Abstract
Bisphenol A (BPA) is an endocrine disruptor that inhibits growth of mouse ovarian follicles and disrupts steroidogenesis at a dose of 438 μM. However, the effects of lower doses of BPA and its mechanism of action in ovarian follicles are unknown. We hypothesized that low doses of BPA inhibit follicular growth and decrease estradiol levels through the aryl hydrocarbon receptor (AHR) pathway. Antral follicles from wild-type and Ahr knock-out (AhrKO) mice were cultured for 96 hours. Follicle diameters and estradiol levels then were compared in wild-type and AhrKO follicles ± BPA (0.004 - 438 μM). BPA inhibited follicle growth (110 - 438 μM) and decreased estradiol levels (43.8 - 438 μM) in wild-type and AhrKO follicles. However, at BPA 110 μM, inhibition of growth in AhrKO follicles was attenuated compared to wild-type follicles. These data suggest that BPA may inhibit follicle growth partially via the AHR pathway, whereas its effects on estradiol synthesis likely involve other mechanisms.
Keywords: BPA, AHR, antral follicle, ovary, low dose, estradiol
1. Introduction
Antral follicles are the most mature types of mammalian ovarian follicles. They are essential for reproduction and endocrine homeostasis because they are the only follicle type capable of ovulation and they are the main source of estradiol production in the female body. Alterations in proper growth or function of the antral follicles may result in infertility. While there are many causes of infertility, environmental chemical exposures may lead to impaired follicular growth and reduced estradiol levels, and in turn, lead to infertility.
Bisphenol A (BPA) is a chemical widely used by plastic and epoxy resin manufacturers. It is incorporated in numerous products such as plastic containers, toys, building materials, and the lining of metal cans such as those used for food and baby formulas [1]. Unfortunately, BPA can leach out of the products it is incorporated in and end up in food and beverages [1]. Several studies indicate that BPA is detectable in different human tissues and fluids, including ovarian follicular fluid [2]. These findings are of concern because BPA is known to have endocrine disrupting properties and to be a reproductive toxicant [3]. For example, BPA exposure has been associated with adverse reproductive outcomes such as infertility [4, 5] and polycystic ovarian syndrome in women [6]. Further, postnatal exposure to BPA (150 μg/pup of 1.5 gram body weight) causes polyovular follicles in mice [7].
The calculated lowest observable adverse effect level (LOAEL) dose according to the United States Environmental Protection Agency (EPA) was determined to be 50 mg/kg/day. Though there is not much known yet on the pharmacokinetics of BPA in rodents or humans and it is difficult to extrapolate LOAEL doses obtained by in vivo studies to in vitro studies, it is still important to study the effects of a wide range of BPA doses in vitro to get a better understanding of the potential mechanism of action of BPA.
To date, studies have shown that in vitro treatment with BPA (100 pM-100 μM) decreases viability of mouse granulosa cells [8]. Recently, BPA also has been shown to have toxic effects on cultured mouse ovarian follicles [9-11]. Specifically, BPA at 438 μM inhibits growth of mouse antral follicles and BPA at 43.8 or 438 μM decreases estradiol synthesis compared to vehicle control treated follicles [9]. However, not many studies have examined the effects of BPA on ovarian follicles using a wide dose range. Thus, one goal of the current study was to examine the effects of a wide range of doses of BPA (0.004 - 438 μM) on ovarian follicle function by focusing on follicle growth and estradiol levels. Since it has been suggested that BPA may act in a non-monotonic manner [12], we specifically tested the hypothesis that a wide range of BPA doses causes U-shaped dose-response effects on follicle growth and estradiol levels.
Though BPA has been shown to act on various tissues and cell types such as MCF-7 cells [13], adipose tissue [14], and pituitary cell lines [15], the receptor types that mediate the effects of BPA on ovarian follicles have not been determined yet. A few studies have focused on whether BPA exerts its action through nuclear receptors such as estrogen [13], androgen [16], and thyroid [17, 18] receptors. Further, Peretz et al. [10] have shown that BPA does not exert its toxic effects via the genomic estrogenic pathway in mouse ovarian follicles. Thus, another goal of this study was to determine if BPA exerts its toxicity in mouse antral follicles via the aryl hydrocarbon receptor (AHR) pathway.
We focused on the AHR pathway because it mediates the effects of various environmental chemicals, including its most potent ligand 2,3,7,8,- tetrachlorodibenzo-p-dioxin (TCDD) [19]. Upon ligand binding, cytoplasmic AHR enters the nucleus, partners with its translocator (AHR nuclear translocator; ARNT), and binds to specific DNA response elements, leading to transcription of several genes including cytochrome P450, family 1, subfamily b, polypeptide 1(Cyp1b1). Further, the AHR repressor (AHRR) can heterodimerize with ARNT to terminate the activation of the AHR signaling pathway [20-22].
We also focused on the AHR because it plays a central role in the ovary [23]. For example, the AHR is involved in regulating the growth of pre-antral and antral follicles by increasing granulosa cell proliferation [24]. It is also involved in regulation of estradiol production by the ovary [25]. However, previous studies that have examined the potential role of the AHR in mediating the toxic effects of BPA have been equivocal. For example, Nishizawa et al. [26] reported that following in utero exposure to BPA, Ahr expression was elevated in the gonads harvested from embryos, whereas Bonefeld-Jorgensen et al. [27] reported weak antagonistic effects of BPA with the AHR (AHR-CALUX system; mouse hepatoma cell line).
Nevertheless, no study has examined the direct role of the AHR in mediating the toxic effects of BPA on ovarian follicles. Thus, another goal of the current study was to test the hypothesis that the AHR mediates the toxic effects of BPA on the growth of cultured mouse antral follicles. Specifically, we hypothesized that BPA exposure leads to changes in the expression of main factors in the AHR signaling pathway in antral follicles isolated from wild-type (WT) mice, and that the effects of BPA exposure on the growth of antral follicles are attenuated in follicles lacking the AHR (i.e., follicles isolated from AHR global knock-out mice; AhrKO) compared to WT follicles.
Lastly, activation of the AHR signaling pathway can decrease the levels of estradiol synthesizing enzymes and increase the levels of estradiol metabolizing enzymes (e.g., CYP1B1) [28]. For example, Karman et al. [29] found that exposure of cultured mouse antral follicles to various doses of the potent AHR ligand TCDD decreased estradiol levels compared to control follicles. Interestingly, Peretz et al. [9] reported that antral follicles cultured with BPA (43.8, 438 μM) also synthesized lower estradiol levels compared to control follicles. Thus, it is possible that the AHR mediates the toxic effects of BPA on mouse antral follicles by decreasing estradiol levels. Therefore, a final goal of this study was to test the hypothesis that treatment of cultured mouse antral follicles with BPA leads to lower estradiol levels via mechanisms involving the AHR signaling pathway.
2. Materials and methods
2.1. Chemicals
BPA powder (99%) was purchased from Sigma-Aldrich (St. Louis, MO). Stock solution of BPA was prepared using dimethyl sulfoxide (DMSO) (Sigma-Aldrich) as a solvent to achieve a BPA dose of 438 μM (133 mg/ml). Further dilutions of the stock solution with DMSO generated working dilutions of 0.004, 0.04, 0.44, 4.38, 43.8, 110, and 219 μM respectively. These serial dilutions allowed us to add an equal volume of BPA to each individual well in which a follicle was placed, and to control for solvent concentration.
