). GS967 (10–300 nM) concentration-dependently reduced APD without reducing Vmax (n = 5). *P < .05 vs control.
Effects of GS967 on EADs and triggered activity induced in canine PFs
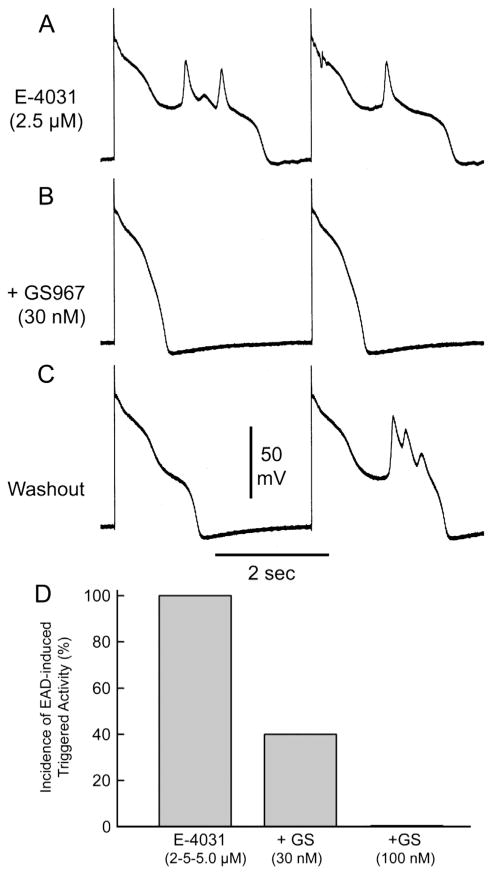
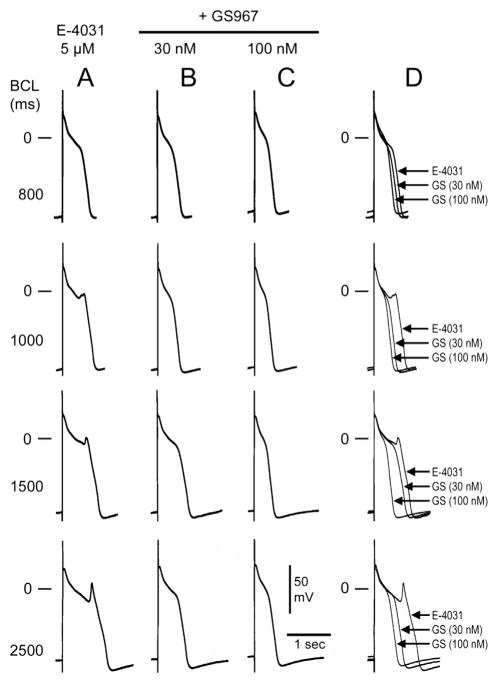
In another series of experiments, we determined the effects of GS967 on EADs and triggered activity induced by the IKr blocker E-4031 (2.5–5 μM) and the late INa agonist ATX-II (10 nM). Figure 2 illustrates a representative example of the effect of GS967 to suppress EADs and triggered activity elicited by exposure to E-4031 in a canine PF. At a BCL of 3500 ms, E-4031 (2.5 μM) induced EADs and triggered activity (Figure 2A). The addition of GS967 (30 nM) suppressed all EADs and triggered activity (Figure 2B). The washout of E-4031 restored EAD and triggered activity (Figure 2C). Figure 3 shows another example of the concentration and rate dependence of the effect of GS967 to reduce APD and suppress EAD-induced triggered activity elicited by exposure to E-4031. Figure 2D illustrates the incidence of EAD-induced triggered activity in 5 PFs exposed to E-4031 (2.5–5 μM) alone or together with 30 or 100 nM GS967. The incidences of EAD-induced triggered activity were 4 of 4 (100%), 2 of 5 (40%), and 0 of 5 (0%) PFs following E-4031, 30 nM GS967, and 100 nM GS967, respectively (P = .029, E-4031 vs 100 nM GS967).
Figure 2.
GS-458967 (GS967) suppression of early afterdepolarizations (EADs) and triggered activity elicited by exposure to E-4031 in a canine Purkinje fiber. A: E-4031(2.5 μM) induced EADs and triggered activity. B: Addition of GS967 (30 nM) abolished all EADs and triggered activity. C: Washout of E-4031 restored EADs and triggered activity. Basic cycle length = 3500 ms. D: Incidence of EAD-induced triggered activity in 5 Purkinje fibers exposed to E-4031 (2.5–5 μM) alone or in combination with 30 and 100 nM GS967.
Figure 3.
