Abstract
Although androgen deprivation therapy for prostate cancer is associated with an increased risk of osteoporosis, the optimal timing and schedule of zoledronic acid has not been identified. This phase II trial randomized 44 men beginning androgen deprivation therapy to 3 schedules of zoledronic acid administration. Earlier or more frequent administration of zoledronic acid was found to stabilize and improve bone mineral density in men treated with androgen deprivation therapy.
Objective
To assess the effects of timing and schedule of zoledronic acid (ZA) administration on bone mineral density (BMD) in patients beginning androgen deprivation therapy (ADT) for the treatment of recurrent prostate cancer.
Patients and Methods
In this randomized, 3-arm trial, we evaluated changes in BMD after 3 different ZA administration schedules in men with recurrent prostate cancer who were beginning ADT. Forty-four patients were enrolled and randomized to receive a single dose of ZA given 1 week before beginning ADT (arm 1), a single dose of ZA given 6 months after beginning ADT (arm 2), or monthly administration of ZA starting 6 months after beginning ADT, for a total of 6 doses (arm 3).
Results
Patients who received ZA before ADT had a significant improvement in BMD at the total proximal femur and trochanter after 6 months compared with the other groups. In addition, only patients in the arm that received multiple doses improved lumbar spine BMD while on ADT, with these findings persisting to 24 months. However, this group also experienced more grade 1 adverse events.
Conclusions
Analysis of these data suggests that ZA administration before initiation of ADT was superior to treatment 6 months after starting ADT in maintaining BMD. In addition, monthly ZA administration can increase BMD above baseline but is associated with more adverse events. Further study is needed to examine whether the timing and frequency of ZA therapy in patients on ADT can reduce fracture risk.
Keywords: Androgen deprivation, Bone mineral density, Gamma delta T cells, Prostate cancer, Zoledronic acid
Introduction
In 2011, prostate cancer was the most commonly diagnosed malignancy in American men and the second leading cause of cancer-related death.1 Although radical prostatectomy or definitive radiation therapies are the most effective treatments of localized prostate cancer, nearly one-third of patients will develop recurrent disease.2–4 Androgen deprivation therapy (ADT) is the first-line therapy for recurrent prostate cancer but is associated with significant adverse effects, including a decrease in bone mineral density (BMD) and, in some cases, osteoporosis.5–7 Analysis of results of previous studies indicate that ADT-associated BMD loss occurs early in the course of treatment, which range from 2% to 4% per year, with increasing BMD loss over long courses of ADT.8–10 This was of additional concern in the elderly male population already at an elevated fracture risk due to age-related loss of BMD.11 Although BMD is an intermediate clinical endpoint for osteoporotic fractures, analysis of results of several studies also indicated a substantially increased risk of fractures, particularly in the spine and hips, in men on prolonged ADT.12,13 Due to the known morbidity and mortality associated with fractures in the elderly population, therapeutic interventions to ameliorate BMD loss are of great interest.
The use of bisphosphonates has been shown to reduce the risk of skeletal-related events in patients with metastatic prostate cancer and has also shown benefit in the treatment of osteoporosis in men without radiographic metastases who received ADT.14–17 Among these agents, zoledronic acid (ZA) has shown superiority over other bisphosphonates in decreasing skeletal-related events in patients with metastatic bone lesions.18 Previous studies by investigators indicated that early administration of ZA could also improve BMD in patients at risk for osteoporosis while on ADT.19–21 The question has been raised as to whether patients beginning ADT should begin treatment with a bisphosphonate regardless of BMD status. However, the appropriate timing and dose of bisphosphonates is unclear because some researchers have investigated the use of these agents monthly, and other researchers, concerned about inhibition of bone remodeling, accumulation of microfractures, atypical femoral fractures, and risk of osteonecrosis of the jaw, advocate less-frequent use.22,23
ZA has also been proposed in preclinical models to have both direct and indirect anticancer activity.24 One indirect mechanism of activity is via the ability of ZA to stimulate expansion of a component of the innate immune system known as Vγ9Vδ2 T lymphocytes, which have potent antitumor effects.25–31 A pilot trial to study this concept randomized 18 patients with metastatic, castrate-resistant prostate cancer to receive ZA at 4 mg, with or without subcutaneous administration of a fixed dose of interleukin-2 at 0.6 × 106 international units, given every 21 days.32 Five of 9 patients in the combination therapy cohort showed sustained or increased absolute numbers of Vγ9Vδ2 T cells that correlated with prostate-specific antigen (PSA) response, which suggests an antitumor response mediated by Vγ9Vδ2 T cells. However, the impact of ADT on the generation of Vγ9Vδ2 T cells is unknown. ADT has previously been shown to lead to regrowth of the thymus and a subsequent increase in the number of naive T cells that can be detected in the peripheral blood.33–35 There has been interest in the field of tumor immunology to take advantage of this increase in naive T cells to expand the number of effector cells with potential to target tumor cells. It thus is possible that this increase in naive T cells could be exploited to specifically increase γδ T cells with antitumor efficacy.36 Subsequent administration with ZA could potentially enhance this population of cells.
This study was designed as a 3-arm, randomized trial to assess whether early intervention with ZA could prevent or ameliorate ADT-associated BMD loss in men with stage D prostate cancer who were beginning ADT. Secondary assessments included serum bone-specific alkaline phosphatase (BSAP) measurements at multiple time points to assess for early serum markers of response and enumeration of circulating Vγ9Vδ2 T cells before and during therapy with ADT and ZA.