The selected dose range is based on previous findings and environmentally relevant levels [30, 31]. Previous studies indicate that BPA at 4.38, 43.8, and 438 μM doses exhibited a selective inhibition of growth of follicles isolated from Friend leukemia virus B (FVB) mice (30-32 days old) [9]. Specifically, BPA at 438 μM, but not at the 4.38 and 43.8 μM doses inhibited follicle growth starting at 72 hours and continuing for 96 hours of culture. In our current study, however, we used a different strain of mice and age of mice than the ones that were used in the study by Peretz et al. [9, 10]. Therefore, BPA at 4.38, 43.8, and 438 μM were included in the current study as well. Additionally, a wide range of other doses (0.004 - 219 μM) were included to fully understand the dose response effects of BPA on ovarian follicles. BPA at 0.004 μM represents BPA levels that have been measured in women’s follicular fluid (i.e., 0.004- 0.008 μM) [2]. BPA at 219 μM was used as a transition dose between the relatively low doses of BPA (up to 43.8 μM) to the higher doses of BPA (greater than 43.8 μM). Moreover, according to the U.S. Environmental Protection Agency the LOAEL of BPA is 50 mg/kg/day. Therefore, treatment with BPA at 219 μM and lower doses is important to get a better understanding of the in vitro effects of BPA at doses that may simulate the LOAEL doses and lower doses.
2.2. Animals
Cycling wild-type (WT) female C57BL/6 mice were obtained from Charles River Laboratories (Charles River, CA). AhrKO mice (official symbol Ahrtm1Bra) were in a C57BL/6 background and were originally generated by Schmidt et al. [32]. The AhrKO (Ahr−/−) mice used in each experiment were generated by intercrossing either heterozygous (Ahr+/−) female and male mice or heterozygous Ahr+/− female mice with AhrKO (Ahr−/−) male mice. Mouse genetic screening was performed using ear tissue punches as previously described [33]. All mice were housed and bred in the core animal facility located at College of Veterinary Medicine, University of Illinois. Mice were maintained in polystyrene cages. Additionally, mice were kept under a 12L:12D photoperiod, at temperature of 22±1°C, with 35% ± 4% relative humidity. Food (Harlan Teklad 8626) and reverse-osmosis purified water were provided ad libitum. Mice were euthanized at 50-54 days of age by carbon dioxide inhalation followed by cervical dislocation. This age range was selected because previous studies indicate that at this age, WT antral follicles and AhrKO antral follicles have similar growth patterns, estradiol levels, and expression levels of a major cell cycle regulator Ccnd2 [34]. All procedures and experimental methods involving animals were approved by the University of Illinois Institutional Animal Care and Use Committee.
2.3. Follicle Culture
Ovaries were removed and antral follicles were mechanically isolated using fine watchmaker forceps and a dissecting microscope. We used 2-3 mice per replicate, and repeated the cultures at least three separate times. We obtained 15-40 antral follicles with relative sizes of 200-400 μm from each mouse. Following isolation, follicles were placed in individual wells of 96-well culture plates. Specifically, follicles from each mouse were equally distributed in all treatment groups and each treatment group included overall a minimum of 8 follicles. Next, follicles were covered with 75 μl of unsupplemented alpha minimal essential media (α-MEM; Invitrogen, Carlsbad, CA) prior to treatment as previously described [35]. After plating, follicles were treated with 150 μl of supplemented media with vehicle control (DMSO) or BPA (0.004, 0.04, 0.4, 4.38, 43.8, 110, 219, and 438 μM). Supplemented media contained unsupplemented α-MEM, 1% ITS (10 ng/ml insulin, 5.5 ng/ml transferrin, and 5.5 ng/ml selenium; Sigma-Aldrich, St. Louis, MO), 5% fetal bovine serum (Atlanta biological, Lawrenceville, GA), 100 U/ml penicillin (Sigma-Aldrich), 100 mg/ml streptomycin (Sigma-Aldrich), and 5 IU/ml human recombinant follicle stimulating hormone (Dr A. F. Parlow, National Hormone and Peptide Program, Harbor- UCLA Medical Center, Torrance, CA). An equal volume of BPA or DMSO (0.75 μl of treatment per 1 ml of media) was added to supplemented α-MEM to keep vehicle concentration at a constant of 0.075% for each treatment. Follicles then were incubated for up to 96 hours, while supplying 5% CO2 at 37°C.
2.4. Analysis of follicular growth
As a measurement of growth, follicle diameter was measured on perpendicular axes every 24 hours. Measurements were done with an inverted microscope equipped with a calibrated ocular micrometer. Follicle diameters were averaged and converted to percent change relative to baseline (0 hour, set as 100%). Data were plotted and statistically analyzed to compare the effects of BPA treatments on growth compared to DMSO per time point. At least three separate cultures were conducted per treatment group.
2.5. Gene expression analyses
At the end of each culture (either 24 hours or 96 hours), follicles were immediately snap frozen and stored at −80°C until further processed for quantitative real-time polymerase chain reactions (qPCR). Total RNA was extracted from at least 8 pooled follicles per treatment group per replicate (n≥3) using RNeasy Micro Kit (Qiagen Inc., CA) following the manufacturer’s protocol. The RNA concentration of each sample was determined at 260 nm using a Nanodrop ND1000 UV-Vis spectrophotometer (Nanodrop Technologies, Wilmington, DE). Unfortunately, we could not isolate enough RNA from follicles treated with 219 and 438 μM BPA following 96 hours of culture or from follicles treated with 438 μM BPA following 24 hours of cultures. RNA Total RNA was reversed transcribed using an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA) according to the manufacturer’s instructions. Each cDNA sample was diluted 1:4 with nuclease-free water prior to further analyses.
Primer sets were either based on previous publications: Ahr [29], beta actin (Actb) [29], Cyp1b1 [29], Arnt [36], B cell leukemia/lymphoma 2 (Bcl2) [37], and BCL2-associated X protein (Bax) [37] or originally designed (Ahrr, NM_009644.2) using a BLASTN search that was performed in GenBank to ensure that primers were unique to the gene of interest and that they spanned a large intron-exon junction. The sequences for the originally designed Ahrr primers were: forward 5′-AGA GCT GTG TCC CCA GGG AAG T - 3′ and reverse 5′- CAG TGA GGA AAG ATG GCT TGT AGG - 3′.
All gene expression analyses were performed using the CFX96 Real-time PCR Detection System C1000 Thermal Cycler (Bio-Rad). All qPCR reactions were done in triplicate in a total volume of 10 μl per sample accordingly: 1μl of diluted cDNA, 1μl of gene specific primer mix (500 nM; Integrated DNA Technologies, INC., Coralville, IA), 3 μl of nuclease free water, and 5 μl of SsoFast EvaGreen Supermix (Bio-Rad). The protocol for the qPCR runs included the following steps: initial denaturation of the cDNA and enzyme activation at 95°C for 1 min, followed by 40 cycles of 10 sec at 95°C, 10 sec at 60°C, and a fluorescent absorbance reading and one final annealing/elongation step for 5 min at 72°C. A heat dissociation curve (from 65°C to 95°C with a fluorescent absorbance reading after each 0.5°C increment) was performed at the end of every run to confirm specificity of each primer pair for the chosen transcript of interest. A standard curve was generated from six serial dilutions of a sample representing the treatment groups to calculate the amplification efficiencies of each primer set. Expression data were generated using the mathematical model developed by Pfaffl [38]. The housekeeping gene Actb was used as a reference gene for each sample because there were no statistical differences in the expression of this gene between any of the treatment groups, including DMSO (data not shown). Mean relative mRNA expression ratios from 3-4 separate follicle culture experiments were reported.