Rate dependence of early afterdepolarizations (EADs) elicited by exposure to E-4031 in a canine Purkinje fiber and their inhibition by GS-967. A: E-4031 (5 μM) induced EADs at basic cycle lengths (BCLs) of 800, 1000, 1500, and 2500 ms. B: Addition of GS-458967 (GS967; 30 nM) suppressed EADs and triggered activity. C: Addition of GS967 (100 nM) further reduced action potential duration. D: Superimposed traces of the effects of E-4031 and GS967.
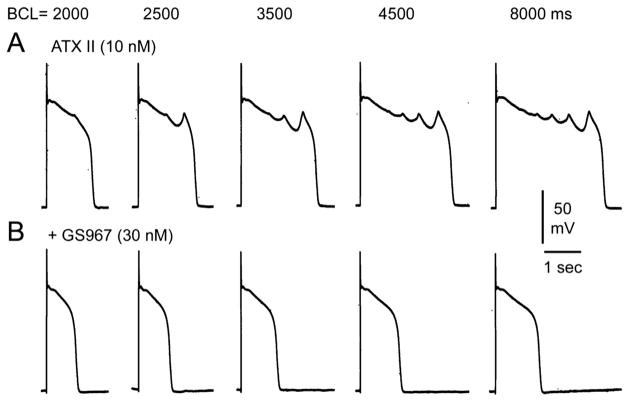
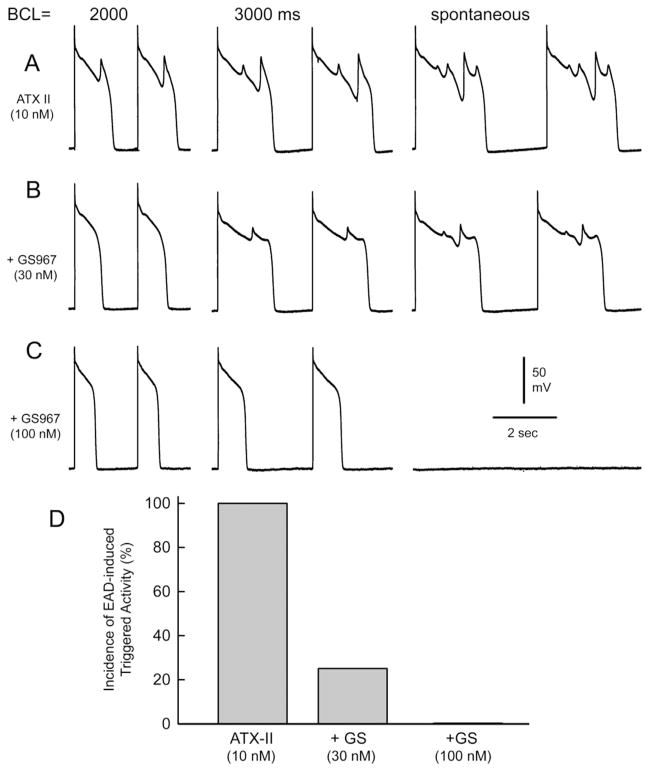
Figures 4 and 5 illustrate the effects of GS967 to suppress EADs and EAD-induced triggered activity elicited by exposure to ATX-II in a canine PF. As shown in Figure 4, ATX-II (10 nM) elicited EADs and EAD-induced triggered activity at BCLs of 2000, 2500, 3500, 4500, and 8000 ms (Figure 4A). The addition of GS967 (30 nM) abolished all EADs and triggered activity (Figure 4B). Figure 5 illustrates the concentration and rate dependence of the effect of GS967 to suppress EADs and EAD-induced triggered activity elicited by exposure to ATX-II in a canine PF. ATX-II (10 nM) induced EADs and triggered activity at BCLs of 2000 and 3000 ms and depressed spontaneous firing (Figure 5A). The addition of GS967 (30 nM) reduced EADs and triggered activity (Figure 5B). The addition of GS967 (100 nM) eliminated all EADs and triggered activity and depressed spontaneous firing of PFs (Figure 5C). Figure 5D illustrates the incidence of EAD-induced triggered activity in 5 PFs exposed to ATX-II (10 nM) alone or in combination with 30 or 100 nM GS967. The incidences of EAD-induced triggered activity were 4 of 4 (100%), 1 of 4 (25%), and 0 of 5 (0%) PFs following ATX-II, 30 nM GS967, and 100 nM G967, respectively (P = .048, ATX-II vs 30 nM GS967; P = .008, ATX-II vs 100 nM GS967).
Figure 4.
GS-458967 (GS967) abolishes early afterdepolarizations (EADs) and EAD-induced triggered activity elicited by exposure to ATX-II in a canine Purkinje fiber. A: ATX-II (10 nM) elicited EADs and EAD-induced triggered activity at basic cycle lengths (BCLs) of 2000, 2500, 3500, 4500, and 8000 ms. GS967 (30 nM) abolished all EADs and triggered activity.
