Abstract
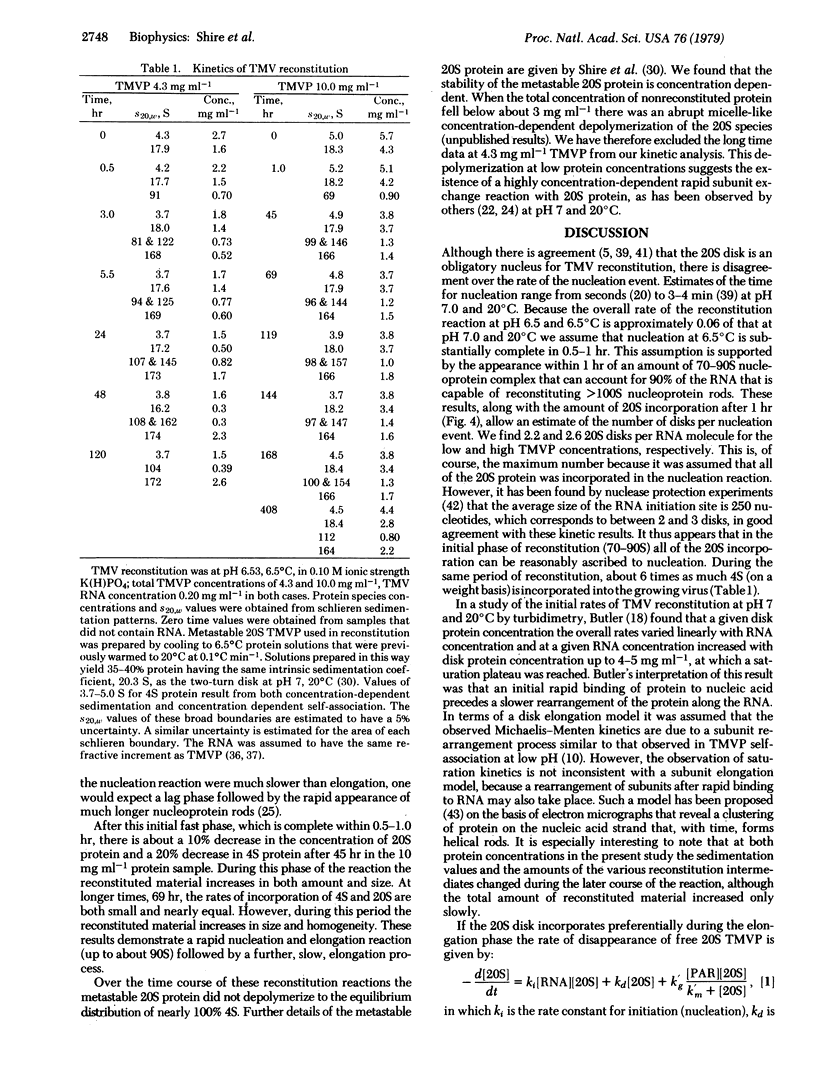
The mechanism of assembly of tobacco mosaic virus has been investigated under conditions in which the rates of incorporation of the 4S and 20S proteins can each be directly measured by analytical centfrifugation. Under these conditions, pH 6.5, 6.5 degrees C, 0.10 M ionic strength potassium orthophosphate, the protein can be made to exist as a metastable 20S aggregate that is necessary for efficient reconstitution. The overall assembly process consists of an initiation (nucleation) reaction that requires two to three 20S disk aggregates per RNA molecule and is followed by an elongation (growth) reaction. In the elongation phase of assembly the 4S protein is incorporated 50 to 70 times faster than the 20S disk, calculated on the basis of a steady-state kinetic analysis. Therefore, under these conditions, in which the rate of assembly is about 0.06 of that at pH 7, 20 degrees C, 0.10 M ionic strength orthophosphate, the 4S protein preferentially participates in the elongation phase. At this slow reconstitution rate intermediate assembly states (about 70-168 S) can be observed. The kinetics of both protein incorporation and nucleoprotein formation suggest that the elongation process is composed of at least two different, possibly sequential, rate-limiting reactions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOEDTKER H. Some physical properties of infective ribose nucleic acid isolated from tobacco mosaic virus. Biochim Biophys Acta. 1959 Apr;32:519–531. doi: 10.1016/0006-3002(59)90629-8. [DOI] [PubMed] [Google Scholar]
- Butler P. J. Assembly of tobacco mosaic virus. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):151–163. doi: 10.1098/rstb.1976.0106. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Finch J. T. Structures and roles of the polymorphic forms of tobacco mosaic virus protein. VII. Lengths of the growing rods during assembly into nucleoprotein with the viral RNA. J Mol Biol. 1973 Aug 25;78(4):637–649. doi: 10.1016/0022-2836(73)90285-4. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Finch J. T., Zimmern D. Configuration of tobacco mosaic virus, RNA during virus assembly. Nature. 1977 Jan 20;265(5591):217–219. doi: 10.1038/265217a0. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Klug A. Assembly of the particle of tobacco mosaic virus from RNA and disks of protein. Nat New Biol. 1971 Jan 13;229(2):47–50. doi: 10.1038/newbio229047a0. [DOI] [PubMed] [Google Scholar]
- Butler P. J., Klug A. Effect of state of polymerisation of the protein component on the assembly of tobacco mosaic virus. Mol Gen Genet. 1973 Jan 18;120(1):91–93. doi: 10.1007/BF00332986. [DOI] [PubMed] [Google Scholar]
- Butler P. J. Structures and roles of the polymorphic forms of tobacco mosaic virus protein. 8. Elongation of nucleoprotein rods of the virus RNA and protein. J Mol Biol. 1974 Jan 25;82(3):333–341. doi: 10.1016/0022-2836(74)90594-4. [DOI] [PubMed] [Google Scholar]
- Butler P. J. Structures and roles of the polymorphic forms of tobacco mosaic virus protein. 8. Initial stages of assembly of nucleoprotein rods from virus RNA and the protein disks. J Mol Biol. 1974 Jan 25;82(3):343–353. doi: 10.1016/0022-2836(74)90595-6. [DOI] [PubMed] [Google Scholar]
