Abstract
Cue-induced cocaine craving in rodents intensifies or “incubates” during the first months of withdrawal from long access cocaine self-administration. This incubation phenomenon is relevant to human users who achieve abstinence but exhibit persistent vulnerability to cue-induced relapse. It is well established that incubation of cocaine craving involves complex neuronal circuits. Here we will focus on neuroadaptations in the nucleus accumbens (NAc), a region of convergence for pathways that control cocaine seeking. A key adaptation is a delayed (~3–4 weeks) accumulation of Ca2+-permeable AMPAR receptors (CP-AMPARs) in synapses on medium spiny neurons (MSN) of the NAc. These CP-AMPARs mediate the expression of incubation after prolonged withdrawal, although different mechanisms must be responsible during the first weeks of withdrawal, prior to CP-AMPAR accumulation. The cascade of events leading to CP-AMPAR accumulation is still unclear. However, several candidate mechanisms have been identified. First, mGluR1 has been shown to negatively regulate CP-AMPAR levels in NAc synapses, and it is possible that a withdrawal-dependent decrease in this effect may help explain CP-AMPAR accumulation during incubation. Second, an increase in phosphorylation of GluA1 subunits (at the protein kinase A site) within extrasynaptic homomeric GluA1 receptors (CP-AMPARs) may promote their synaptic insertion and oppose their removal. Finally, elevation of brain-derived neurotrophic factor (BDNF) levels in the NAc may contribute to maintenance of incubation after months of withdrawal, although incubation-related increases in BDNF accumulation do not account for CP-AMPAR accumulation. Receptors and pathways that negatively regulate incubation, such as mGluR1, are promising targets for the development of therapeutic strategies to help recovering addicts maintain abstinence.
Keywords: BDNF, cocaine, incubation, metabotropic glutamate receptor, nucleus accumbens, protein kinase A
1. Introduction
One of the major challenges in treating cocaine addiction is the propensity for abstinent users to relapse upon re-exposure to drug-associated cues (Hunt, 1971; Mendelson and Mello, 1996; Reichel and Bevins, 2009). It has been hypothesized that this may be because craving elicited by drug-related cues increases after acute abstinence and persists even after periods of prolonged abstinence (Gawin and Kleber, 1986). A similar phenomena referred to as “incubation” of cue-induced craving has been identified and extensively characterized in animals with a history of cocaine self-administration (e.g., Lu et al., 2004a; Pickens et al., 2011). These studies found that, in rats that underwent long access cocaine self-administration, context- and cue-induced cocaine seeking progressively increased (incubated) during withdrawal, peaking after about 1 month, persisting at similarly elevated levels at the 3 month time-point, and remaining elevated compared to withdrawal day (WD) 1 for at least 6 months (Grimm et al., 2001; Grimm et al., 2003; Lu et al., 2004b). Incubation of cue-induced craving has also been shown to occur in animals with a history of self-administering other drugs, including heroin, methamphetamine, and nicotine (see Pickens et al., 2011 for an extensive review), and has recently been shown to occur in abstinent human smokers (Bedi et al., 2011).
Alterations in neuronal activity and signaling pathways in the ventral tegmental area (VTA), medial prefrontal cortex (mPFC), amygdala, and nucleus accumbens (NAc) have been linked to various aspects of incubated cocaine craving (Pickens et al., 2011). For instance, activity of the glial cell line-derived neurotrophic factor (GDNF) in the VTA plays a role in the development of incubation (Lu et al., 2009). Neuronal activity in the ventral mPFC (Koya et al., 2009) and the central nuclei of the amygdala (CeA) (Lu et al., 2005a; Lu et al., 2007) is important for the expression of incubated cocaine craving. Finally, as will be discussed in more detail (Section 5), incubation is associated with time-dependent changes in brain-derived neurotrophic factor (BDNF) levels in the VTA, NAc and amygdala (Grimm et al., 2003).
This review will focus on adaptations in the NAc that contribute to incubated cocaine craving. We will begin by describing a key adaptation, the withdrawal-dependent accumulation of Ca2+-permeable AMPARs (CP-AMPARs), that mediates the expression of incubation after prolonged withdrawal. Then we will discuss specific mechanisms that are candidates for regulating this AMPAR plasticity, namely mGluR1 mediated synaptic depression, phosphorylation of GluA1 at the protein kinase A (PKA) site, and BDNF transmission in the NAc. For a more comprehensive review that covers incubation-related neuroadaptations in multiple brain regions, please see Pickens et al. (2011).