Figure 5.
Concentration and rate dependence of the effect of GS-458967 (GS967) to suppress early afterdepolarizations (EADs) and EAD-induced triggered activity elicited by exposure to ATX-II in canine Purkinje fibers. A: ATX-II (10 nM)-induced EADs and triggered activity at basic cycle lengths (BCLs) of 2000 and 3000 ms and during spontaneous firing. B: GS967 (30 nM) reduced EADs and triggered activity. C: GS967 (100 nM) abolished all EADs and triggered activity and depressed spontaneous firing of the Purkinje pacemaker. D: Incidence of EAD-induced triggered activity in 5 Purkinje fibers exposed to ATX-II (10 nM) alone or in combination with 30 and 100 nM GS967.
Effects of GS967 on DADs and DAD-induced triggered activity in canine PFs
Figure 6 shows a representative example of the effect of GS967 on DADs and DAD-induced triggered activity in a canine PF. High calcium (5.4 mM) and isoproterenol (1 μM) induced prominent DADs and DAD-induced triggered activity following 20 beats at BCLs of 1000, 800, 500, and 300 ms (Figure 6A). The addition of GS967 (30 nM) markedly reduced DADs and triggered activity (Figure 6B). A higher concentration of GS967 (100 nM) abolished all DADs and triggered activity (Figure 6C). Figure 6D illustrates the incidence of DAD-induced triggered activity in 4 PFs exposed to high calcium (5.4 mM), isoproterenol (l μM), or their combination with 30 or 100 nM GS4967. The incidences of DAD-induced triggered activity were 4 of 4 (100%), 2 of 4 (50%), and 0 of 5 (0%) PFs following high calcium + isoproterenol, 30 nM GS967, and 100 nM GS967, respectively.
Figure 6.
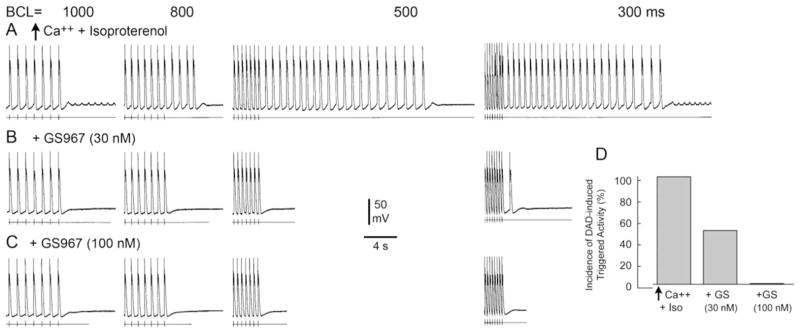
Effect of GS-458967 (GS967) on delayed afterdepolarizations (DADs) and DAD-induced triggered activity elicited by exposure to high calcium and isoproterenol in a canine Purkinje fiber. A: High calcium (5.4 mM) and isoproterenol (1 μM) induced prominent DADs and DAD-induced triggered activity in a pulmonary vein sleeve preparation following 20 beats at basic cycle lengths (BCLs) of 1000, 800, 500, and 300 ms. B: GS967 (30 nM) markedly reduced DADs and triggered activity. C: GS967 (100 nM) completely abolished all DADs and triggered activity. D: Incidence of DAD-induced triggered activity in 4 Purkinje fibers exposed to high calcium (5.4 mM) + isoproterenol (l μM) alone or in combination with 30 and 100 nM GS967.
Effects of GS967 on DADs and DAD-induced triggered activity induced in PV and SVC sleeve preparations
We also studied the effects of GS967 on DADs and DAD-induced triggered activity elicited by exposure of PV and SVC sleeve preparations exposed to high calcium and isoproterenol. As shown in Figures 7 and 8, GS967 suppressed DADs and DAD-induced triggered activity elicited in PV (Figure 7) and SVC (Figure 8) sleeve preparations. Figure 7D illustrates the incidence of DAD-induced triggered activity in 4 PV sleeve preparations exposed to high calcium (5.4 mM), isoproterenol (l μM), or their combination with 30 or 100 nM GS967. The incidences of DAD-induced triggered activity were 4 of 4 (100%), 1 of 4 (25%), and 0 of 4 (0%) following high calcium + isoproterenol, 30 nM GS967, and +100 nM GS967, respectively.
Figure 7.