Patients and Methods
Patient Population and Study Design
A single-institution, randomized, 3-arm trial was conducted at the University of Wisconsin Carbone Cancer Center. Patients with adenocarcinoma of the prostate who had not been previously treated with ADT but who were preparing to begin therapy were invited to participate. All the subjects were treated with single-agent pharmacologic castration with a gonadotropin-releasing hormone (GnRH) analogue (leuprolide or goserelin) administered as 3-month depot injections. For patients without radiographic evidence of metastatic disease, PSA recurrent disease was defined by the following. In patients treated by using surgery, serum PSA values had to have been >0.2 ng/mL by 2 measurements at least 2 weeks apart. In patients treated with ablative radiation therapy, 3 consecutive increases in serum PSA levels were documented, with at least a 1-month interval between values as evidence of biochemical PSA failure. Patients who had not had prior primary therapy, such as radiation or surgery, were required to have a detectable PSA level of ≥2 ng/mL. Patients with evidence of regional lymph node involvement (clinical stage D1) or distant metastatic disease (clinical stage D2) identified pathologically or on bone scan or computed tomography before study enrollment, were considered eligible irrespective of serum PSA level. Normal liver, cardiac, and marrow function as well as an Eastern Cooperative Oncology Group ECOG Performance Status of 0 or 1 were required of all the patients. Current or prior treatment with a GnRH analogue, antiandrogen, bisphosphonate, calcitonin, or other bone resorptive and/or anabolic agents was not allowed. All the patients signed informed consent before enrollment approved by the University of Wisconsin Institutional Review Board. Dose modifications and delays were based on observed toxicities and laboratory parameters. The National Cancer Institute Common Terminology Criteria version 2.0 was used for toxicity and adverse event reporting. In the absence of treatment delays due to adverse events, treatment was continued until disease progression, intercurrent illness that prevented further administration of treatment, unacceptable adverse events, patient withdrawal, or investigator determination that other therapies were warranted.
Treatment Plan and Randomization
All the subjects received at least 1 year of treatment with a GnRH analogue administered as four 3-month depot injections (Figure 1). The subjects were randomized to receive 1 of the 3 treatment arms:
Figure 1.
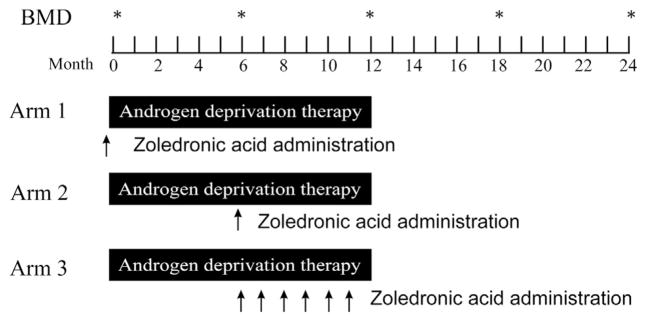
Study Schema
Abbreviation: BMD = bone mineral density.
Arm 1: GnRH analogue 3-month depot injection, every 3 months for 1 year; ZA 4 mg intravenous (I.V.) once, given 7 days before beginning ADT
Arm 2: GnRH analogue 3-month depot injection, every 3 months for 1 year; ZA 4 mg I.V., once, given at month 6
Arm 3: GnRH analogue 3-month depot injection, every 3 months for 1 year; ZA 4 mg I.V. given monthly for 6 total doses, beginning in month 6
These schedules were chosen to permit a comparison of timing of ZA administration relative to beginning ADT (arms 1 and 2), and a comparison of frequency of administration (arms 2 and 3). The timing of ZA beginning at month 6 in arms 2 and 3 was chosen given previous reports that demonstrated detectable bone mineral loss after 6 months of ADT.16,20 Patient treatment was conducted in an open-label fashion for the duration of the study. While receiving protocol therapy, the patients were recommended to take an oral calcium supplement of at least 500 mg calcium and a multivitamin that contained 400 IU of vitamin D daily. Although the study did not mandate treatment with ADT beyond 12 months for patients without radiographic metastases, the patients were permitted to remain on ADT during the following year of BMD evaluation.
Assessments, Follow-up, and Radiographic Monitoring: BMD Evaluation
All the participants were to undergo BMD testing of the lumbar spine (L1–4), total proximal femur, femoral neck, and trochanter at baseline, 6, 12, 18, and 24 months. All scans were performed on a single machine with dedicated research technologists by using a single Prodigy bone densitometer (GE Medical Systems, Lunar, Madison, WI) and by using standard adult software, version 6.6. The SD in the precision of this instrument was 1.75% at the lumbar (L1–L4) spine and 1.35% at the femoral neck. Based on World Health Organization criteria and the official position statement of the International Society for Clinical Densitometry, the lowest T score of the lumbar spine, total proximal femur, and femoral neck was used to define osteopenia (T score, −1.1 to −2.4) and osteoporosis (T score, less than to equal to −2.5).37
Human BSAP Enzyme-linked Immunosorbent Assay
Patient BSAP was measured by ELISA (enzyme-linked immunosorbent assay) kit (TSZ ELISA, Framingham, MA) from patient sera at the indicated time points according to the manufacturer’s instructions. Briefly, sera were collected at the indicated time points and cryopreserved at −20°C until all study samples had been collected. Sera samples were diluted 5-fold and evaluated in triplicate. Raw data were collected, and protein concentrations were determined by comparison of optical density with a serial dilution of BSAP standard. Standardized values were then reported from each time point as percentage change from baseline.
Vγ9Vδ2 T-cell Evaluation
Peripheral blood mononuclear cells were isolated by density gradient centrifugation over Ficoll-Paque (Little Chalfont, UK) by using standard techniques and were stained with the following fluorochrome-labeled monoclonal antibodies for respective experiments: Vγ9-FITC, Vδ2-PE, (Beckton Dickinson Bioscience Pharmingen, San Jose, CA), or isotype controls (immunoglobulin G1-FITC, immunoglobulin G1-PE [Sigma, St Louis, MO]). After labeling, the cells were washed and fixed with paraformaldehyde (Sigma), and the samples were analyzed on a FACSCalibur (Becton–Dickinson Biosciences, Mountain View, CA). At least 20,000 events were collected for all samples and were analyzed by using FlowJo software (TreeStar, San Carlos, CA). Lymphocytes were isolated based on forward and side scatter and gates set based on isotype controls. Events were then analyzed for Vγ9-FITC and Vδ2-PE. Cells that stained positive for both Vγ9 and Vδ2 were counted as a percentage of total analyzed lymphocytes.