- Butler P. J. Structures and roles of the polymorphic forms of tobacco mosaic virus protein. VI. Assembly of the nucleoprotein rods of tobacco mosaic virus from the protein disks and RNA. J Mol Biol. 1972 Dec 14;72(1):25–35. doi: 10.1016/0022-2836(72)90065-4. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L. ASSEMBLY AND STABILITY OF THE TOBACCO MOSAIC VIRUS PARTICLE. Adv Protein Chem. 1963;18:37–121. doi: 10.1016/s0065-3233(08)60268-5. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Crowther R. A., Amos L. A. Harmonic analysis of electron microscope images with rotational symmetry. J Mol Biol. 1971 Aug 28;60(1):123–130. doi: 10.1016/0022-2836(71)90452-9. [DOI] [PubMed] [Google Scholar]
- Diener T. O., Schneider I. R. Virus degradation and nucleic acid release in single-phase phenol systems. Arch Biochem Biophys. 1968 Mar 20;124(1):401–412. doi: 10.1016/0003-9861(68)90344-5. [DOI] [PubMed] [Google Scholar]
- Durham A. C., Finch J. T., Klug A. States of aggregation of tobacco mosaic virus protein. Nat New Biol. 1971 Jan 13;229(2):37–42. doi: 10.1038/newbio229037a0. [DOI] [PubMed] [Google Scholar]
- Durham A. C., Klug A. Polymerization of tobacco mosaic virus protein and its control. Nat New Biol. 1971 Jan 13;229(2):42–46. doi: 10.1038/newbio229042a0. [DOI] [PubMed] [Google Scholar]
- Durham A. C., Klug A. Structures and roles of the polymorphic forms of tobacco mosaic virus protein. 3. A model for the association of A-protein into disks. J Mol Biol. 1972 Jun 20;67(2):315–332. doi: 10.1016/0022-2836(72)90244-6. [DOI] [PubMed] [Google Scholar]
- Durham A. C. Structures and roles of the polymorphic forms of tobacco mosaic virus protein. I. Sedimentation studies. J Mol Biol. 1972 Jun 20;67(2):289–305. doi: 10.1016/0022-2836(72)90242-2. [DOI] [PubMed] [Google Scholar]
- ENGLANDER S. W., EPSTEIN H. T. Optical methods for measuring nucleoprotein and nucleic acid concentrations. Arch Biochem Biophys. 1957 May;68(1):144–149. doi: 10.1016/0003-9861(57)90334-x. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B. Reconstitution of tobacco mosaic virus. III. Improved methods and the use of mixed nucleic acids. Biochim Biophys Acta. 1959 Jun;33(2):359–370. doi: 10.1016/0006-3002(59)90126-x. [DOI] [PubMed] [Google Scholar]
- FRAENKEL-CONRAT H., SINGER B., TSUGITA A. Purification of viral RNA by means of bentonite. Virology. 1961 May;14:54–58. doi: 10.1016/0042-6822(61)90131-3. [DOI] [PubMed] [Google Scholar]
- Fraenkel-Conrat H., Williams R. C. RECONSTITUTION OF ACTIVE TOBACCO MOSAIC VIRUS FROM ITS INACTIVE PROTEIN AND NUCLEIC ACID COMPONENTS. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):690–698. doi: 10.1073/pnas.41.10.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonathan P., Butler G., Durham A. C. Tobacco mosaic virus protein aggregation and the virus assembly. Adv Protein Chem. 1977;31:187–251. doi: 10.1016/s0065-3233(08)60219-3. [DOI] [PubMed] [Google Scholar]
- Lauffer M. A., Stevens C. L. Structure of the tobacco mosaic virus particle; polymerization of tobacco mosaic virus protein. Adv Virus Res. 1968;13:1–63. doi: 10.1016/s0065-3527(08)60250-x. [DOI] [PubMed] [Google Scholar]
- Lebeurier G., Morel M. C., Hirth L. Tobacco mosaic virus reconstitution in the presence of 8 S TMV-protein component. FEBS Lett. 1974 Apr 15;41(1):25–29. doi: 10.1016/0014-5793(74)80945-2. [DOI] [PubMed] [Google Scholar]
- Lebeurier G., Nicolaieff A., Richards K. E. Inside-out model for self-assembly of tobacco mosaic virus. Proc Natl Acad Sci U S A. 1977 Jan;74(1):149–153. doi: 10.1073/pnas.74.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcinka K. Application of permeation chromatography on controlled-pore glass in the purification of plant viruses. Acta Virol. 1972 Jun;16(1):53–62. [PubMed] [Google Scholar]