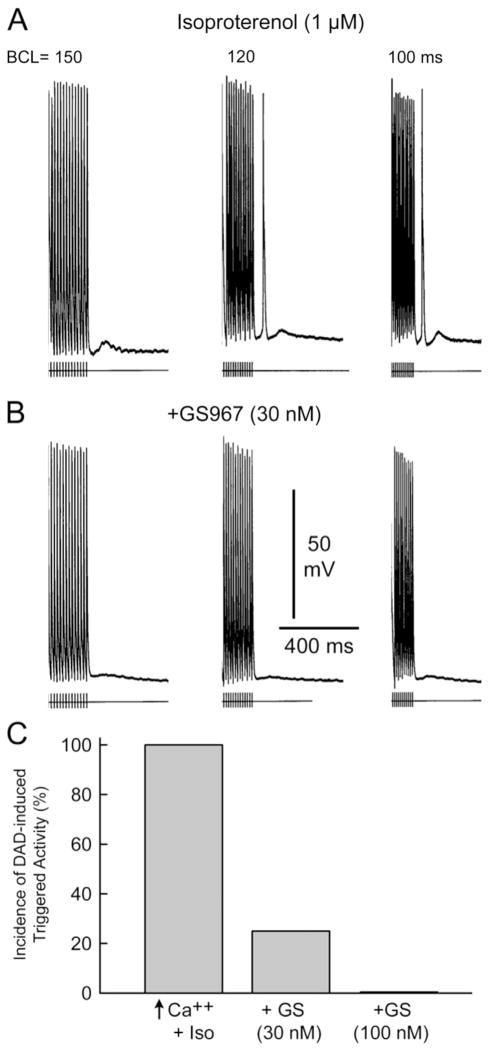
GS-458967 (GS967) suppresses delayed afterdepolarizations (DADs) and DAD-induced triggered activity elicited by exposure to isoproterenol in a canine pulmonary vein (PV) sleeve preparation. A: Isoproterenol (1 μM) elicited a DAD following 20 beats at a basic cycle length (BCL) of 150 ms and a DAD-induced triggered response after 20 beats at BCLs of 120 or 100 ms. B: GS967 (30 nM) suppressed the triggered response and markedly reduced DAD activity. C: Incidence of DAD-induced triggered activity in 4 PV sleeve preparations exposed to high calcium (5.4 mM) + isoproterenol (l μM) alone or in combination with 30 and 100 nM GS967.
Figure 8.
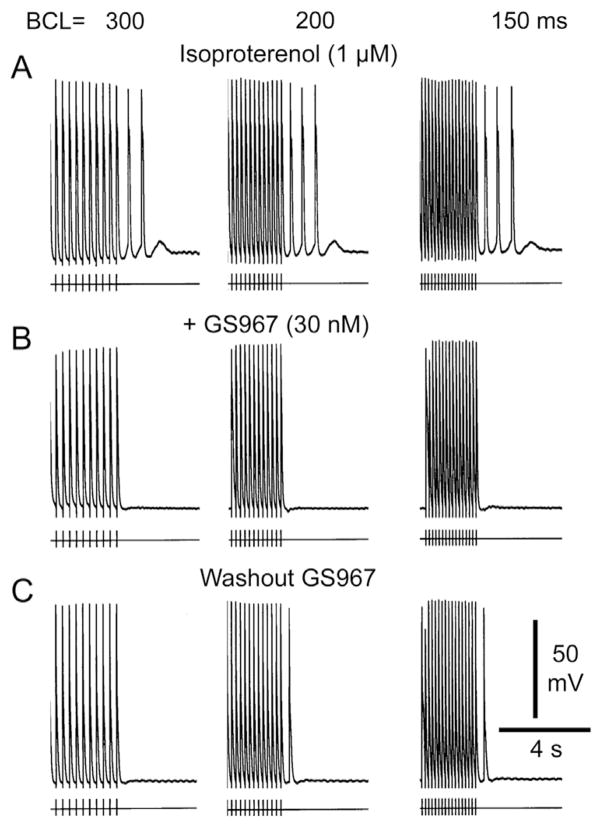
GS-458967 (GS967) suppresses delayed afterdepolarizations (DADs) and DAD-induced triggered activity elicited by exposure to isoproterenol in a canine superior vena cava sleeve preparation. A: Isoproterenol (1 μM) elicited DAD-induced triggered activity after 20 beats at basic cycle lengths (BCLs) of 300, 200, and 150 ms. B: GS967 (30 nM) abolished all triggered responses and markedly reduced DAD activity. C: Partial washout of the drug restored triggered activity.
Discussion
Our data demonstrate that GS967, at concentrations of 30–100 nM, concentration-dependently reduces APD without altering Vmax. In addition, GS967 was effective in (1) fully suppressing ATX-II and E-4031-induced EADs and EAD-induced triggered activity in canine PFs, (2) partially suppressing DADs induced by exposure to high calcium or high calcium + isoproterenol, and (3) fully suppressing DAD-induced triggered activity in PFs and PV and SVC sleeve preparations. Thus, the data point to a potent antiarrhythmic effect of the late sodium channel blocker GS967 in canine PFs and PV and SVC sleeve preparations. In addition, considering the high selectivity of GS96711 and the effects of relatively low concentrations (30 nM) of this compound, these data point to an important role for late INa in the genesis of EADs and DADs.