Statistical Analysis
The study was designed as a 3-arm, randomized trial with a maximum accrual of 15 subjects per study arm. The assignment to the 3 treatment arms was blinded until subject entry into the study. A blocked randomization procedure was used to achieve a balanced treatment assignment. The planned sample size provided 80% power to detect an anticipated mean difference of at least 2% in the rate change in BMD in the spine from the baseline to the 12-month follow-up assessment for the pairwise comparisons between arms at the 2-sided 5% significance level by assuming an overall SD of 1.75% and a loss of follow-up rate of up to 10%.
This analysis of BMD parameters and secondary endpoints was conducted on an intent-to-treat basis. Categorical data were summarized as proportions and percentages. Continuous data were summarized and reported as mean (SD), median, and range. Percentage changes in BMD parameters from the baseline to the 6-, 12-, 18-, and 24-month assessment were calculated and summarized by using standard descriptive statistics; and, as analogous, percentage changes in BSAP measurements from the baseline to the 1-, 6-, 12-, and 24-month time points were calculated and analyzed. A paired t test was used to evaluate changes in BMD parameters within each arm. The comparison of changes in BMD parameters among arms was performed by using an analysis of variance model. Because arms 2 and 3 were treated identically at months 0 and 6, these data points were combined for comparison to arm 1 at only these time points. Changes from baseline in BSAP parameters were evaluated by using a nonparametric Wilcoxon signed rank test. The comparison of changes from baseline in BSAP parameters among arms was performed by using a nonparametric Kruskal-Wallis test. Nonparametric Spearman rank correlation analysis was conducted to evaluate the association between changes in BMD parameters and changes in BSAP parameters. All data analyses were performed by using SAS version 9.2 (SAS Institute Inc, Cary, NC). A 2-sided significance level of .05 was used for all statistical tests. The percentage of Vγ9Vδ2 T cells among circulating lymphocytes for arm 1 and arms 2 and 3 was analyzed by using a paired t test with a 2-sided significance level set at .05.
Results
Patient Characteristics
Between 2003 and 2009, this 3-arm randomized trial enrolled 44 subjects with recurrent and/or metastatic prostate cancer. The patients were well balanced among arms with respect to age, body surface area, and Eastern Cooperative Oncology Group ECOG Performance Status. The majority of patients had stage D0 prostate cancer (Table 1). There were 4 patients with evidence of osteopenia in arm 1, 3 patients in arm 2, and 5 patients in arm 3. There were 2 patients with evidence of osteoporosis in arm 1, one patient in arm 2, and no patients in arm 3. Patients in arm 2 had a higher mean baseline T score in the lumbar spine, total proximal femur, and trochanter compared with arms 1 and 3. The data from patients accrued to this trial were thus consistent with other reports that men with recurrent prostate cancer are at a high risk of osteopenia or osteoporosis even before initiation of ADT.8
Table 1.
Patient Characteristics (n = 44)
| Arm 1 (n = 14) | Arm 2 (n = 15) | Arm 3 (n = 15) | |
|---|---|---|---|
| Mean (SD) age, years | 67.1 ± 7.8 | 66.5 ± 8.5 | 64.4 ± 11.9 |
| Race, no. patients | |||
| African American | 0 | 1 | 0 |
| White | 13 | 13 | 15 |
| Unknown | 1 | 1 | 0 |
| Mean (SD) BSA, m2 | 2.18 ± 0.27 | 2.12 ± 0.18 | 2.14 ± 0.19 |
| ECOG PS score | |||
| 0 | 11 | 13 | 12 |
| 1 | 3 | 2 | 2 |
| Clinical stage, no. patients | |||
| D0 | 10 | 8 | 9 |
| D1 | 3 | 4 | 3 |
| D2 | 1 | 3 | 3 |
| LN mets | 1 | 1 | 1 |
| Bone mets | 0 | 2 | 3 |
| Gleason score | |||
| Unknown | 1 | 1 | 1 |
| ≤6 | 5 | 4 | 1 |
| 7 | 5 | 6 | 5 |
| 8 | 0 | 2 | 4 |
| 9 | 3 | 2 | 4 |
| 10 | 0 | 0 | 0 |
| Mean (SD) baseline T score | |||
| Lumbar spine | 1.06 ± 1.71 | 1.72 ± 1.85 | 0.77 ± 1.38 |
| Femoral neck | −0.42 ± 1.94 | −0.27 ± 1.11 | −0.27 ± 1.14 |
| Total proximal femur | 0.26 ± 1.61 | 0.36 ± 1.22 | 0.13 ± 0.85 |
| Trochanter | 0.69 ± 1.95 | 0.83 ± 1.49 | 0.26 ± 1.22 |
| Number of patients with low BMD at baseline | |||
| Osteopenia (T score, | 4 | 3 | 5 |
| −1.0 to −2.4) | |||
| Osteoporosis (T score, less than or equal to −2.5) | 2 | 1 | 0 |
Abbreviations: BMD = bone mineral density; BSA = body surface area; ECOG PS = Eastern Cooperative Oncology Group ECOG Performance Status; LN = lymph node.
Study Conduct and Adverse Events
The adverse events reported in Table 2 are those that were deemed at least possibly related to ZA. The study medications were generally well tolerated with no grade 3/4 toxicities attributed to ZA or ADT. A greater frequency of grade 1 toxicities, including fatigue and myalgias, was observed in patients who received 6 infusions of ZA in arm 3. In the course of the trial, 2 patients in arm 2 came off the study, one due to intolerance of ADT and one due to comorbidities unrelated to disease or treatment. Five patients came off the study due to disease progression, which necessitated a change in therapy. Of these 5 patients, 2 were in arm 1, one was in arm 2, and 2 were in arm 3. One patient in arm 3, who was without evidence of distal metastatic disease, came off the study to receive salvage radiation therapy not related to disease progression. Finally, 3 patients missed their month-24 dual-energy x-ray absorptiometry scans for reasons unrelated to disease, 1 patient in arm 1 and 2 patients in arm 3.
Table 2.