2.6. Measurement of estradiol levels
The media from follicles in each treatment group were collected at the end of the cultures, and stored at −80°C until performing measurements of estradiol levels. Randomly selected media samples per treatment group were subjected to enzyme linked immunosorbent assays (ELISA) for estradiol levels according to the manufacturer’s guidelines (DRG International, Mountainside, NJ). The minimum detection limits were 9.71 pg/ml, and intra-assay and inter-assay coefficients of variation were 4.7%, and 7.8%, respectively. Individual follicle estradiol level was normalized to follicle diameter. Averaged estradiol levels represent normalized values from 12 individual follicles per treatment group obtained from three separate culture experiments.
2.7. Statistical analyses
Data were expressed as the mean ± SEM from at least three separate experiments. Differences between DMSO and treatment groups were statistically analyzed using SPSS software (SPSS Inc., Chicago, IL). For all comparisons, statistical significance was assigned at p≤0.05. Treatment group data were statistically compared to DMSO control data. When data were normally distributed and the homogeneity of variance assumption was met, we used the one-way analysis of variance (ANOVA) test followed by a Dunnett’s post hoc test. When data were not normally distributed and/or when the homogeneity of variance assumption was not met, we used Kruskal-Wallis non-parametric test, followed by a Mann-Whitney test.
3. Results
3.1. Effect of BPA exposure on antral follicle growth in WT follicles (C57BL/6)
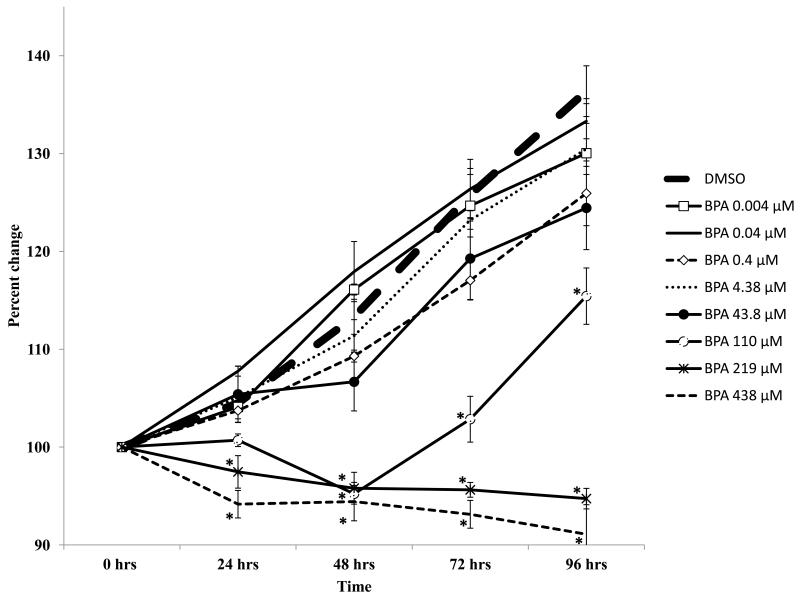
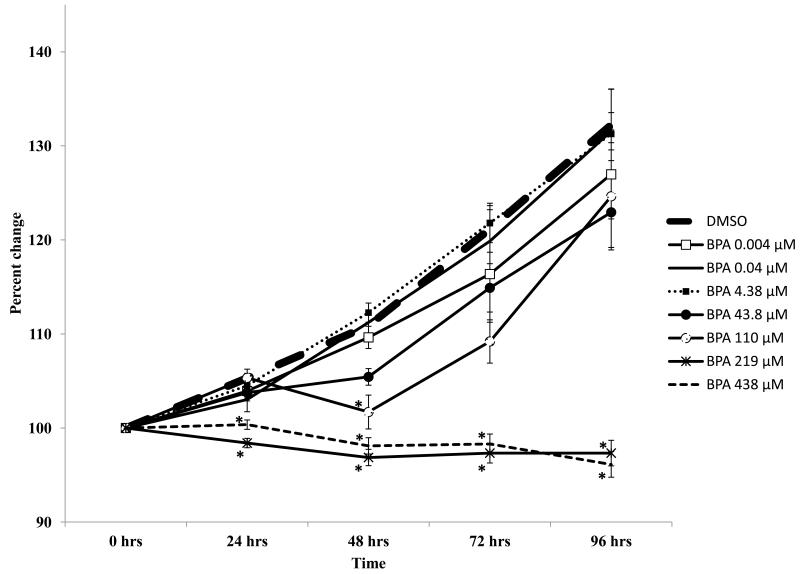
The results indicate that BPA selectively inhibited the growth of cultured WT antral follicles (Figure 1). Specifically, doses of BPA up to 43.8 μM did not alter follicle growth compared to DMSO, whereas doses of BPA from 110 - 438 μM significantly inhibited follicle growth, starting at 24 hours (219, 438 μM) or 48 hours (110 μM) and continuing throughout the culture (Figure 1).
Figure 1.
Effect of in vitro BPA exposure on wild-type mouse antral follicle growth. Antral follicles mechanically isolated from wild-type mice were individually cultured with vehicle control (DMSO) or BPA (0.004 - 438 μM) for 96 hours. During the culture, follicle diameters were measured and then were converted to percent change from baseline (time 0). Graph represents means ± SEM from 3-4 separate experiments (at least five follicles per treatment per replicate). Asterisks represent significant differences from DMSO per time point; p≤0.05.
3.2. Effect of BPA exposure on estradiol levels in WT follicles
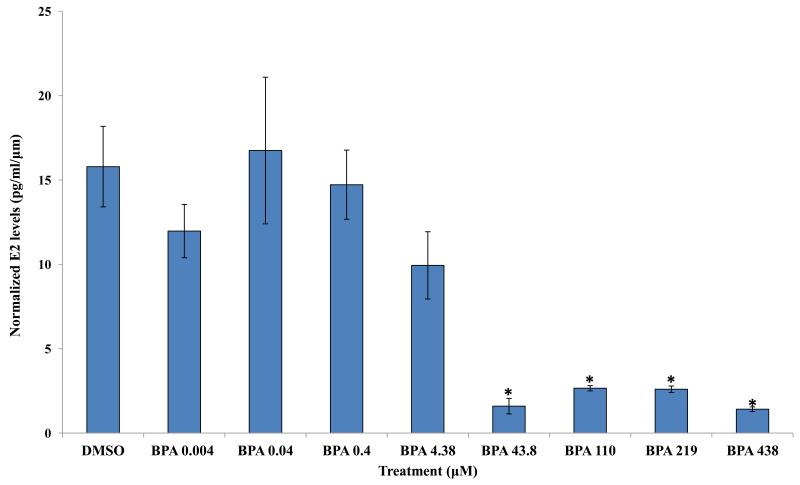
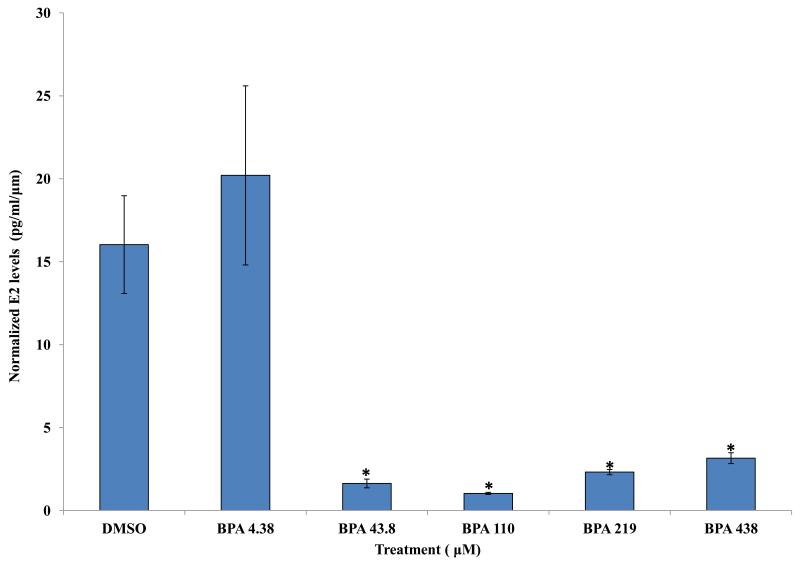
Previous studies indicate that BPA exposure at 43.8 and 438 μM decreases estradiol levels in mouse antral follicle cultures [9]. Therefore, we compared estradiol levels in the media from vehicle and BPA-treated follicles to expand our knowledge about potential effects of a wide range of BPA treatments on estradiol levels. The results indicate that exposure to 43.8, 110, 219, and 438 μM BPA for 96 hours significantly decreased estradiol levels synthesized by WT antral follicles compared to WT follicles treated with DMSO (Figure 2). In contrast, exposure to 0.004, 0.04, 0.4, and 4.38 μM BPA did not alter estradiol levels synthesized by WT antral follicles compared to WT follicles treated with DMSO (Figure 2).