The present study extends observations of an antiarrhythmic effect of GS967 previously made by using isolated rabbit myocytes and a rabbit in vivo model11 to canine PFs and PV and SVC sleeve preparations. The data suggest that the effect of GS967 to suppress EAD-induced triggered activity also applies to canine PFs. The effect of GS967 to suppress DAD-mediated extrasystoles in PV and SVC sleeve preparations has not been previously reported.
Effect of GS967 in PFs
In canine PFs isolated from the right and left ventricles, GS967 shortened the AP without altering Vmax, APA, or MDP even at fast rates and a high dose of GS967, suggesting selective block of late INa with little or no effect on peak INa at the drug concentrations evaluated. GS967 is reported to inhibit ATX-II-induced late INa in ventricular myocytes and isolated rabbit hearts with IC50 values of 0.13 and 0.21 μM, respectively, with minimal inhibition (≤7.5% at 10 μM) of peak INa and IKr (16.7% at 10 μM).11 Interestingly, the block of peak INa by GS967 was voltage dependent, with minimal or no use-dependent effect.
Antiarrhythmic effects of GS967 in PFs exposed to QT prolonging agents
In isolated rabbit hearts and myocytes, GS967 abbreviates the duration of the ventricular AP, does not prolong the QRS interval, prevents and suppresses proarrhythmic activity, including EAD-induced triggered activity and torsades de pointes (TdP) arrhythmias, induced by the late INa enhancer ATX-II, the IKr inhibitor E-4031, and ischemia.11 Our data illustrating the suppression of EADs and EAD-induced triggered activity demonstrate a similar effect of GS967 in canine PFs exposed to QT prolonging agents, such as E-4031 and ATX-II, to eliminate EAD-dependent extrasystoles.
Antiarrhythmic effects of GS967 under calcium overload conditions
In addition to eliminating EAD activity, GS967 was found to markedly reduce DADs and suppress DAD-induced triggered activity in canine PFs as well as in PV and SVC canine preparations. The development of DADs and DAD-induced triggered activity has been demonstrated under calcium-loading conditions in PFs.1,2 DADs and DAD-induced triggered activity have been reported under conditions of sympathetic simulation, parasympathetic stimulation, and calcium overload conditions in canine- and rabbit-isolated single PV myocytes,13–15 canine PV sleeve superfused preparations,7 coronary-perfused atrial preparations,16 and SVC sleeve preparations.8
DADs are known to be caused by calcium loading-induced transient inward current (ITi).17 The suppression of DADs and DAD-induced triggered activity by GS967 is likely due to a reduction in intracellular calcium loading (via the sodium-calcium exchanger) secondary to a decrease in intracellular sodium activity.18 This interpretation is consistent with a previous report by Song et al,19 showing that in guinea pig-isolated atrial myocytes, ATX-II-induced ITi and DADs are suppressed by late INa block with agents such as ranolazine.
Late INa and cardiac arrhythmias
An increase in late INa has been shown to delay cardiac repolarization in ventricular cells, tissues, and in vivo experimental models. We demonstrate the effect of the late INa agonist ATX-II to markedly prolong APD and induce EADs and EAD-induced triggered responses in canine PFs (Figures 4 and 5). Similar results have been reported in other experimental models including multicellular canine M cell preparations, canine wedge models, rabbit isolated myocytes, and in vivo and anesthetized rabbit hearts.10,11 Like GS967, late INa blockers such as mexiletine and ranolazine are known to be effective in reversing AP prolongation and suppressing EADs as well as TdP.10 The late INa blocker ranolazine has also been shown to reduce dispersion of ventricular repolarization, EADs, and TdP induced by drugs that block IKr.20,21 In a rabbit model of TdP,22 ranolazine terminated and prevented the recurrence of TdP induced by the IKr blocker clofilium.23 Similarly, in a canine chronic atrioventricular block model, TdP induced by the IKr blocker dofetilide was suppressed by ranolazine.24 Ranolazine has been reported to suppress TdP in several experimental models of long QT syndrome by reducing transmural dispersion of repolarization.25–27 Arrhythmogenic effects occurring when late INa is increased by congenital (mainly long QT syndrome) or acquired pathophysiological conditions have also been shown to be reversed or prevented by ranolazine.19
The observed effect of the late INa inhibitor GS967 to suppress EADs (Figures 2 and 3) and TdP arrhythmias11 when repolarization reserve is reduced secondary to IKr block28 can be explained by the inhibition of intrinsic late INa present under physiological conditions. The inhibition of late INa is expected to be even more effective in cases in which late INa is augmented as a result of pathophysiological remodeling.