Maximum Severity of Adverse Events (n = 44). All Grades 1–4 Adverse Events Observed in This Trial are Showna,b
| Grade 1 | Grade 2 | |||||
|---|---|---|---|---|---|---|
| Arm 1 (n = 14) | Arm 2 (n = 15) | Arm 3 (n = 15) | Arm 1 (n = 14) | Arm 2 (n = 15) | Arm 3 (n = 15) | |
| Constitutional | ||||||
| Flu-like symptoms | 2 | 1 | 1 | |||
| Anorexia | 1 | |||||
| Fatigue | 1 | 1 | 9 | |||
| Nausea | 3 | |||||
| Infection | 1 | 1 | ||||
| Musculoskeletal | ||||||
| Myalgia | 4 | 1 | 4 | 1 | ||
| Arthralgia | 1 | 8 | 1 | |||
| Bone pain | 1 | 1 | 1 | 1 | ||
| Hand edema | 1 | |||||
| Pain NOS | 1 | 1 | ||||
| Neurologic | ||||||
| Mood alteration | 1 | 1 | ||||
| Insomnia | 1 | |||||
| Genitourinary: urinary frequency | 1 | |||||
Abbreviation: NOS = not otherwise specified.
The numbers represent the number of patients within each arm who experienced a particular event at any point during the treatment period, with the highest grade reported for any single individual.
Adverse event grade is according to National Cancer Institute Common Terminology Criteria version 2.0.
BMD Assessments
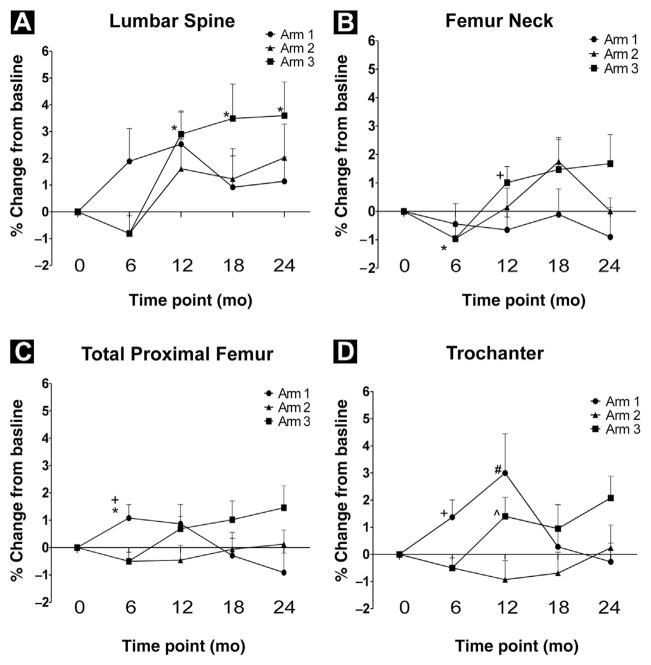
As shown in Figure 2, there was a significant increase in BMD in the total proximal femur at 6 months compared with baseline in patients who received a single dose of ZA before beginning ADT (1.1% ± 1.9%; P = .047). As patients in arms 2 and 3 were not treated with ZA until after their month six scans, data from these groups was combined for the 6-month analysis. A significant decrease in BMD was observed after 6 months of ADT in the femur neck in arms 2 and 3 (−1.0% ± 2.4%; P = .04). There was a statistically significant improvement in BMD in arm 1 compared to arms 2 and 3 in both the total proximal femur (1.1% ± 1.9% vs. −0.5% ± 1.8%; P = .008) and trochanter (1.4% ± 2.4% vs. 0.5% ± 2.1%; P = .016).
Figure 2.
Bone Mineral Density (BMD) Results. (A) Percentage Change From Baseline BMD in the Lumbar Spine. Note That the Data Points for Arms 2 and 3 at Month 0 and Month 6 are Grouped as Described in the Statistical Analysis for All Measured Sites. *Denotes That Arm 3 is Significantly Different From Baseline at 12 mo (P = .009), 18 mo (P = .043), and 24 mo (P = .044). (B) Percentage Change From Baseline BMD in the Femoral Neck. *Denotes That Arms 2 and 3 are Significantly Different From Baseline (P = .04). +Denotes That Arm 1 is Significantly Different From Arm 3 (P = .036). (C) Percentage Change From Baseline BMD in the Total Proximal Femur. *Denotes That Arm 1 is Significantly Different From Baseline (P = .047). +Denotes That Arm 1 is Significantly Different Compared With Arms 2 and 3 (P = .008). (D) Percentage Change From Baseline BMD in the Trochanter. +Denotes That Arm 1 is Significantly Different Compared With Arms 2 and 3 (P = .016). #Denotes That Arm 1 is Significantly Different Compared With Arm 2 (P = .026). ^Denotes That Arm 3 Is Significantly Different Compared With Arm 2 (P = .035)
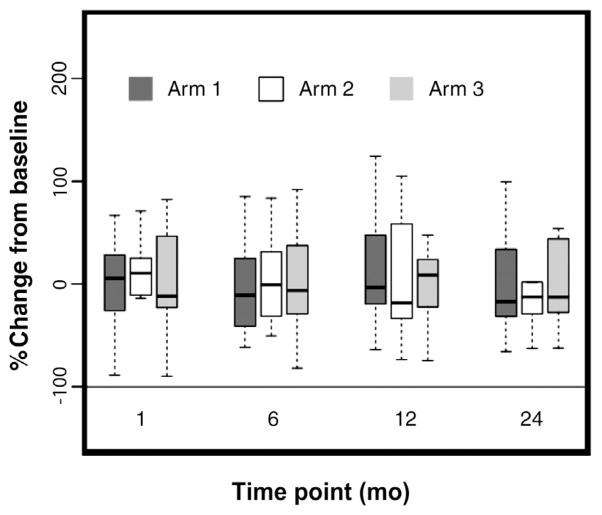
After 6 months of ADT, patients in arm 2 received a single infusion of ZA whereas patients in arm 3 received monthly infusions of ZA for a total of 6 doses. Both arms 2 and 3 showed improved BMD in the lumbar spine at the 12-month measurement compared with baseline, although only arm 3 was statistically significant (2.9% ± 3.4%; P = .009). As shown in Figure 2, patients in arm 3 had significantly increased BMD in the lumbar spine relative to baseline, up to the 24-month measurement (3.5% ± 5.0% at month 18, P = .043; 3.6% ± 4.9% at month 24, P = .044). BMD was significantly increased in the femoral neck at month 12 for patients on arm 3 relative to arm 1, (1.0% ± 2.2% vs. 0.7% ± 1.7%; P = .036), whereas no such difference was found in arm 2. Unexpectedly, BMD in the trochanter for patients on arm 2 (−0.9% ± 2.7%) was significantly lower at 12 months than both arm 1 (3.0% ± 5.4%; P = .026) and arm 3 (1.4% ± 2.7%; P = .035). It should be noted that a single dose of ZA after 6 months of ADT did not improve BMD at any site or at any time point measured up to 24 months. Sera were obtained at baseline, 1-, 6-, 12-, and 24-month time points for evaluation of BSAP to determine whether changes in BSAP might predict benefit of ZA with regard to improved BMD. There were no statistically significant changes in BSAP among any of the arms at any posttreatment time point measured (Figure 3).