Figure 2.
Effect of in vitro BPA exposure on estradiol synthesis by wild-type mouse antral follicles. Antral follicles mechanically isolated from wild-type mice were individually cultured with vehicle control (DMSO) or BPA (0.004 - 438 μM). After 96 hours, the media were collected and analyzed for estradiol levels (at least three follicles per treatment, per experiment). Graph represents means ± SEM from three separate experiments. Asterisks represent significant differences from DMSO; p≤0.05.
3.3. Effect of BPA exposure on expression levels of Bcl2 and Bax in WT follicles
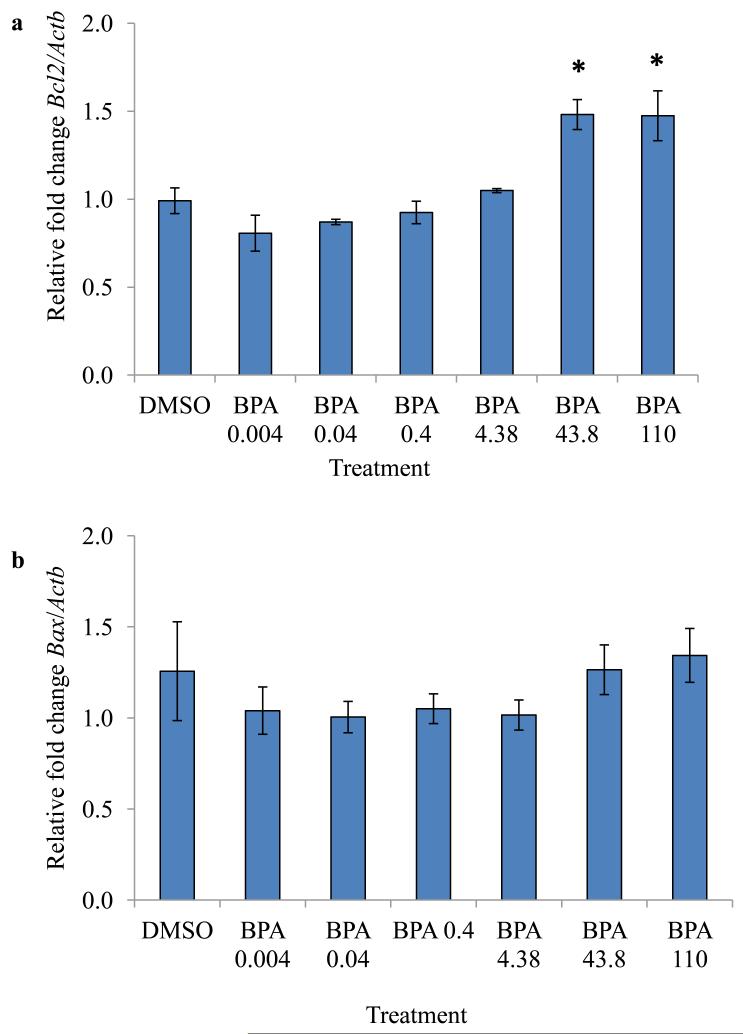
Inhibition of follicle growth and low estradiol levels can be a result of underlying atresia (programmed cell death of the ovarian cells). Further, previous studies indicate that BPA at 438 μM causes atresia and increases expression of apoptotic factors [10]. Thus, we measured selected apoptotic factors levels at 96 hours in the current study. The results indicate that at 96 hours, 43.8 and 110 μM BPA significantly up-regulated expression levels of the anti-apoptotic factor Bcl2 in WT follicles compared to DMSO treated WT follicles (Figure 3). In contrast, BPA did not alter the expression of the pro-apoptotic factor Bax at any of the examined doses compared to DMSO (Figure 3).
Figure 3.
Effect of 96 hours in vitro BPA exposure on expression of Bcl2 and Bax. Antral follicles isolated from wild-type mice were treated with vehicle control (DMSO) or BPA (0.004 - 438 μM) and cultured for 96 hours. Expression levels of Bcl2 and Bax were measured using qPCR. Data represent means ± SEM from at least three separate experiments. Asterisks represent significant differences from DMSO; p≤0.05.
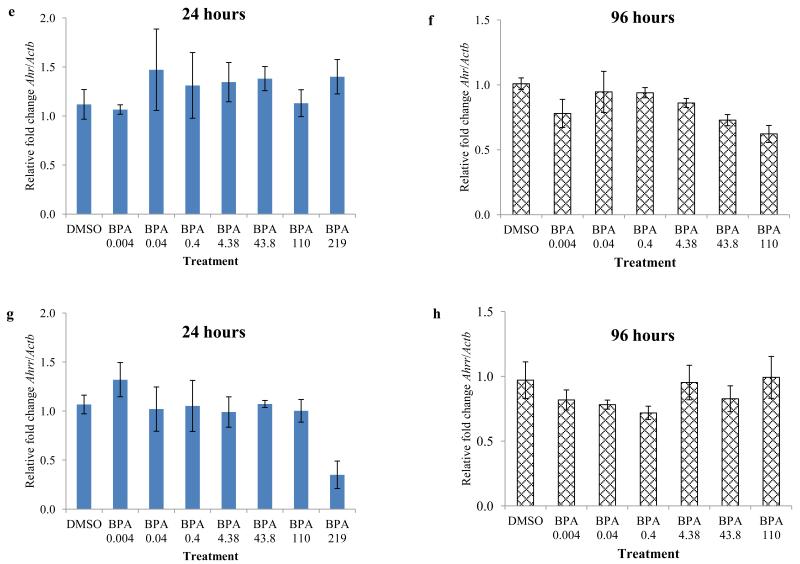
3.4. Effect of BPA exposure on expression of factors in the AHR signaling pathway in WT follicles
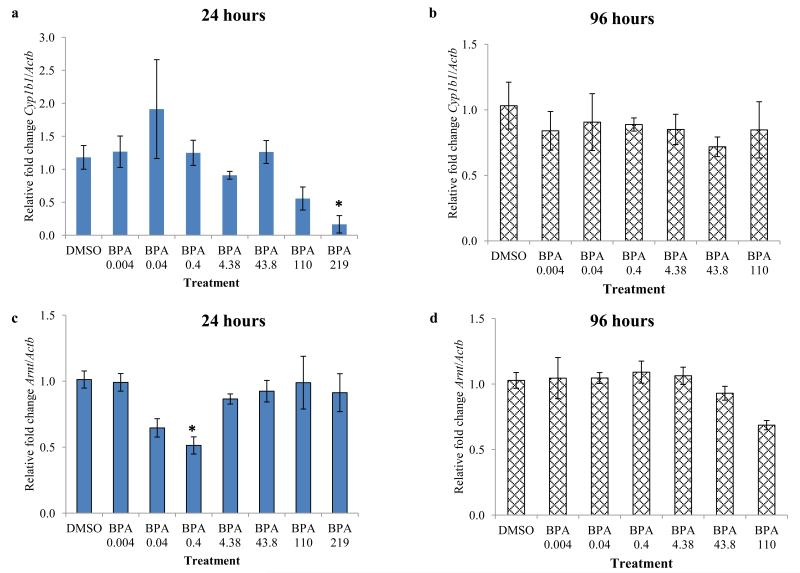
To determine the potential role of the AHR in mediating the toxic effects of BPA, we compared relative mRNA levels of selected genes in the AHR signaling pathway (Ahr, Ahrr, Arnt, and Cyp1b1) in DMSO and BPA treated WT follicles. Because we observed inhibition of follicle growth beginning at 24 hours with BPA 219 and 438 μM compared to control and because this inhibition continued for 96 hours, we compared expression levels at 24 and 96 hours per treatment group. After 24 hours of culture, BPA at 219 μM significantly down-regulated Cyp1b1 levels in WT follicles and BPA 110 μM exposure resulted in a trend toward reducing Cyp1b1 levels in WT follicles compared to DMSO treated WT follicles (Figure 4a). BPA at 0.4 μM significantly down-regulated Arnt levels in WT follicles compared to DMSO treated WT follicles (Figure 4c). However, none of the selected doses of BPA altered the expression of Ahr or Ahrr in WT follicles compared to DMSO treated WT follicles (Figure 4 e and g). Similarly, at 96 hours, none of the selected doses of BPA altered the expression of Cyp1b1, Ahr, Ahrr, and Arnt in WT follicles compared to DMSO treated WT follicles (Figure 4 b, d, f, and h).