In addition to prolonging APD and inducing repolarization abnormalities, enhanced late INa increases Na influx and, via the sodium-calcium exchanger, increases intracellular calcium, resulting in calcium overload, which in turn promotes the spontaneous release of calcium from the sarcoplasmic reticulum, induction of ITi, DADs, and triggered activity. Increases in late INa also induce intracellular acidosis and activation of calmodulin-dependent protein kinase II in rat ventricular myocytes.29 Low concentrations of GS967 are reported to reduce ATX-II-induced late INa and reverse the sodium-dependent calcium overload in rabbit myocytes.11 The inhibition of late INa has also been shown to decrease spontaneous diastolic depolarizations because of the inhibition of a slowly inactivating tetrodotoxin-sensitive sodium current in atrial myocytes30 and rabbit sinoatrial node cells.31
Late INa is increased in heart failure, and inhibitors such as ranolazine have been shown to abbreviate the AP, improve contraction, and eliminate EAD- and DAD-mediated extra-systoles in this setting.32 In ventricular myocytes from canine failing hearts, prolonged calcium transients are abbreviated and spontaneous calcium releases are suppressed by ranolazine and tetrodotoxin.33
Comparison with other late sodium channel blockers
The inhibition of late INa per se has no arrhythmogenic effects in normal or diseased hearts. Arrhythmogenic effects of INa inhibitors such as flecainide can be ascribed to their effects to cause marked use-dependent reduction in peak INa and/or reduction in IKr. In contrast to flecainide, GS967 has little or no effect on fast INa or IKr at the concentrations used and known to exert antiarrhythmic effects in experimental models. The IC50 for the inhibition of ATX-II-induced late INa in isolated rabbit ventricular myocytes is much greater for GS967 than for flecainide (0.13 μM vs 3.4 μM). Our data indicate that 0.1–0.3 μM is sufficient to inhibit arrhythmias induced in canine PFs and PV and SVC sleeve preparations whereas 5–10 μM of ranolazine is needed to suppress EAD-or DAD-induced triggered activity in experimental models of arrhythmias.34,35 Thus, GS967 is a more than 10-fold more potent and more than 70-fold more selective inhibitor of late INa compared to previously described late INa inhibitors.
Conclusions
Our data indicate that the potent (IC50 = 130 nM) and highly selective late INa blocker GS967 exerts antiarrhythmic effects by suppressing EAD- and DAD-mediated extrasystoles in PFs and PV and SVC sleeve preparations. The data add to the growing evidence for a prominent role of late INa inhibitors as potential agents in the treatment of ventricular arrhythmias associated with long QT intervals or intracellular calcium loading.
Acknowledgments
This work was supported by grants from Gilead Sciences, National Heart, Lung, and Blood Institute (grant HL 47678 from the National Institutes of Health; to Dr Antzelevitch), NYSTEM (grant C026424; to Dr Antzelevitch), and Masons of New York, Florida, Massachusetts, and Connecticut. Dr Antzelevitch received a research grant and serves as a consultant to Gilead Sciences. Dr Belardinelli is employed by Gilead Sciences.
We are grateful to Judith Hefferon and Robert J. Goodrow, Jr, for technical assistance.