2. AMPAR transmission in the NAc during incubation
2.1. Ca2+-permeable AMPA receptors (CP-AMPARs) are incorporated into NAc synapses during incubation and mediate its expression after prolonged withdrawal
AMPARs are tetramers comprised of GluA1-4 subunits. Their properties are dramatically altered by the presence or absence of the GluA2 subunit. Receptors lacking this subunit are Ca2+-permeable, exhibit larger single channel conductance and faster kinetics than GluA2-containing AMPARs, and display inward rectification due to voltage-dependent block by intracellular polyamines. Interest in CP-AMPARs has intensified in recent years as a result of studies demonstrating their involvement in certain forms of long-term potentiation (LTP), long-term depression (LTD) and synaptic scaling, as well as excitotoxicity associated with disorders such as epilepsy and Alzheimer’s disease (Cull-Candy et al., 2006; Isaac et al., 2007; Liu and Zukin, 2007; Lee, 2012). Intriguingly, the synaptic incorporation of CP-AMPARs not only strengthens synapses and alters postsynaptic signaling (due to their higher conductance and Ca2+-permeability, respectively) - it also changes the rules for induction of subsequent synaptic plasticity (Mameli et al., 2011). Thus, modifying CP-AMPAR levels in synapses has the potential to qualitatively alter the function of neuronal circuits and behavioral output.
In the NAc of adult drug-naïve rodents, GluA2-containing, Ca2+-impermeable AMPARs (CI-AMPARs) mediate the majority of excitatory synaptic transmission onto medium spiny neurons (MSN) (Boudreau et al., 2007; Kourrich et al., 2007; Conrad et al., 2008; Reimers et al., 2011). However, there is a minority population of GluA2-lacking, Ca2+-permeable AMPARs (CP-AMPARs) in this region (Boudreau et al., 2007; Conrad et al., 2008; Reimers et al., 2011; Ferrario et al., 2011a). Since NAc AMPARs are required for cocaine seeking behavior (Kalivas and Volkow, 2005; Wolf, 2010a; Wolf and Ferrario, 2010), alterations in AMPAR levels and/or AMPAR subunit composition in the NAc could contribute to incubation of cocaine seeking. This possibility was first examined by Lu et al. (2003), who measured AMPAR levels in NAc homogenates after different periods of withdrawal from a long access cocaine self-administration regimen (6 h/day for 10 days) previously shown to lead to incubation (e.g., Grimm et al., 2001; 2003). Sucrose self-administration was the control condition. Lu et al. (2003) found that GluA1 was increased on withdrawal day (WD) 1 and WD90 (~20% over controls) but not WD30, while GluA2 was increased on WD1 and WD30 (~50% over control; there was also a trend towards increased GluA2 on WD90).
In 2008, we re-assessed this issue by measuring cell surface expression of AMPAR subunits after 1 or 45 days of withdrawal from the same cocaine self-administration regimen; saline self-administration was our control condition (Conrad et al., 2008). For the studies described in this section, we used a protein crosslinking assay to distinguish surface from intracellular receptor pools (Boudreau and Wolf, 2005; Boudreau et al., 2012) in whole NAc tissue from each rat (core plus shell). On WD1, when cocaine seeking was low, cocaine rats showed modest decreases in surface, intracellular and total GluA1 compared to saline controls. We speculate that this could represent synaptic scaling (scaling down) in response to high levels of synaptic activity during cocaine self-administration training. However, on WD45, when cocaine seeking had incubated, rats exhibited significant increases in all of these GluA1 measures relative to saline controls, while GluA2 measures were unchanged (Conrad et al., 2008). It is possible that our results for total AMPAR subunit protein levels differed from prior results (Lu et al., 2003) because we used saline self-administration, rather than sucrose self-administration, as a control condition, although Lu et al. (2003) found that sucrose controls did not differ in any measure from drug-naïve rats.