Figure 4.
Effect of in vitro BPA exposure on expression of major factors in the AHR signaling pathway, following 24 and 96 hours of culture. Antral follicles isolated from wild-type mice were treated with vehicle control (DMSO) or BPA (0.004 - 438 μM) and cultured for 24 or 96 hours. Expression levels of Cyp1b1 (a ,b), Arnt (c, d), Ahr (e, f), and Ahrr (g, h) were measured using qPCR. Data represent means ± SEM from at least three separate experiments. Asterisks represent significant differences from DMSO; p≤0.05.
3.5. Effect of BPA exposure on antral follicle growth in AhrKO follicles
As a direct way to investigate the role of the AHR as a potential mediator of the toxic effects of BPA, we cultured follicles from AhrKO mice with a wide range of doses of BPA and measured their growth over time. The data indicate that BPA at doses between 0.04 - 43.8 μM did not alter the growth of AhrKO follicles compared to DMSO treated AhrKO follicles (Figure 5). This growth pattern was similar to the growth of WT follicles following treatment with BPA at doses between 0.04 - 43.8 μM (Figure 1). BPA at doses of 219 and 438 μM significantly inhibited the growth of AhrKO follicles compared to AhrKO follicles treated with DMSO starting at 24 hours, and continuing throughout the culture time (Figure 5). Again, these results are similar to what was observed with the WT follicles at the same doses (Figure 1). Lastly, BPA at 110 μM inhibited the growth of AhrKO follicles compared to AhrKO follicles treated with DMSO at 48 hours only (Figure 5). Interestingly, BPA at 110 μM did not significantly alter the growth of AhrKO follicles compared to AhrKO follicles treated with DMSO at any of the other time points (Figure 5). These results are different than what we observed with WT follicles treated with BPA 110 μM, in which inhibition of growth started at 48 hours, but lasted throughout the culture time (Figure 1).
Figure 5.
Effect of in vitro BPA exposure on AhrKO mouse antral follicle growth. Antral follicles mechanically isolated from AhrKO mice were individually cultured with vehicle control (DMSO) or BPA (0.004 - 438 μM) for 96 hours. During the culture, follicle diameters were measured and then were converted to percent change from baseline (time 0). Graph represents means ± SEM from 3-4 separate experiments (at least five follicles per treatment per experiment). Asterisks represent significant differences from DMSO per time point; p≤0.05.
3.6. Effect of BPA exposure on estradiol levels in AhrKO follicles
Previous studies indicate that BPA exposure at 43.8 and 438 μM decreases estradiol levels in mouse antral follicle cultures [9], but the mechanism by which BPA leads to decreased estradiol synthesis is unknown. Therefore, we measured estradiol levels in the media from vehicle and BPA-treated AhrKO follicles to expand our knowledge about the potential role of the AHR signaling pathway in mediating BPA toxic effects on estradiol synthesis. The results indicate that 96 hours exposure to BPA at 43.8, 110, 219, and 438 μM significantly decreased estradiol levels synthesized by AhrKO antral follicles compared to AhrKO follicles treated with DMSO (Figure 6). In contrast, exposure to BPA at 4.38 μM did not alter estradiol levels synthesized by AhrKO antral follicles compared to AhrKO follicles treated with DMSO (Figure 6). These results are similar to the results of WT follicles treated with these doses of BPA (Figure 2).
Figure 6.
Effect of in vitro BPA exposure on estradiol synthesis by AhrKO mouse antral follicles. Antral follicles mechanically isolated from AhrKO mice were individually cultured with vehicle control (DMSO) or BPA (4.38 - 438 μM). After 96 hours, the media were collected and analyzed for estradiol levels. Graph represents means ± SEM from three separate experiments (four follicles per treatment, per experiment). Asterisks represent significant differences from DMSO; p≤0.05.
4. Discussion
The current study expands on the limited published data regarding the direct toxic effects of a wide dose range of BPA on cultured mouse antral follicles. It also provides information on the potential mechanism by which BPA may exert its toxic effects in antral follicles. In this study, we utilized a follicle culture system to closely examine the direct effects of exposure to a wide range of doses of BPA, including environmentally relevant doses, on antral follicles [2, 10, 11, 34]. We also compared the growth of antral follicles isolated from WT and AhrKO mice to examine the role of the AHR in mediating the toxic effects of BPA. The results suggest that BPA inhibits the growth and decreases estradiol levels of WT follicles in a dose dependent manner. Furthermore, the data suggest that BPA at 110 μM may selectively act through the AHR signaling pathway to exert its toxic effects. The maximum tolerated dose for BPA is considered to be 1,000 mg/kg/body weight/day and the calculated reference dose according to the U.S. EPA was determined to be 50 μg/kg/day based on the LOAEL. Though there is not much known yet on the pharmacokinetics of BPA in mice or humans and it is difficult to extrapolate from LOAELs determined in vivo to in vitro studies, our study adds valuable data on the effects of BPA exposure at levels lower than the LOAEL and the reference dose.
In our study, BPA inhibited the growth of WT follicles at doses of 110, 219, and 438 μM, but not at doses of 43.8 μM or less. These findings indicate that in our antral follicle bioassay, BPA has a linear dose-response effect on follicular growth. This finding is in contrast to our original hypothesis that BPA exhibits non-monotonic effects on follicles as it does in other cell lines/tissues such as pituitary cell lines [39] and adipose explants [14]. The reasons for different dose-response effects with BPA are unknown, but studies have shown that BPA can act differently in different tissues and this appears to be the case with ovarian follicle cultures versus pituitary cell lines and adipose explants [14, 39]. Yet, our findings are in agreement with other studies that examined the effects of BPA on mouse ovarian follicles. Xu et al. [8] observed that BPA in a dose dependent manner decreases viability of mouse cultured granulosa cells. Specifically, treatment with doses of 0.1 nM, 0.1 μM, and 100 μM of BPA resulted in cytotoxic effects [8]. Moreover, the toxic effects of BPA at 100 μM (which is close to our BPA 110 μM dose) were observed as early as 24 hours [8]. Further, according to Lenie et al. [11], BPA exposure at 3 nM - 30 μM (doses similar to our doses of BPA 0.004 - 43.8 μM) did not affect early preantral follicle growth compared to control after 4 days of culture. Peretz et al. [9] reported that BPA at 438 μM inhibited follicle growth as it did in our current study. Interestingly, BPA at 438 μM inhibited follicle growth starting at 72 hours of culture in the Peretz et al. study [9], while it inhibited follicle growth beginning at 24 hours in the current study. This difference in the timing of BPA effects could be due to differences in strains of mice and animal ages in Peretz et al. [9] compared to our study. Peretz et al. [9] used FVB mice, whereas we used C57BL/6 mice. Additionally, Peretz et al. [9] used 32 day old mice, whereas we used 50-54 day old mice.