- Okada Y., Ohno T., Nonomura Y. Assembly of tobacco mosaic virus in vitro. Improved model for the elongation process by protein subunits. J Biochem. 1975 Jun;77(6):1157–1163. [PubMed] [Google Scholar]
- Ono T., Inoue H., Okada Y. Assembly of rod-shaped virus in vitro: reconstitution with cucumber green mottle mosaic virus protein and tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3680–3683. doi: 10.1073/pnas.69.12.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki Y., Takebe I., Ohno T., Fukuda M., Okada Y. Reconstitution of tobacco mosaic virus rods occurs bidirectionally from an internal initiation region: demonstration by electron microscopic serology. Proc Natl Acad Sci U S A. 1977 May;74(5):1913–1917. doi: 10.1073/pnas.74.5.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paglini S., Lauffer M. A. Polymerization-depolymerization of tobacco mosaic virus protein. XI. Osmotic pressure studies of solutions in water and in deuterium. Biochemistry. 1968 May;7(5):1827–1835. doi: 10.1021/bi00845a030. [DOI] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C. Assembly of tobacco mosaic virus in vitro: effect of state of polymerization of the protein component. Proc Natl Acad Sci U S A. 1972 May;69(5):1121–1124. doi: 10.1073/pnas.69.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards K. E., Williams R. C. Assembly of tobacco mosaic virus rods in vitro. Elongation of partially assembled rods. Biochemistry. 1973 Nov 6;12(23):4574–4581. doi: 10.1021/bi00747a005. [DOI] [PubMed] [Google Scholar]
- Scheele R. B., Lauffer M. A. Acid-base titrations of tobacco mosaic virus and tobacco mosaic virus protein. Biochemistry. 1967 Oct;6(10):3076–3081. doi: 10.1021/bi00862a014. [DOI] [PubMed] [Google Scholar]
- Scheele R. B., Schuster T. M. Kinetics of protein subunit interactions: simulation of a polymerization overshoot. Biopolymers. 1974;13(2):276–288. doi: 10.1002/bip.1974.360130204. [DOI] [PubMed] [Google Scholar]
- Scheele R. B., Schuster T. M. Letter: Hysteresis of proton binding to tobacco mosaic virus protein associated with metastable polymerization. J Mol Biol. 1975 May 25;94(3):519–525. doi: 10.1016/0022-2836(75)90218-1. [DOI] [PubMed] [Google Scholar]
- Schuster T. M., Scheele R. B., Khairallah L. H. Mechanism of self-assembly of tobacco mosaic virus protein. I. Nucleation-controlled kinetics of polymerization. J Mol Biol. 1979 Feb 5;127(4):461–485. doi: 10.1016/0022-2836(79)90232-8. [DOI] [PubMed] [Google Scholar]
- Shire S. J., Steckert J. J., Schuster T. M. Mechanism of self-assembly of tobacco mosaic virus protein. II. Characterization of the metastable polymerization nucleus and the initial stages of helix formation. J Mol Biol. 1979 Feb 5;127(4):487–506. doi: 10.1016/0022-2836(79)90233-x. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Klug A. Electron microscopy of the stacked disk aggregate of tobacco mosaic virus protein. I. Three-dimensional image reconstruction. J Mol Biol. 1974 Aug 25;87(4):641–656. doi: 10.1016/0022-2836(74)90075-8. [DOI] [PubMed] [Google Scholar]
- Vogel D., Jaenicke R. Conformational changes and proton uptake in the reversible aggregation of tobacco-mosaic-virus protein. Eur J Biochem. 1974 Feb 1;41(3):607–615. doi: 10.1111/j.1432-1033.1974.tb03303.x. [DOI] [PubMed] [Google Scholar]
- Zimmern D. The region of tobacco mosaic virus RNA involved in the nucleation of assembly. Philos Trans R Soc Lond B Biol Sci. 1976 Nov 30;276(943):189–204. doi: 10.1098/rstb.1976.0110. [DOI] [PubMed] [Google Scholar]