Our biochemical results showing withdrawal-dependent increases in cell surface and total GluA1 but not GluA2 indicated that GluA2-lacking AMPARs, i.e., CP-AMPARs, might be accumulating in NAc synapses of rats that underwent long access cocaine self-administration and prolonged withdrawal (hereafter referred to as “incubated rats”). Supporting this, co-IP studies showed an increase in the portion of GluA1 not associated with GluA2 or any other subunit, suggesting that at least some of the new CP-AMPARs are homomeric GluA1 receptors. Next, patch clamp recordings established that CP-AMPARs contribute to synaptic transmission after incubation. Thus, on WD42-47, inward rectification and sensitivity of evoked EPSCs to the CP-AMPAR antagonist Naspm were observed in MSN recorded in the NAc core of the cocaine group, but not MSN from saline controls. Finally, these CP-AMPARs were shown to mediate the expression of incubated cocaine craving by demonstrating that injections of the selective CP-AMPAR blocker Naspm into the NAc core markedly depressed the expression of incubated cue-induced cocaine seeking on WD45 (Conrad et al., 2008). It should be mentioned that CP-AMPARs appear to be added “on top” of existing CI-AMPARs in the NAc, as no decrease in surface or total GluA2 was detected on WD45 (Conrad et al., 2008).
Together with other findings (Mameli et al., 2009; Wolf and Tseng, 2012), these results demonstrate that CP-AMPARs accumulate in NAc synapses after 3–4 weeks of withdrawal from long access cocaine self-administration and thereafter mediate the expression of incubated cocaine craving. Their role in mediating incubation can be understood based on evidence that synaptic incorporation of CP-AMPARs increases the responsiveness of MSNs to glutamate inputs. This was first suggested by an increase in the number of high amplitude spontaneous excitatory postsynaptic currents (sEPSC) in MSNs from “incubated rats” compared to saline controls (Conrad et al., 2008). More recently, we showed that MSNs from “incubated rats” exhibit an increase in EPSC amplitude in response to synaptic stimulation compared to saline controls, indicating increased baseline responsiveness of these neurons to excitatory drive (Purgianto et al., 2013). Together, these findings support the idea that synaptic incorporation of CP-AMPARs increases the ability of MSNs to respond and engage the motor circuitry when presentation of a cocaine-related cue leads to glutamate release in the NAc, leading to incubated cocaine seeking. However, it remains unclear which attribute of CP-AMPARs is more important to incubation of cocaine craving: their higher single channel conductance or their ability to qualitatively change Ca2+ signaling during synaptic transmission.
As described in Section 1, incubation of craving occurs following self-administration of many drugs of abuse besides cocaine. Whether these other drugs elicit CP-AMPAR accumulation in the NAc remains to be determined. Another interesting question is whether excitatory synapses in the NAc are further strengthened (beyond the increase in basal synaptic strength produced by withdrawal-dependent accumulation of CP-AMPARs) when “incubated rats” express incubated cue-induced cocaine craving. This possibility is raised by the demonstration of a rapid, transient synaptic potentiation in the NAc during cue-induced reinstatement of cocaine seeking (Gipson et al., 2013), similar to a phenomenon previously demonstrated during cocaine-primed reinstatement (Anderson et al., 2008), although the use of extinction training in these studies complicates comparisons to the incubation model.
2.2. CP-AMPAR accumulation during incubation of cocaine craving does not appear to be restricted to a particular population of MSNs in the NAc
In our original study, the most in depth biochemical analysis of AMPAR distribution and composition during incubation was performed in whole NAc tissue (core plus shell) and all electrophysiological studies were conducted in NAc core – these are the results described in Section 2.1 (Conrad et al., 2008). However, to extend these findings, we also determined AMPAR subunit surface, intracellular and total levels in separately dissected core and shell tissue samples from cocaine rats on WD1 versus WD45. The core showed a nearly identical pattern to that described in Section 2.1 for whole NAc. In shell, there was a significant increase in surface GluA1 (albeit less of an increase than in observed in core) but no other statistically significant changes (Conrad et al., 2008). We also extended our original electrophysiological results by comparing NAc shell and core on WD30-47 from the same regimen. We found that nearly all MSN recorded from the core exhibited an elevated rectification index consistent with CP-AMPAR accumulation; a statistically significant increase in the rectification index was also observed in shell, but there was more variability (McCutcheon et al., 2011a). One interpretation of these findings is that CP-AMPARs accumulate more slowly in the shell than core. While we have conducted a detailed time course of their emergence in core and found that they are detectable as early as WD25-30 (Wolf and Tseng, 2012), our only electrophysiological data in shell were obtained on WD30-47 (McCutcheon et al., 2011a). Alternatively, the phenomenon may be less robust in shell, or affect only a portion of MSN, perhaps reflecting different excitatory inputs to core and shell. In mice, patch clamp recordings have demonstrated synaptic incorporation of CP-AMPARs in the NAc shell after 35 days of withdrawal from extended access cocaine self-administration, but the core was not examined (Mameli et al., 2009).