Figure 3.
Percentage Change in Bone-specific Alkaline Phosphatase (BSAP) From Baseline. Sera Were Collected at Baseline, 1-, 6-, 12-, and 24-mo and Were Assayed for BSAP Concentration by Enzyme-Linked Immunosorbent Assay. Box Plots Represent the Percentage Change From Baseline in Serum BSAP for Patients at the Indicated Time Points for Each Arm of the Study. The Black Line Within the Box Represents the Median of the Percentage Changes. The Top and Bottom Edges of the Boxes Represent the 25th and 75th Percentiles of the Percentage Changes. The 5th and 95th Percentiles Are Represented by the Whiskers Extending From the Top and Bottom of the Box
Vγ9Vδ2 T-Cell Evaluation
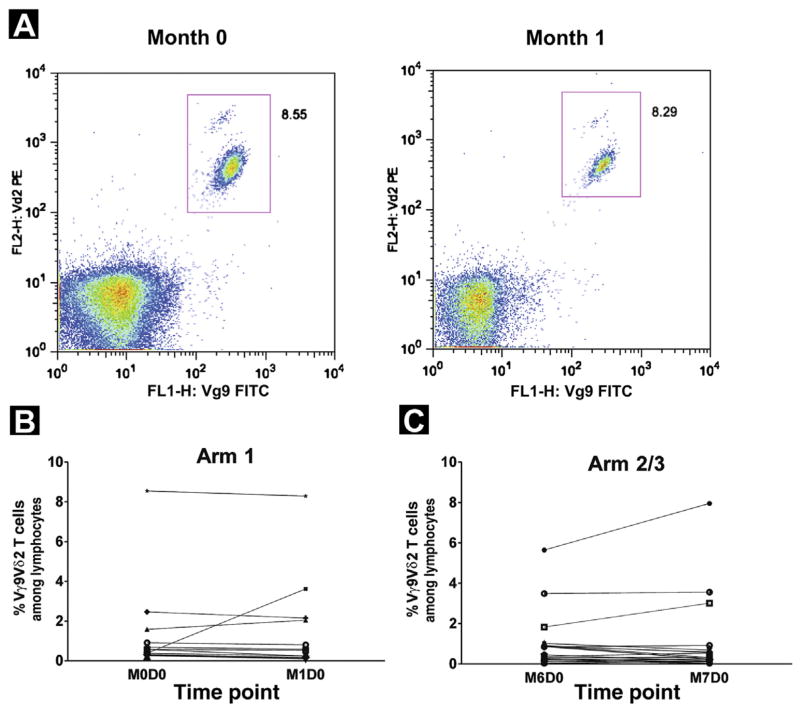
We next tested whether the frequency of Vγ9Vδ2 T cells in the peripheral blood of patients was increased 1 month after ZA infusion and whether this was affected by concomitant ADT. For patients in arm 1, Vγ9Vδ2 T cells were enumerated before initiation of ADT and 1 month after infusion of ZA. For patients in arms 2 and 3, Vγ9Vδ2 T cells were assessed at month 6 (6 months after initiation of ADT) and 1 month after the first infusion of ZA. As shown in Figure 4B, only 2 of 14 patients in arm 1 had an increase in the frequency of circulating Vγ9Vδ2 T cells after infusion of ZA, ranging from 0.46% to 3.23% of circulating lymphocytes. Seven patients in arm 1 had a decline in Vγ9Vδ2 T cells 1 month after initiation of ADT, but this decrease was less than 0.5% of circulating Vγ9Vδ2 T cells in all but 1 patient and was not statistically significant. After 6 months of ADT, Vγ9Vδ2 T cells were increased in only 4 of 30 patients in arms 2 and 3 (range, 0.03%–2.41%) after the infusion of ZA (Figure 4C).
Figure 4.
Percentage of Vγ9Vδ2 T Cells Among Lymphocytes From Peripheral Blood. Peripheral Blood Mononuclear Cells Were Collected From Patients With Prostate Cancer Before and After Zoledronic Acid (ZA) Therapy and Stained With Vγ9-FITC- and Vδ2-PE-Labeled Antibodies. Lymphocytes Were Gated by Forward and Side Scatter, and the Frequency of Vγ9Vδ2 T Cells was Directly Assessed by Dual Parameter Scatter Plots as Exemplified by One Patient in (A). (B) Data Points Shown Represent Percentage of Vγ9Vδ2 T Cells Among Circulating Lymphocytes From Peripheral Blood for Each Patient in Arm 1 at Month 0 and Month 1. (C) Data Points Shown Represent Percentage of Vγ9Vδ2 T Cells Among Lymphocytes From Peripheral Blood for Each Patient in Arms 2 and 3 at Month 6 and Month 7. No Statistically Significant Changes From Baseline to Posttreatment Were Observed
Discussion
We report the results of a 3-arm, randomized phase II trial that assessed different schedules of ZA administration to prevent bone loss in men with stage D (recurrent and/or metastatic) prostate cancer who were beginning ADT. We found, consistent with prior reports, that ADT significantly decreased BMD in the femoral neck relative to baseline after only 6 months. However, patients who received a single 4-mg dose of ZA before ADT did not exhibit such loss. These results were consistent across other measured sites, including the total proximal femur and trochanter. Importantly, ADT-associated bone loss could be recovered after a single dose of ZA but only repeated administration of ZA could improve lumbar spine BMD above baseline. An assessment of serum BSAP as a marker of bone turnover did not reveal any significant differences among treatment groups at any of the measured time points. Although other studies have shown a decrease in bone turnover (both bone formation and bone resorption) after ZA administration,38–40 the performance of these markers is limited in a smaller study size such as ours due to their known high variability.41 Further investigation of circulating Vγ9Vδ2 T cells, a subclass of T cells known to proliferate after treatment with bisphosphonates, also did not show any consistent changes across the treatment groups, and this was not affected by prior ADT, a treatment known to increase the number of circulating naive T cells.