Despite differences in the timing of inhibition of growth between our current study and the study by Peretz et al. [9], both studies showed that BPA treatment decreases estradiol levels. Estradiol is one of the main factors needed to stimulate follicle growth and to protect follicles from atresia [40]. Thus, decreased estradiol levels are another indication of impaired function of the follicles that can be observed even when follicle growth is not inhibited. In the current study, after 96 hours of culture, BPA at 43.8 - 438 μM significantly decreased estradiol levels in a dose dependent manner (Figure 2). Specifically, BPA at 43.8 μM significantly decreased estradiol levels, even though it did not affect follicle growth compared to controls, whereas BPA at 110, 219, and 438 μM decreased both follicle growth and estradiol levels (Figures 1,2). These findings are in agreement with the results by Peretz et al. [9], who reported that BPA at 43.8 and 438 μM significantly decreased estradiol levels compared to controls after 120 hours of culture. These data may suggest that exposure to BPA at 43.8, 110, 219, and 438 μM pushes the follicles into cellular stress that results with impaired steroidogenesis followed by inhibition of growth with higher doses of BPA (110, 219, and 438 μM). BPA at 43.8 μM still allows the follicles to recover their growth, but not estradiol synthesis.
Estradiol can protect follicles from atresia [40] by suppressing the transcription of pro-apoptotic factors such as Bax [41]. Previous studies also indicate that overexpression of the anti-apoptotic factor Bcl2 enhances follicular growth and decreases apoptosis [42]. Studies also indicate that the balance between pro- and anti-apoptotic factors is a main determinant in whether the process of atresia will be initiated in follicles [43]. In our current study, Bcl2 levels (Figure 3) were significantly increased at BPA 43.8 and 110 μM, whereas the pro-apoptotic factor Bax levels were not altered (Figure 3) with any of the examined BPA treatments compared to controls. Therefore, it is likely that BPA-induced changes in the ratio of pro- and anti-apoptotic factors to favor an anti-apoptotic state may be an attempt of the follicles to restore normal growth following treatment with BPA 43.8 and 110 μM. This attempt is not completely successful because follicles were only able to retain normal growth at BPA 43.8 μM, but not at the higher tested doses. At higher doses, it may be that the follicular damage caused by BPA (110 - 438 μM) was too profound to allow the follicles to be rescued or even partially protected by increasing expression of anti-apoptotic factors such as Bcl2. Xu et al. [8] have shown that BPA can induce apoptosis in isolated granulosa cells at concentration as low as 100 pM [8]. Therefore the difference in doses required to cause apoptosis in our study versus that by Xu et al. [8] can be due to the fact that we cultured whole follicles, whereas Xu et al. [8] cultured isolated granulosa cells. Interestingly, Peretz et al. [10] measured expression levels of Bax and Bcl2 in isolated antral follicles cultured with doses of BPA at 4.38, 43.8, and 438 μM from 32-35 day old FVB mice. Their results indicate that at 96 hours, BPA at 438 μM treatment significantly increased expression of both Bax and Bcl2. Again, this difference between the study by Peretz et al. [10] and the current study could be due to difference in the age and strain of mice that were used in both studies.
The AHR signaling pathway plays a major role in regulating follicle growth and estradiol levels in the ovary and in mediating the toxic effects of other environmental chemicals (e.g., dioxins, polycyclic aromatic hydrocarbons) [20-22]. A few studies have examined the AHR as a potential mediator of the toxic effects of BPA [26, 27, 44-46]. However, only Nishizawa et al. [26] have examined the AHR-BPA relationship in the gonads. The researchers observed elevated gonadal Ahr expression following in utero exposure to BPA. Therefore, we investigated expression levels of major factors in the AHR signaling pathway following BPA exposure in our isolated follicle culture system. Interestingly, there were no striking differences in the expression of major factors in the AHR signaling pathway in WT follicles treated with BPA versus WT follicles treated with DMSO. These results are similar to what has been observed following treatment of cultured mouse antral follicles even with the potent ligand of the AHR TCDD, in which the Ahr expression in TCDD treated follicles was not different than controls [47].
In the absence of the Ahr, however, antral follicles may have been rescued from BPA (110 μM) induced inhibition of growth. This recovery occurs only at BPA 110 μM and only starts at 72 hours. Interestingly, the rescue of follicular growth was not observed at BPA 219 or 438 μM. This may be because different doses of BPA may act through different signaling pathways to exert its toxic effects. It may also be that higher doses of BPA are cytotoxic to the follicles and that the follicles cannot be rescued from BPA exposure at higher doses even when removing the Ahr. These results are in agreement with other studies that have shown that disruption of the normal activity of the AHR reduces the toxic effects of other chemicals in the ovary. For example, α-napthoflavone, a known AHR inhibitor, reduces the toxic effects of benzo(a)pyrene, 3-methylcholanthrene, and 7,12-dimethylbenz(a)anthracene on the ovary [48-50], whereas TCDD, a potent AHR agonist, decreases tumorgenic cell proliferation [51]. Further, Basavarajappa et al. [47], who used a similar bioassay to the one that was used in our current study reported that AhrKO follicles treated with the pesticide methoxychlor are also partially protected from inhibition of growth. These findings suggest that the AHR differentially modulates the toxic effects on follicular growth caused by BPA as well as other chemicals such as methoxychlor.
Lastly, unlike the case with follicular growth, BPA treatment of AhrKO follicles resulted in similar estradiol levels to what we observed with WT follicles treated with BPA. These data are highly suggestive that the AHR signaling pathway is not a major route through which BPA exerts its toxic effects on the steroidogenic capacity of the follicles. These results add to a number of publications on the other potential targets through which BPA exerts its toxic effects on steroidogenesis. For example, Peretz et al. [9] concluded that BPA treatment of antral follicles may interfere with steroidogenesis by inhibiting cholesterol uptake and metabolism by decreasing the expression of the rate limiting factors steroidogenic acute regulatory protein (StAR) and cytochrome P450 cholesterol side chain cleavage (Cyp11a1). Similar conclusions were also published by other researchers in other species [52]. Our results indicate that it is unlikely that any of these effects of BPA on steroidogenesis are mediated by the AHR.
5. Conclusions
Overall findings from the current study expand our knowledge regarding the toxic effects of a wide range of BPA doses in ovarian follicles. The examined range of doses represents both human exposure and environmentally relevant levels. Results from our study indicate that BPA in a linear dose-response manner decreases in vitro antral follicle growth and decreases synthesis of estradiol. Interestingly, BPA (0.004 and 0.04 μM) at doses similar to the levels measured in women’s ovarian follicular fluid undergoing in vitro fertilization treatments [2] did not affect follicular growth or estradiol synthesis compared to the control group. BPA also alters expression levels of Bcl2, a factor that is involved in the process of follicular atresia. However, BPA treatment did not dramatically alter genes in the AHR signaling pathway. BPA-induced inhibition of follicle growth, however, was partially rescued in the absence of the Ahr, indicating that the AHR signaling pathway may be partially involved in mediating the toxic effects of BPA on the growth of cultured mouse antral follicles. Further studies, however, are needed to better understand the mechanism by which the AHR signaling pathway mediates the toxic effects of BPA (110 μM).