ABBREVIATIONS
- AF
atrial fibrillation
- AP
action potential
- APA
action potential amplitude
- APD90
action potential duration at 90% repolarization
- BCL
basic cycle length
- DAD
delayed afterdepolarization
- EAD
early afterdepolarization
- GS-458967
GS967
- IKr
rapidly activating delayed rectifier potassium channel current
- INa
sodium channel current
- ITi
transient inward current
- MDP
maximum diastolic potential
- PF
Purkinje fiber
- PV
pulmonary vein
- SVC
superior vena cava
- TdP
torsades de pointes
- Vmax
maximum rate of rise of the action potential upstroke
References
- 1.Cranefield PF. Action potentials, afterpotentials and arrhythmias. Circ Res. 1977;41:415–423. doi: 10.1161/01.res.41.4.415. [DOI] [PubMed] [Google Scholar]
- 2.Wit AL, Rosen MR. Afterdepolarizations and triggered activity. In: Fozzard HA, Haber E, Jennings RB, et al., editors. The Heart and Cardiovascular System. New York: Raven Press; 1986. pp. 1449–1491. [Google Scholar]
- 3.Ferrier GR, Saunders JH, Mendez C. A cellular mechanism for the generation of ventricular arrhythmias by acetylstrophanthidin. Circ Res. 1973;32:600–609. doi: 10.1161/01.res.32.5.600. [DOI] [PubMed] [Google Scholar]
- 4.Rosen MR, Gelband H, Merker C, et al. Mechanisms of digitalis toxicity: effects of ouabain on phase four of canine Purkinje fiber transmembrane potentials. Circulation. 1973;47:681–689. doi: 10.1161/01.cir.47.4.681. [DOI] [PubMed] [Google Scholar]
- 5.Haissaguerre M, Jais P, Shah DC, et al. Spontaneous initiation of atrial fibrillation by ectopic beats originating in the pulmonary veins. N Engl J Med. 1998;339:659–666. doi: 10.1056/NEJM199809033391003. [DOI] [PubMed] [Google Scholar]
- 6.Corrado A, Bonso A, Madalosso M, et al. Impact of systematic isolation of superior vena cava in addition to pulmonary vein antrum isolation on the outcome of paroxysmal, persistent, and permanent atrial fibrillation ablation: results from a randomized study. J Cardiovasc Electrophysiol. 2010;21:1–5. doi: 10.1111/j.1540-8167.2009.01577.x. [DOI] [PubMed] [Google Scholar]
- 7.Sicouri S, Glass A, Belardinelli L, et al. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeve preparations. Heart Rhythm. 2008;5:1019–1026. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sicouri S, Blazek J, Belardinelli L, et al. Electrophysiological characteristics of canine superior vena cava sleeve preparations: effect of ranolazine. Heart Rhythm. 2012;9:S255. doi: 10.1161/CIRCEP.111.969493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morita H, Zipes DP, Morita ST, et al. Epicardial ablation eliminates ventricular arrhythmias in an experimental model of Brugada syndrome. Heart Rhythm. 2009;6:665–671. doi: 10.1016/j.hrthm.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 10.Antzelevitch C, Burashnikov A, Sicouri S, et al. Electrophysiological basis for the antiarrhythmic actions of ranolazine. Heart Rhythm. 2011;8:1281–1290. doi: 10.1016/j.hrthm.2011.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belardinelli L, Liu G, Smith-Maxwell C, et al. A novel, potent, and selective inhibitor of cardiac late sodium current suppresses experimental arrhythmias. J Pharmacol Exp Ther. 2013;344:23–32. doi: 10.1124/jpet.112.198887. [DOI] [PubMed] [Google Scholar]
- 12.Sicouri S, Blazek J, Belardinelli L, et al. Antiarrhythmic effects of the highly-selective late sodium channel current blocker GS 458967 in canine Purkinje fibers and pulmonary vein sleeve preparations. Heart Rhythm. 2012;9:S186. doi: 10.1016/j.hrthm.2013.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YJ, Chen SA, Chang MS, et al. Arrhythmogenic activity of cardiac muscle in pulmonary veins of the dog: implication for the genesis of atrial fibrillation. Cardiovasc Res. 2000;48:265–273. doi: 10.1016/s0008-6363(00)00179-6. [DOI] [PubMed] [Google Scholar]
- 14.Chen YJ, Chen SA. Electrophysiology of pulmonary veins. J Cardiovasc Electrophysiol. 2006;17:220–224. doi: 10.1111/j.1540-8167.2005.00317.x. [DOI] [PubMed] [Google Scholar]
- 15.Patterson E, Po SS, Scherlag BJ, et al. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005;2:624–631. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 16.Burashnikov A, Sicouri S, Di Diego JM, et al. Synergistic effect of the combination of dronedarone and ranolazine to suppress atrial fibrillation. J Am Coll Cardiol. 2010;56:1216–1224. doi: 10.1016/j.jacc.2010.08.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Katra RP, Laurita KR. Cellular mechanism of calcium-mediated triggered activity in the heart. Circ Res. 2005;96:535–542. doi: 10.1161/01.RES.0000159387.00749.3c. [DOI] [PubMed] [Google Scholar]