Men with recurrent prostate cancer are at an elevated fracture risk by virtue of both age and ADT-related bone loss. Nearly a third of the patients enrolled in this randomized phase II trial had evidence of osteopenia or osteoporosis on pretreatment dual-energy x-ray absorptiometry scans. After initiation of ADT, BMD was significantly reduced compared with baseline in the femoral neck. Interestingly, patients who received a single dose of ZA before initiation of ADT exhibited a higher BMD at the total proximal femur and trochanter compared with those who did not receive ZA. This result suggests that administration of ZA can ameliorate ADT-associated BMD loss early in the course of therapy. Administration of a single dose of ZA did not increase BMD when given after initiation of ADT at any site or time point up to 24 months.
We observed that monthly administration of ZA for a total of 6 doses given to patients in arm 3 significantly increased BMD in the lumbar spine compared with baseline, with a trend to improvement in the femoral neck. These results suggest that more frequent dosing and/or a higher cumulative dose is more effective in increasing BMD than a single dose. Campbell et al42 found that administration of ZA every 3 months for 1 year was adequate to improve BMD in patients with prostate cancer who were on ADT, with a prior diagnosis of severe osteopenia or osteoporosis. Given that the U.S. Food and Drug Administration has approved only yearly doses of ZA for men with evidence of osteoporosis,43 it is unclear whether this dosing regimen is adequate for individuals with prostate cancer who are receiving ADT. A phase III trial could directly address the questions raised in this study by randomizing high-risk patients with osteopenia or osteoporosis who are beginning ADT to receive ZA once yearly per current guidelines or on a more frequent dosing schedule. In the absence of phase III data, results of our study indicate that clinicians should consider more frequent BMD monitoring for patients at highest risk of osteoporotic fractures.
Whether higher BMD increases provide superior fracture risk reduction remains controversial.44 Some bisphosphonate studies found the fracture risk reduction in those patients with stable BMD to be similar to those with an increase in BMD.45,46 Watts et al. reported that a greater increase in BMD further reduces fracture risk.47 The American Association of Clinical Endocrinology guidelines recommend considering stability or an increase in BMD as treatment success.47 This trial was unable to answer the question of whether an increase in BMD is superior to BMD stability in this patient population because it was not powered or designed to assess a fracture risk reduction. It should be noted that, in the 2-year follow-up on patients in this trial, there were no osteoporotic fractures. However, this short duration of follow-up is not adequate to draw long-term conclusions on this dosing strategy to impact osteoporotic fractures, and, although no grade 3/4 toxicities were observed in any of the patients enrolled to this trial, there were more grade 1 toxicities in arm 3, including fatigue, myalgias, and arthralgias. As such, our data highlight the need for further research that examines whether a more frequent administration of ZA does reduce fracture risk without causing unacceptable rates of adverse events.
We also assessed for markers of early bone remodeling and hypothesized that ZA would reduce serum measures of bone remodeling. In ADT-related bone loss and its inhibition by bisphosphonate treatment, bone remodeling largely remains coupled (ie, if bone resorption is inhibited, then bone formation will also decrease).20,48 As such, BSAP is expected to increase with ADT but decrease with resorption inhibition by ZA. This has been observed in several randomized trials that used ZA in the treatment of osteoporosis.38–40,49 However, we did not detect any significant differences in serum BSAP among treatment groups at any of the measured time points. The performance of these markers of bone turnover is limited in a smaller study size such as ours due to their known high variability and, potentially, by the presence of bone metastases in a subset of our patients.41,50,51
Further laboratory analysis of circulating Vγ9Vδ2 T cells was conducted to determine if the administration of ZA could amplify this population of effector T cells with a known ability to lyse tumor cells in vitro.26 A previous report found that frequent administration of ZA every 21 days in conjunction with low-dose interleukin-2 significantly increased circulating Vγ9Vδ2 T cells at 3, 6, and 9 months during treatment, and this correlated with PSA responses.32 We did not observe a significant increase in Vγ9Vδ2 T cells after 1 month in patients who received ZA naive to ADT or previously treated with ADT. It is unclear whether ZA-induced expansion of circulating Vγ9Vδ2 T cells could have occurred earlier than the 1-month time point assessed or if interleukin-2 administration is required to expand circulating Vγ9Vδ2 T cells, as suggested by Dieli et al.32 We have recently reported that higher doses of ZA can exhaust the proliferative capacity of circulating Vγ9Vδ2 T cells, which raises the possibility that the dose and timing of ZA administration may be critical to enhance Vγ9Vδ2 T-cell proliferation.52
Conclusion
In summary, we report a randomized, 3-arm trial to assess if early administration of ZA could blunt ADT-associated loss of BMD in men with stage D prostate cancer. We observed that administration of ZA before initiation of ADT could prevent ADT-associated loss of BMD. However, only repeated dosing of ZA could significantly increase BMD above baseline levels in men previously initiated on ADT. This frequent administration was associated with an increased frequency of low-grade toxicities. No significant change from baseline was found in a serum marker of bone turnover (BSAP). Analysis of circulating Vγ9Vδ2 T cells did not show expansion of this T-cell subset in peripheral blood. The results of this study suggest that, in men beginning ADT, early and comprehensive assessment and management of bone density can prevent bone loss. These results may guide larger, randomized trials to define the optimal timing and frequency of ZA to prevent and treat ADT-associated bone loss and determine whether these interventions delay the occurrence of other skeletal-related events.