HIGHLIGHTS.
BPA (110-438 μM) inhibits mouse ovarian antral follicle growth.
BPA (43-438 μM) decreases mouse ovarian antral follicle estradiol levels.
BPA may inhibit ovarian follicle growth partially via the AHR signaling pathway.
Acknowledgements
This work was supported by: National Institute on Environmental Health NIH grants R01ES019178 (J.A.F.), K99ES021467 (Z.R.C.), and T32ES007326 (W.W.), an Environmental Toxicology Fellowship (A.Z.G.), and the Billie A. Field Fellowship in Reproductive Biology (W.W.) The authors also wish to thank Dr. Liying Gao for her continuous support and technical assistance and all the other members of the Flaws’ lab for their assistance and constructive input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement: The authors have no conflicts of interest or any financial disclosures to declare.
References
- [1].Cao XL, Perez-Locas C, Dufresne G, Clement G, Popovic S, Beraldin F, et al. Concentrations of bisphenol A in the composite food samples from the 2008 Canadian total diet study in Quebec City and dietary intake estimates. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:791–8. doi: 10.1080/19440049.2010.513015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–41. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- [3].Craig ZR, Wang W, Flaws JA. Endocrine-disrupting chemicals in ovarian function: effects on steroidogenesis, metabolism and nuclear receptor signaling. Reproduction. 2011;142:633–46. doi: 10.1530/REP-11-0136. [DOI] [PubMed] [Google Scholar]
- [4].Ehrlich S, Williams PL, Missmer SA, Flaws JA, Berry KF, Calafat AM, et al. Urinary bisphenol A concentrations and implantation failure among women undergoing in vitro fertilization. Environ Health Perspect. 2012;120:978–83. doi: 10.1289/ehp.1104307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ehrlich S, Williams PL, Missmer SA, Flaws JA, Ye X, Calafat AM, et al. Urinary bisphenol A concentrations and early reproductive health outcomes among women undergoing IVF. Hum Reprod. 2012;27:3583–92. doi: 10.1093/humrep/des328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Takeuchi T, Tsutsumi O, Ikezuki Y, Takai Y, Taketani Y. Positive relationship between androgen and the endocrine disruptor, bisphenol A, in normal women and women with ovarian dysfunction. Endocr J. 2004;51:165–9. doi: 10.1507/endocrj.51.165. [DOI] [PubMed] [Google Scholar]
- [7].Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, et al. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol. 2002;16:107–16. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- [8].Xu J, Osuga Y, Yano T, Morita Y, Tang X, Fujiwara T, et al. Bisphenol A induces apoptosis and G2-to-M arrest of ovarian granulosa cells. Biochem Biophys Res Commun. 2002;292:456–62. doi: 10.1006/bbrc.2002.6644. [DOI] [PubMed] [Google Scholar]
- [9].Peretz J, Gupta RK, Singh J, Hernandez-Ochoa I, Flaws JA. Bisphenol A impairs follicle growth, inhibits steroidogenesis, and downregulates rate-limiting enzymes in the estradiol biosynthesis pathway. Toxicol Sci. 2011;119:209–17. doi: 10.1093/toxsci/kfq319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Peretz J, Craig ZR, Flaws JA. Bisphenol A inhibits follicle growth and induces atresia in cultured mouse antral follicles independently of the genomic estrogenic pathway. Biol Reprod. 2012;87:63. doi: 10.1095/biolreprod.112.101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Lenie S, Cortvrindt R, Eichenlaub-Ritter U, Smitz J. Continuous exposure to bisphenol A during in vitro follicular development induces meiotic abnormalities. Mutat Res. 2008;651:71–81. doi: 10.1016/j.mrgentox.2007.10.017. [DOI] [PubMed] [Google Scholar]
- [12].Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Sengupta S, Obiorah I, Maximov P, Curpan R, Jordan V. Molecular mechanism of action of bisphenol and bisphenol A mediated by oestrogen receptor alpha in growth and apoptosis of breast cancer cells. Br J Pharmacol. 2013;169:167–78. doi: 10.1111/bph.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hugo ER, Brandebourg TD, Woo JG, Loftus J, Alexander JW, Ben Jonathan N. Bisphenol A at environmentally relevant doses inhibits adiponectin release from human adipose tissue explants and adipocytes. Environ Health Perspect. 2008;116:1642–7. doi: 10.1289/ehp.11537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Vinas R, Watson CS. Bisphenol S disrupts estradiol-induced nongenomic signaling in a rat pituitary cell line: effects on cell functions. Environ Health Perspect. 2013;121:352–8. doi: 10.1289/ehp.1205826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Teng C, Goodwin B, Shockley K, Xia M, Huang R, Norris J, et al. Bisphenol A affects androgen receptor function via multiple mechanisms. Chem Biol Interact. 2013 doi: 10.1016/j.cbi.2013.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, et al. Thyroid hormone action is disrupted by bisphenol A as an antagonist. J Clin Endocrinol Metab. 2002;87:5185–90. doi: 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- [18].Rubin BS. Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. J Steroid Biochem Mol Biol. 2011;127:27–34. doi: 10.1016/j.jsbmb.2011.05.002. [DOI] [PubMed] [Google Scholar]
- [19].Poland A, Knutson JC. 2,3,7,8-tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons: examination of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–54. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- [20].Puga A, Ma C, Marlowe JL. The aryl hydrocarbon receptor cross-talks with multiple signal transduction pathways. Biochem Pharmacol. 2009;77:713–22. doi: 10.1016/j.bcp.2008.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Abel J, Haarmann-Stemmann T. An introduction to the molecular basics of aryl hydrocarbon receptor biology. Biol Chem. 2010;391:1235–48. doi: 10.1515/BC.2010.128. [DOI] [PubMed] [Google Scholar]
- [22].Pollenz RS. The mechanism of AH receptor protein down-regulation (degradation) and its impact on AH receptor-mediated gene regulation. Chem Biol Interact. 2002;141:41–61. doi: 10.1016/s0009-2797(02)00065-0. [DOI] [PubMed] [Google Scholar]
- [23].Hernandez-Ochoa I, Karman BN, Flaws JA. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77:547–59. doi: 10.1016/j.bcp.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Bussmann UA, Bussmann LE, Baranao JL. An aryl hydrocarbon receptor agonist amplifies the mitogenic actions of estradiol in granulosa cells: evidence of involvement of the cognate receptors. Biol Reprod. 2006;74:417–26. doi: 10.1095/biolreprod.105.043901. [DOI] [PubMed] [Google Scholar]
- [25].Safe S, Wormke M. Inhibitory aryl hydrocarbon receptor-estrogen receptor alpha cross-talk and mechanisms of action. Chem Res Toxicol. 2003;16:807–16. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- [26].Nishizawa H, Morita M, Sugimoto M, Imanishi S, Manabe N. Effects of in utero exposure to bisphenol A on mRNA expression of arylhydrocarbon and retinoid receptors in murine embryos. J Reprod Dev. 2005;51:315–24. doi: 10.1262/jrd.16008. [DOI] [PubMed] [Google Scholar]