Is one subregion or the other more important for incubation? It is clear that CP-AMPAR accumulation in the core plays a critical role, at least in the rat, because intra-core injection of Naspm reduced cue-induced cocaine seeking nearly to WD1 levels, although some diffusion of naspm to the shell cannot be ruled out (Conrad et al., 2008). In mice, support for a relationship between CP-AMPARs in the shell and incubated craving was obtained based on a comparison of control and NR1DATCreERT2 mice (Mameli et al., 2009). NMDARs are lacking in DA neurons of the mutant mice, which prevents the NMDAR-dependent plasticity in the VTA that is normally observed in the early days of cocaine withdrawal (Engblom et al., 2008; Mameli et al., 2009). Following cocaine self-administration (4 h/day for 8 days) and a withdrawal period consistent with incubation (35 days), control mice showed significantly greater cue-induced seeking than NR1DATCreERT2 mice. Recordings performed within 48 h of the behavioral test revealed CP-AMPARs in NAc shell MSNs of the control cocaine-exposed mice, but not in the NR1DATCreERT2 cocaine-exposed mice. Most importantly, a significant correlation was observed between the number of lever presses during the seeking test and the rectification index in the subsequent recordings. These findings indicated that the magnitude of CP-AMPAR accumulation in the NAc shell is correlated with the magnitude of cue-induced cocaine craving. They also demonstrated that NMDAR-dependent activation of DA neurons in the VTA is a prerequisite for CP-AMPAR synaptic incorporation in MSN (Mameli et al., 2009), as discussed further in Section 2.4.
Beyond core and shell distinctions, MSN in the NAc can be divided into D1 receptor (D1R) and D2 receptor (D2R) expressing populations. However, the situation in NAc is not exactly analogous to the dorsal striatum’s clearly defined D1R-expressing ‘direct pathway’ and D2R-expressing ‘indirect pathway’ MSNs. Thus, while NAc MSN expressing enkephalin/D2R mRNA project selectively to the ventral pallidum, those expressing substance P/D1R mRNA can project to both the ventral pallidum and ventral tegmental area (Lu et al., 1998, Sesack and Grace, 2010). Nevertheless, given evidence that D1R and D2R expressing populations in the NAc play different roles in reward and can undergo different drug-induced plasticity (e.g., Lobo et al., 2010; Kravitz et al., 2012; Grueter et al. 2012), it is of interest to know whether CP-AMPARs accumulate selectively in either population. While we have not studied this directly, we view this possibility as unlikely because we detect CP-AMPARs in nearly every MSN recorded from the NAc core of “incubated rats” (see above). This apparent lack of D1R or D2R selectivity may reflect the fact that weeks pass between the last cocaine self-administration session (a time of strong DA receptor activation) and the accumulation of CP-AMPARs. Therefore, rather than envisioning CP-AMPAR accumulation as a consequence of signaling downstream of either D1 or D2 DA receptors, it is more reasonable to view CP-AMPAR accumulation as a form of plasticity evoked by delayed alterations in the activity of glutamate inputs to the NAc (see Wolf and Tseng, 2012 for more discussion).
The considerations summarized above do not mean that there is no selectivity in CP-AMPAR accumulation. Thus, even if every MSN from the NAc core of an “incubated rat” expresses CP-AMPARs, these receptors may accumulate only at certain synapses onto that MSN. For example, CP-AMPARs may accumulate at synapses between basolateral amygdala inputs and the MSN, but not synapses between prefrontal cortical inputs and the MSN. This can be addressed using optogenetic techniques and is an important issue for future studies.