Clinical Practice Points.
Men with advanced prostate cancer are already at risk for osteopenia and osteoporosis on the basis of advanced age. ADT significantly raises the risk of further BMD loss and may raise the risk of osteoporotic fractures.
ZA is currently approved by the U.S. Food and Drug Administration for the treatment of osteoporosis, administered on a yearly basis. Analysis of the results from this randomized phase II trial indicates that yearly administration of ZA, if given after initiation of ADT, does not improve BMD density and may be inadequate for men at highest risk for osteoporotic fractures.
Administration of ZA, either before initiation of ADT or with more frequent dosing may be required to stabilize or improve BMD for men with advanced prostate cancer who require ADT who have existing osteoporosis or osteopenia.
The results from this study should be used to guide further randomized trials that assess early intervention for ADT-associated BMD loss.
Acknowledgments
We are grateful to Beth A. Fredricks and Mahazarin R. Kaikobad for their invaluable help, and Novartis Pharmaceuticals for funding support, the DOD Prostate Cancer Research Program (W81XWH-04-1-0770, W81XWH-05-1-0147), and the NIH (T32 CA009614-21).
Footnotes
Disclosure
The authors have stated that they have no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.D’Amico AV, Cote K, Loffredo M, et al. Determinants of prostate cancer specific survival following radiation therapy during the prostate specific antigen era. J Urol. 2003;170:S42–6. doi: 10.1097/01.ju.0000094800.63501.15. discussion S46–7. [DOI] [PubMed] [Google Scholar]
- 3.Roehl KA, Han M, Ramos CG, et al. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172:910–4. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 4.Lee WR, Hanlon AL, Hanks GE. Prostate specific antigen nadir following external beam radiation therapy for clinically localized prostate cancer: the relationship between nadir level and disease-free survival. J Urol. 1996;156:450–3. doi: 10.1097/00005392-199608000-00033. [DOI] [PubMed] [Google Scholar]
- 5.Shahinian VB, Kuo YF, Freeman JL, et al. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 6.Holzbeierlein JM. Managing complications of androgen deprivation therapy for prostate cancer. Urol Clin North Am. 2006;33:181–90. vi. doi: 10.1016/j.ucl.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 7.Smith MR, Lee WC, Brandman J, et al. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 8.Eriksson S, Eriksson A, Stege R, et al. Bone mineral density in patients with prostatic cancer treated with orchidectomy and with estrogens. Calcif Tissue Int. 1995;57:97–9. doi: 10.1007/BF00298427. [DOI] [PubMed] [Google Scholar]
- 9.Maillefert JF, Sibilia J, Michel F, et al. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–22. [PubMed] [Google Scholar]
- 10.Morote J, Morin JP, Orsola A, et al. Prevalence of osteoporosis during long-term androgen deprivation therapy in patients with prostate cancer. Urology. 2007;69:500–4. doi: 10.1016/j.urology.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 11.Panju AH, Breunis H, Cheung AM, et al. Management of decreased bone mineral density in men starting androgen-deprivation therapy for prostate cancer. BJU Int. 2009;103:753–7. doi: 10.1111/j.1464-410X.2008.08156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daniell HW, Dunn SR, Ferguson DW, et al. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–6. [PubMed] [Google Scholar]
- 13.Dhanapal V, Reeves DJ. Bone health management in prostate cancer patients receiving androgen deprivation therapy. J Oncol Pharm Pract. 2012;18:84–90. doi: 10.1177/1078155211402105. [DOI] [PubMed] [Google Scholar]
- 14.Morabito N, Gaudio A, Lasco A, et al. Neridronate prevents bone loss in patients receiving androgen deprivation therapy for prostate cancer. J Bone Miner Res. 2004;19:1766–70. doi: 10.1359/JBMR.040813. [DOI] [PubMed] [Google Scholar]
- 15.Saad F, Gleason DM, Murray R, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94:1458–68. doi: 10.1093/jnci/94.19.1458. [DOI] [PubMed] [Google Scholar]
- 16.Diamond TH, Winters J, Smith A, et al. The antiosteoporotic efficacy of intravenous pamidronate in men with prostate carcinoma receiving combined androgen blockade: a double blind, randomized, placebo-controlled crossover study. Cancer. 2001;92:1444–50. doi: 10.1002/1097-0142(20010915)92:6<1444::aid-cncr1468>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 17.Greenspan SL, Nelson JB, Trump DL, et al. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–24. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 18.Rosen LS, Gordon DH, Dugan W, Jr, et al. Zoledronic acid is superior to pamidronate for the treatment of bone metastases in breast carcinoma patients with at least one osteolytic lesion. Cancer. 2004;100:36–43. doi: 10.1002/cncr.11892. [DOI] [PubMed] [Google Scholar]
- 19.Smith MR, Eastham J, Gleason DM, et al. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–12. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 20.Smith MR, McGovern FJ, Zietman AL, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–55. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 21.Michaelson MD, Kaufman DS, Lee H, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–42. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pataki A, Muller K, Green JR, et al. Effects of short-term treatment with the bisphosphonates zoledronate and pamidronate on rat bone: a comparative histomorphometric study on the cancellous bone formed before, during, and after treatment. Anat Rec. 1997;249:458–68. doi: 10.1002/(SICI)1097-0185(199712)249:4<458::AID-AR5>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 23.Reid IR. Bisphosphonates in the treatment of osteoporosis: a review of their contribution and controversies. Skeletal Radiol. 2011;40:1191–6. doi: 10.1007/s00256-011-1164-9. [DOI] [PubMed] [Google Scholar]