- 18.Li D, Melnyk P, Feng J, et al. Effects of experimental heart failure on atrial cellular and ionic electrophysiology. Circulation. 2000;101:2631–2638. doi: 10.1161/01.cir.101.22.2631. [DOI] [PubMed] [Google Scholar]
- 19.Song Y, Shryock JC, Belardinelli L. An increase of late sodium current induces delayed afterdepolarizations and sustained triggered activity in atrial myocytes. Am J Physiol Heart Circ Physiol. 2008;294:H2031–H2039. doi: 10.1152/ajpheart.01357.2007. [DOI] [PubMed] [Google Scholar]
- 20.Wu L, Shryock JC, Song Y, et al. Antiarrhythmic effects of ranolazine in a guinea pig in vitro model of long-QT syndrome. J Pharmacol Exp Ther. 2004;310:599–605. doi: 10.1124/jpet.104.066100. [DOI] [PubMed] [Google Scholar]
- 21.Wu L, Shryock JC, Song Y, et al. An increase in late sodium current potentiates the proarrhythmic activities of low-risk QT-prolonging drugs in female rabbit hearts. J Pharmacol Exp Ther. 2006;316:718–726. doi: 10.1124/jpet.105.094862. [DOI] [PubMed] [Google Scholar]
- 22.Carlsson L, Almgren O, Duker GD. Qtu-prolongation and torsades-de-pointes induced by putative class-III antiarrhythmic agents in the rabbit—etiology and interventions. J Cardiovasc Pharmacol. 1990;16:276–285. doi: 10.1097/00005344-199008000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Wang WQ, Robertson C, Dhalla AK, et al. Antitorsadogenic effects of (±)-N-(2,6-dimethylphenyl)-(4[2-hydroxy-3-(2-methoxyphenoxy)propyl]-1-piperazine (ranolazine) in anesthetized rabbits. J Pharmacol Exp Ther. 2008;325:875–881. doi: 10.1124/jpet.108.137729. [DOI] [PubMed] [Google Scholar]
- 24.Antoons G, Oros A, Beekman JDM, et al. Late Na+ current inhibition by ranolazine reduces torsades de pointes in the chronic atrioventricular block dog model. J Am Coll Cardiol. 2010;55:801–809. doi: 10.1016/j.jacc.2009.10.033. [DOI] [PubMed] [Google Scholar]
- 25.Lindegger N, Hagen BM, Marks AR, et al. Diastolic transient inward current in long QT syndrome type 3 is caused by Ca2+ overload and inhibited by ranolazine. J Mol Cell Cardiol. 2009;47:326–334. doi: 10.1016/j.yjmcc.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Antzelevitch C, Belardinelli L, Zygmunt AC, et al. Electrophysiologic effects of ranolazine: a novel anti-anginal agent with antiarrhythmic properties. Circulation. 2004;110:904–910. doi: 10.1161/01.CIR.0000139333.83620.5D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belardinelli L, Antzelevitch C, Fraser H. Inhibition of late (sustained/persistent) sodium current: a potential drug target to reduce intracellular sodium-dependent calcium overload and its detrimental effects on cardiomyocyte function. Eur Heart J Suppl. 2004;6:i3–i7. [Google Scholar]
- 28.Wu L, Rajamani S, Li H, et al. Reduction of repolarization reserve unmasks the pro-arrhythmic role of endogenous late sodium current in the heart. Am J Physiol Heart Circ Physiol. 2009;297:H1048–H1057. doi: 10.1152/ajpheart.00467.2009. [DOI] [PubMed] [Google Scholar]
- 29.Yao L, Fan P, Jiang Z, et al. Nav1. 5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol. 2011;301:C577–C586. doi: 10.1152/ajpcell.00125.2011. [DOI] [PubMed] [Google Scholar]
- 30.Song Y, Shryock JC, Belardinelli L. A slowly inactivating sodium current contributes to spontaneous diastolic depolarization of atrial myocytes. Am J Physiol Heart Circ Physiol. 2009;297:H1254–H1262. doi: 10.1152/ajpheart.00444.2009. [DOI] [PubMed] [Google Scholar]
- 31.Baruscotti M, DiFrancesco D, Robinson RB. Na(+) current contribution to the diastolic depolarization in newborn rabbit SA node cells. Am J Physiol Heart Circ Physiol. 2000;279:H2303–H2309. doi: 10.1152/ajpheart.2000.279.5.H2303. [DOI] [PubMed] [Google Scholar]
- 32.Undrovinas AI, Belardinelli L, Undrovinas NA, et al. Ranolazine improves abnormal repolarization and contraction in left ventricular myocytes of dogs with heart failure by inhibiting late sodium current. J Cardiovasc Electrophysiol. 2006;17:S161–S177. doi: 10.1111/j.1540-8167.2006.00401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Undrovinas NA, Maltsev VA, Belardinelli L, et al. Late sodium current contributes to diastolic cell Ca2+ accumulation in chronic heart failure. J Physiol Sci. 2010;60:245–257. doi: 10.1007/s12576-010-0092-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sicouri S, Timothy KW, Zygmunt AC, et al. Cellular basis for the electrocardiographic and arrhythmic manifestations of Timothy syndrome: effects of ranolazine. Heart Rhythm. 2007;4:638–647. doi: 10.1016/j.hrthm.2006.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sicouri S, Glass A, Pedulla P, et al. Antiarrhythmic effects of ranolazine in canine pulmonary vein sleeves. Heart Rhythm. 2007;4:S166. doi: 10.1016/j.hrthm.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]