2.3 Long access but not short access cocaine self-administration leads to CP-AMPAR accumulation in the NAc of adult rats
Work from the Shaham lab has shown that the nature of cocaine exposure influences the development of incubation. Although it is difficult to establish exact requirements for triggering incubation (see below), it is clear that incubation is far more robust when rats self-administer cocaine for 6 h/day (long access) as compared to 2 h/day (short access) (Lu et al., 2004). Recently, we have evaluated the type of cocaine regimen required for the accumulation of CP-AMPARs in the NAc after prolonged withdrawal. In our first study, we compared rats exposed to our standard long access regimen (6 h/day for 10 days) with rats given 8 non-contingent (i.p.) cocaine injections. After 35–49 days of withdrawal, which is more than sufficient for CP-AMPAR accumulation after our long access self-administration regimen, we failed to detect increased CP-AMPAR levels in core or shell of rats that received non-contingent cocaine treatment (McCutcheon et al., 2011a). Next we evaluated CP-AMPAR transmission in the NAc core after >40 days of withdrawal from four different self-administration regimens, named according to whether sessions were short access (ShA, 2 h/day) or long access (LgA, 6 h/day) and the total number of daily sessions: 1) LgA/10d (our standard regimen, already shown to elicit CPAMPAR accumulation; Conrad et al., 2008), 2) ShA/11d, 3) ShA/20-24d, and 4) LgA/20-24d. In the last two regimens, rats began with 10 days of short access sessions (2 h/day) and then entered a differential phase (10–14 days) in which some rats continued with 2 h/day sessions and others switched to 6 h/day sessions. After >40 days of withdrawal, whole-cell patch-clamp recordings were performed in the NAc core to assess the contribution of CP-AMPARs to synaptic transmission, based on the magnitude of synaptic suppression elicited by naspm. We found that naspm produced significant synaptic attenuation (25–30%) after both of the long access regimens (LgA/10d and LgA/20-24d), but not after the short access regimens or in saline controls (Purgianto et al., 2013). Furthermore, by comparing cocaine infusions in each regimen, we determined that the duration of the daily session was more important than the total number of sessions or total cocaine intake in determining whether or not CP-AMPARs accumulated. For example, total cocaine intake was comparable between the ShA/20-24d group and our standard incubation regimen (LgA/10d), yet only the latter group exhibited elevated CP-AMPAR levels (Purgianto et al., 2013).
Although our results indicate that non-contingent and short access cocaine exposure do not produce CP-AMPAR accumulation, this does not mean that they have no effect on AMPAR transmission. It has been known for many years that non-contingent cocaine exposure leading to behavioral sensitization increases cell surface and synaptic levels of the CI-AMPARs (GluA1A2) that normally mediate the vast majority of synaptic transmission in the NAc. This occurs during the first week of withdrawal, persists through WD21, but dissipates by WD41 (Boudreau and Wolf, 2005; Boudreau et al., 2007, 2009; Kourrich et al., 2007; Ghasemzadeh et al., 2009; Schumann and Yaka, 2009; Ferrario et al., 2010; McCutcheon et al., 2011a). Thus, non-contingent cocaine exposure produces a moderately persistent upregulation of CI-AMPARs during withdrawal, whereas long access cocaine self-administration produces a more delayed but ultimately more persistent increase in CP-AMPARs. Although we have shown that short access cocaine self-administration does not elicit CP-AMPAR accumulation, it may resemble non-contingent cocaine exposure in producing CI-AMPAR upregulation. Supporting this, elevated AMPA/NMDA ratios were found in NAc shell after 23–30 days of withdrawal from a cocaine self-administration regimen in which 2 h/day sessions were conducted for several weeks, first on an FR1 schedule and then an FR5 schedule (Ortinski et al., 2012). A simpler short access regimen (2 h sessions for ~2 weeks) followed by 2–3 weeks of abstinence prevented electrically induced LTP but not LTD in NAc core, which would be consistent with AMPAR upregulation leading to occlusion of postsynaptically expressed LTP (Knackstedt et al., 2010). Interestingly, earlier studies, in which NAc MSN were recorded from awake rats on WD30 after cocaine self-administration (2 h/day for 2–3 weeks), demonstrated heightened activation of MSN during resumption of cocaine self-administration or presentation of cocaine-associated cues (Hollander and Carelli, 2005, 2007). Moreover, these rats exhibited incubation of cue-induced craving on WD30 compared to WD1. This raises questions about whether the heightened activation of MSN was due to upregulation of CI-AMPARs versus CP-AMPARs (of course non-AMPAR changes cannot be ruled out), and whether certain regimens may lead to incubation but not CP-AMPAR accumulation. In such a case, we would speculate that incubation would not be as persistent. More generally, it is important to keep in mind the wide variety of cocaine self-administration regimens used in the literature. Many are “intermediate” to the ones we have tested (in terms of session duration or number). They also differ in other ways (e.g., use of prior food training, cocaine dose, and more). Thus, empirical tests are required to conclusively determine whether a particular regimen leads to incubation or to CP-AMPAR accumulation. Nevertheless, our results do establish that CP-AMPAR accumulation and related neuroadaptations (McCutcheon et al., 2011b) do not occur after standard short access regimens used in the literature, and that it is therefore inaccurate to generalize findings from short access and long access regimens when attempting to describe the state of excitatory synaptic transmission in the NAc after cocaine withdrawal. Generalizations between animals subjected to withdrawal in the home cage versus extinction training are also quite problematic (e.g., Knackstedt et al., 2010).