- 24.Gnant M, Clezardin P. Direct and indirect anticancer activity of bisphosphonates: a brief review of published literature. Cancer Treat Rev. 2012;38:407–15. doi: 10.1016/j.ctrv.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Fisch P, Malkovsky M, Braakman E, et al. Gamma/delta T cell clones and natural killer cell clones mediate distinct patterns of non-major histocompatibility complex-restricted cytolysis. J Exp Med. 1990;171:1567–79. doi: 10.1084/jem.171.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunzmann V, Bauer E, Feurle J, et al. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96:384–92. [PubMed] [Google Scholar]
- 27.Gougeon ML, Malkovsky M, Casetti R, et al. Innate T cell immunity to HIV-infection. Immunotherapy with phosphocarbohydrates, a novel strategy of immune intervention? Vaccine. 2002;20:1938–41. doi: 10.1016/s0264-410x(02)00070-1. [DOI] [PubMed] [Google Scholar]
- 28.Lanier LL, Ruitenberg J, Bolhuis RL, et al. Structural and serological heterogeneity of gamma/delta T cell antigen receptor expression in thymus and peripheral blood. Eur J Immunol. 1988;18:1985–92. doi: 10.1002/eji.1830181218. [DOI] [PubMed] [Google Scholar]
- 29.Malkovsky M, Bartz SR, MacKenzie D, et al. Are gamma delta T cells important for the elimination of virus-infected cells? J Med Primatol. 1992;21:113–8. [PubMed] [Google Scholar]
- 30.Kobayashi H, Tanaka Y, Yagi J, et al. Gamma/delta T cells provide innate immunity against renal cell carcinoma. Cancer Immunol Immunother. 2001;50:115–24. doi: 10.1007/s002620100173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dieli F, Gebbia N, Poccia F, et al. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102:2310–1. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 32.Dieli F, Vermijlen D, Fulfaro F, et al. Targeting human gamma delta T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 2007;67:7450–7. doi: 10.1158/0008-5472.CAN-07-0199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viselli SM, Stanziale S, Shults K, et al. Castration alters peripheral immune function in normal male mice. Immunology. 1995;84:337–42. [PMC free article] [PubMed] [Google Scholar]
- 34.Morse MD, McNeel DG. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum Immunol. 2010;71:496–504. doi: 10.1016/j.humimm.2010.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morse MD, McNeel DG. T cells localized to the androgen-deprived prostate are T(H) 1 and T(H) 17 biased. Prostate. 2012;72:1239–47. doi: 10.1002/pros.22476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McNeel DG, Malkovsky M. Immune-based therapies for prostate cancer. Immunol Lett. 2005;96:3–9. doi: 10.1016/j.imlet.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Baim S, Binkley N, Bilezikian JP, et al. Official positions of the International Society for Clinical Densitometry and executive summary of the 2007 ISCD Position Development Conference. J Clin Densitom. 2008;11:75–91. doi: 10.1016/j.jocd.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 38.Orwoll ES, Miller PD, Adachi JD, et al. Efficacy and safety of a once-yearly i.v. Infusion of zoledronic acid 5 mg versus a once-weekly 70-mg oral alendronate in the treatment of male osteoporosis: a randomized, multicenter, double-blind, active-controlled study. J Bone Miner Res. 2010;25:2239–50. doi: 10.1002/jbmr.119. [DOI] [PubMed] [Google Scholar]
- 39.Israeli RS, Rosenberg SJ, Saltzstein DR, et al. The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer. 2007;5:271–7. doi: 10.3816/CGC.2007.n.003. [DOI] [PubMed] [Google Scholar]
- 40.Ryan CW, Huo D, Demers LM, et al. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol. 2006;176:972–8. doi: 10.1016/j.juro.2006.04.078. discussion 978. [DOI] [PubMed] [Google Scholar]
- 41.Vasikaran S, Eastell R, Bruyere O, et al. Markers of bone turnover for the prediction of fracture risk and monitoring of osteoporosis treatment: a need for international reference standards. Osteoporos Int. 2011;22:391–420. doi: 10.1007/s00198-010-1501-1. [DOI] [PubMed] [Google Scholar]
- 42.Campbell SC, Bhoopalam N, Moritz TE, et al. The use of zoledronic acid in men receiving androgen deprivation therapy for prostate cancer with severe osteopenia or osteoporosis. Urology. 2010;75:1138–43. doi: 10.1016/j.urology.2009.11.083. [DOI] [PubMed] [Google Scholar]
- 43.Zometa/Reclast (Zoledronic Acid) [package insert] East Hanover, NJ: Novartis Pharmaceuticals; 2001. [Google Scholar]
- 44.Sebba AI. Significance of a decline in bone mineral density while receiving oral bisphosphonate treatment. Clin Ther. 2008;30:443–52. doi: 10.1016/j.clinthera.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 45.Watts NB, Cooper C, Lindsay R, et al. Relationship between changes in bone mineral density and vertebral fracture risk associated with risedronate: greater increases in bone mineral density do not relate to greater decreases in fracture risk. J Clin Densitom. 2004;7:255–61. doi: 10.1385/jcd:7:3:255. [DOI] [PubMed] [Google Scholar]
- 46.Miller PD, Delmas PD, Huss H, et al. Increases in hip and spine bone mineral density are predictive for vertebral antifracture efficacy with ibandronate. Calcif Tissue Int. 2010;87:305–13. doi: 10.1007/s00223-010-9403-y. [DOI] [PubMed] [Google Scholar]
- 47.Watts NB. Bone: bone density screening leads to reduced fracture risk. Nat Rev Endocrinol. 2010;6:17–8. doi: 10.1038/nrendo.2009.246. [DOI] [PubMed] [Google Scholar]
- 48.Greenspan SL, Nelson JB, Trump DL, et al. Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapy. J Clin Oncol. 2008;26:4426–34. doi: 10.1200/JCO.2007.15.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 50.Seibel MJ. Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev. 2005;26:97–122. [PMC free article] [PubMed] [Google Scholar]
- 51.Seibel MJ. Clinical use of markers of bone turnover in metastatic bone disease. Nat Clin Pract Oncol. 2005;2:504–17. doi: 10.1038/ncponc0320. quiz 501 p following 533. [DOI] [PubMed] [Google Scholar]
- 52.Lang JM, Kaikobad MR, Wallace M, et al. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60:1447–60. doi: 10.1007/s00262-011-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]