- [27].Bonefeld-Jorgensen EC, Long M, Hofmeister MV, Vinggaard AM. Endocrine-disrupting potential of bisphenol A, bisphenol A dimethacrylate, 4-n-nonylphenol, and 4-n-octylphenol in vitro: new data and a brief review. Environ Health Perspect. 2007;115(Suppl 1):69–76. doi: 10.1289/ehp.9368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Swedenborg E, Pongratz I. AhR and ARNT modulate ER signaling. Toxicology. 2010;268:132–8. doi: 10.1016/j.tox.2009.09.007. [DOI] [PubMed] [Google Scholar]
- [29].Karman BN, Basavarajappa MS, Craig ZR, Flaws JA. 2,3,7,8-Tetrachlorodibenzo-p-dioxin activates the aryl hydrocarbon receptor and alters sex steroid hormone secretion without affecting growth of mouse antral follicles in vitro. Toxicol Appl Pharmacol. 2012;261:88–96. doi: 10.1016/j.taap.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Flint S, Markle T, Thompson S, Wallace E. Bisphenol A exposure, effects, and policy: a wildlife perspective. J Environ Manage. 2012;104:19–34. doi: 10.1016/j.jenvman.2012.03.021. [DOI] [PubMed] [Google Scholar]
- [31].Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Cien Saude Colet. 2012;17:407–34. doi: 10.1590/s1413-81232012000200015. [DOI] [PubMed] [Google Scholar]
- [32].Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Benedict JC, Lin TM, Loeffler IK, Peterson RE, Flaws JA. Physiological role of the aryl hydrocarbon receptor in mouse ovary development. Toxicol Sci. 2000;56:382–8. doi: 10.1093/toxsci/56.2.382. [DOI] [PubMed] [Google Scholar]
- [34].Hernandez-Ochoa I, Barnett-Ringgold KR, Dehlinger SL, Gupta RK, Leslie TC, Roby KF, et al. The ability of the aryl hydrocarbon receptor to regulate ovarian follicle growth and estradiol biosynthesis in mice depends on stage of sexual maturity. Biol Reprod. 2010;83:698–706. doi: 10.1095/biolreprod.110.087015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Miller KP, Gupta RK, Greenfeld CR, Babus JK, Flaws JA. Methoxychlor directly affects ovarian antral follicle growth and atresia through Bcl-2- and Bax-mediated pathways. Toxicol Sci. 2005;88:213–21. doi: 10.1093/toxsci/kfi276. [DOI] [PubMed] [Google Scholar]
- [36].Abbott BD, Schmid JE, Brown JG, Wood CR, White RD, Buckalew AR, et al. RT-PCR quantification of AHR, ARNT, GR, and CYP1A1 mRNA in craniofacial tissues of embryonic mice exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin and hydrocortisone. Toxicol Sci. 1999;47:76–85. doi: 10.1093/toxsci/47.1.76. [DOI] [PubMed] [Google Scholar]
- [37].Craig ZR, Hannon PR, Wang W, Ziv-Gal A, Flaws JA. Di-n-butyl phthalate disrupts the expression of genes involved in cell cycle and apoptotic pathways in mouse ovarian antral follicles. Biol Reprod. 2013;88:23. doi: 10.1095/biolreprod.112.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wozniak AL, Bulayeva NN, Watson CS. Xenoestrogens at picomolar to nanomolar concentrations trigger membrane estrogen receptor-alpha-mediated Ca2+ fluxes and prolactin release in GH3/B6 pituitary tumor cells. Environ Health Perspect. 2005;113:431–9. doi: 10.1289/ehp.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Quirk SM, Cowan RG, Harman RM, Hu CL, Porter DA. Ovarian follicular growth and atresia: the relationship between cell proliferation and survival. J Anim Sci. 2004;82(E-Suppl):E40–E52. doi: 10.2527/2004.8213_supplE40x. [DOI] [PubMed] [Google Scholar]
- [41].Matsuda F, Inoue N, Manabe N, Ohkura S. Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Dev. 2012;58:44–50. doi: 10.1262/jrd.2011-012. [DOI] [PubMed] [Google Scholar]
- [42].Hsu SY, Lai RJ, Finegold M, Hsueh AJ. Targeted overexpression of Bcl-2 in ovaries of transgenic mice leads to decreased follicle apoptosis, enhanced folliculogenesis, and increased germ cell tumorigenesis. Endocrinology. 1996;137:4837–43. doi: 10.1210/endo.137.11.8895354. [DOI] [PubMed] [Google Scholar]
- [43].Riou C, Tonoli H, Bernier-Valentin F, Rabilloud R, Fonlupt P, Rousset B. Susceptibility of differentiated thyrocytes in primary culture to undergo apoptosis after exposure to hydrogen peroxide: relation with the level of expression of apoptosis regulatory proteins, Bcl-2 and Bax. Endocrinology. 1999;140:1990–7. doi: 10.1210/endo.140.5.6725. [DOI] [PubMed] [Google Scholar]
- [44].Nishizawa H, Imanishi S, Manabe N. Effects of exposure in utero to bisphenol a on the expression of aryl hydrocarbon receptor, related factors, and xenobiotic metabolizing enzymes in murine embryos. J Reprod Dev. 2005;51:593–605. doi: 10.1262/jrd.17026. [DOI] [PubMed] [Google Scholar]
- [45].Kruger T, Long M, Bonefeld-Jorgensen EC. Plastic components affect the activation of the aryl hydrocarbon and the androgen receptor. Toxicology. 2008;246:112–23. doi: 10.1016/j.tox.2007.12.028. [DOI] [PubMed] [Google Scholar]
- [46].Imanishi S, Manabe N, Nishizawa H, Morita M, Sugimoto M, Iwahori M, et al. Effects of oral exposure of bisphenol A on mRNA expression of nuclear receptors in murine placentae assessed by DNA microarray. J Reprod Dev. 2003;49:329–36. doi: 10.1262/jrd.49.329. [DOI] [PubMed] [Google Scholar]
- [47].Basavarajappa MS, Hernandez-Ochoa I, Wang W, Flaws JA. Methoxychlor inhibits growth and induces atresia through the aryl hydrocarbon receptor pathway in mouse ovarian antral follicles. Reprod Toxicol. 2012;34:16–21. doi: 10.1016/j.reprotox.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Cooper AR, Moley KH. Maternal tobacco use and its preimplantation effects on fertility: more reasons to stop smoking. Semin Reprod Med. 2008;26:204–12. doi: 10.1055/s-2008-1042959. [DOI] [PubMed] [Google Scholar]
- [49].Shiromizu K, Mattison DR. Murine oocyte destruction following intraovarian treatment with 3-methylcholanthrene or 7,12-dimethylbenz(a)anthracene: protection by alpha-naphthoflavone. Teratog Carcinog Mutagen. 1985;5:463–72. doi: 10.1002/tcm.1770050609. [DOI] [PubMed] [Google Scholar]
- [50].Shiromizu K, Mattison DR. The effect of intraovarian injection of benzo(a)pyrene on primordial oocyte number and ovarian aryl hydrocarbon [benzo(a)pyrene] hydroxylase activity. Toxicol Appl Pharmacol. 1984;76:18–25. doi: 10.1016/0041-008x(84)90025-5. [DOI] [PubMed] [Google Scholar]
- [51].Holcomb M, Safe S. Inhibition of 7,12-dimethylbenzanthracene-induced rat mammary tumor growth by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Cancer Lett. 1994;82:43–7. doi: 10.1016/0304-3835(94)90144-9. [DOI] [PubMed] [Google Scholar]
- [52].Mlynarcikova A, Kolena J, Fickova M, Scsukova S. Alterations in steroid hormone production by porcine ovarian granulosa cells caused by bisphenol A and bisphenol A dimethacrylate. Mol Cell Endocrinol. 2005;244:57–62. doi: 10.1016/j.mce.2005.02.009. [DOI] [PubMed] [Google Scholar]