A final point is that our studies of different cocaine regimens were conducted in adult rats (McCutcheon et al., 2011a; Purgianto et al., 2013). The same rules may not apply in younger animals, as Mameli and colleagues (2009) found that non-contingent cocaine exposure in young mice (P16-P35) leads to CP-AMPAR accumulation in the NAc shell. These findings highlight how the age of the animal can profoundly impact drug-induced plasticity (McCutcheon and Marinelli, 2009) and may be linked to greater addiction vulnerability during development.
2.4 Time-course of CP-AMPAR accumulation in the NAc: Implications for circuitry and mechanisms involved in incubation
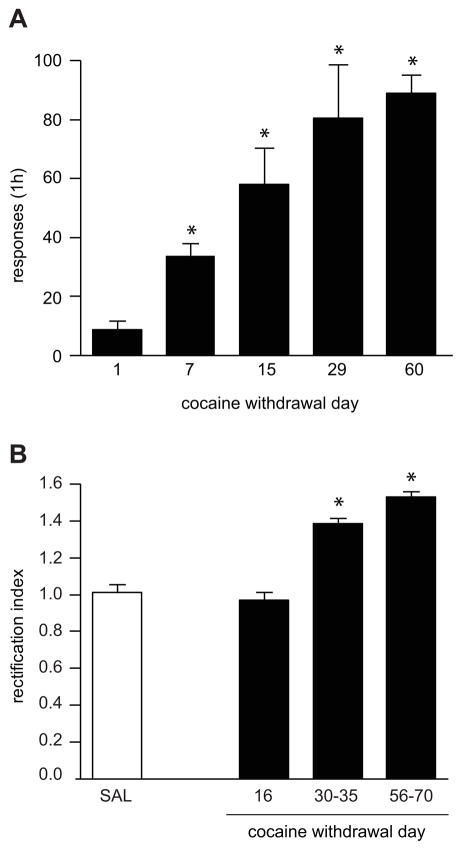
Using the rectification index as a measure of CP-AMPAR synaptic incorporation, we have found that CP-AMPAR levels in the core increase sometime between WD25 and WD35, and then remain high through at least WD70 (Wolf and Tseng, 2012). This concurs with results of biochemical results suggesting detectable increases in GluA1 as early as WD21 following the same regimen (Conrad et al., 2008), whereas no differences in GluA1 levels in synaptic membrane fractions were found after 10–12 days of home cage withdrawal from a similar long access regimen (Ghasemzadeh et al., 2009). The delay before CP-AMPAR accumulation is fascinating, but raises two major issues: 1) CP-AMPAR plasticity lags behind the time-course of incubation, and 2) the delay is difficult to explain based on known mechanisms of synaptic plasticity. These issues will be considered in turn.
Behavioral studies have shown that incubation of cue-induced cocaine craving is already evident by WD7 (e.g., Grimm et al., 2001; Lu et al., 2004a) and therefore occurs more rapidly than CP-AMPAR accumulation in the NAc (see Figure 1). This indicates that different neuroadaptations must be responsible for expression of incubated craving at earlier withdrawal times. As mentioned above, prior work indicates that neuronal activity in the ventral mPFC and CeA is required for expression of incubated cocaine seeking after 3–4 weeks of withdrawal (Lu et al., 2005a, WD30; Lu et al., 2007, WD21; Koya et al. 2009, WD30). However, it is possible that incubation-related activity in these brain regions “kicks in” at even earlier withdrawal times, accounting for early phases of incubation. This still leaves the question of how these observations can be understood in the context of neurocircuitry. As discussed in Koya et al (2009), activation of ventral mPFC is predicted to inhibit rather than activate the CeA (Quirk et al., 2003), which is inconsistent with a simple model in which activity in both regions occurs in concert to mediate expression of incubation. If excitatory transmission in the NAc, particularly core, is ultimately critical for controlling the level of cue-induced cocaine seeking (Milton and Everitt, 2012), the next question is how activity in the ventral mPFC and CeA can be linked to activation of MSN in the NAc. The ventral mPFC sends glutamate projections to the NAc, which innervate both core and shell, although projections to shell are stronger (Berendse et al., 1992; Voorn et al., 2004). While the CeA does not directly project to the NAc, the activity of the CeA is regulated by the activity of the BLA, which does directly project to the NAc (Pitkänen et al., 1997). Furthermore, although enhanced cue-responsiveness of CeA but not BLA has been demonstrated on WD21-30 (Lu et al., 2005a; Lu et al., 2007), both regions show increased AMPAR subunit levels on WD1 and WD30 (Lu et al., 2005b), so an early activation of BLA cannot be ruled out. Based on these considerations, we propose that excitatory transmission in the NAc is increased through presynaptic mechanisms early in withdrawal, i.e., activation of mPFC and perhaps BLA leads to enhanced activity of their glutamate projections to the NAc. However, once CP-AMPARs accumulate in the NAc (~WD25-30), their stimulation is required for expression of incubation, i.e., expression of incubation now has a postsynaptic basis. It must be emphasized that this is quite speculative, and that there is a major gap in our understanding of the activity of glutamate afferents to the NAc during incubation.
Figure 1. Incubation of cue-induced cocaine craving begins prior to CP-AMPAR detection in the NAc.
A) Time-dependent increase in cue-induced responding over the first 2 months of withdrawal from cocaine self-administration (6 h/day for 10 days). Data are presented as mean (± SEM) number of non-reinforced responses on the active lever during a seeking test. During the test, lever pressing resulted in contingent presentations of a tone-light discrete cue that had been previously paired with cocaine infusions. *Different from withdrawal day 1 (p<0.01), n=9–11 rats per withdrawal period. Data are from Grimm et al. (2001). B) Changes in AMPAR-mediated synaptic transmission rectification index (RI) measured in NAc core MSNs following withdrawal from cocaine self-administration (6 h/day for 10 days). Compared to control rats that self-administered saline [n=17, pooled from withdrawal days (WD) 35–70], no apparent changes in RI were observed during the first two weeks of withdrawal (WD16, n=5). However, a significant increase in RI was observed after a month of withdrawal (WD30-35, n=7). The increased RI persisted through WD70 (WD35-45, n=10; WD45-55, n=13; WD55-70, n=7), the latest time-point measured. *P<0.01 vs. saline, Tukey post-hoc test after significant ANOVA. Data are from Wolf and Tseng (2012). Together, data in panels A and B indicate that adaptations apart from CP-AMPAR accumulation must be responsible for incubation of craving during the first several weeks of withdrawal. As discussed in the text, a withdrawal-dependent decrease in mGluR1 transmission in the NAc may help explain the delayed onset of CP-AMPAR accumulation (Section 3). The time-course of GluA1 serine 845 phosphorylation during incubation remains to be determined, although it is elevated in extrasynaptic membrane fractions on WD45 (Section 4). The time-course of increased BDNF protein levels in the NAc differs between core and shell subregions, but does not correlate well with CP-AMPAR accumulation (Section 5).
While the previous paragraph focused on forebrain circuits, adaptations in the VTA also contribute to incubation of cocaine craving. In particularly, neurotrophin transmission in the VTA plays a critical role (Lu et al., 2004a; Ghitza et al., 2010; Pickens et al., 2011). Lu and colleagues showed that chronically interfering with GDNF activity in the VTA over the first 14 days of withdrawal from long access cocaine self-administration blocked the development of incubated craving (Lu et al. 2009). Furthermore, a single microinjection of GDNF into the VTA on the last day of cocaine self-administration enhanced cue-induced cocaine seeking at early (WD3 and WD10) withdrawal time points (Lu et al., 2009). Similarly, a single injection of BDNF on the last day of self-administration enhanced cocaine seeking at both early (WD3 and WD10) and later (WD30) withdrawal time points (Lu et al., 2004c). These effects of exogenous GDNF and BDNF on cocaine seeking were both dependent on ERK activity in the VTA (Lu et al., 2004c; Lu et al., 2009). A progressive increase in BDNF levels in the VTA (elevated on WD30 and WD90 but not WD1) is also observed during withdrawal, which is similar to the time course of incubation of cue-induced cocaine seeking (Grimm et al., 2003). In addition, studies of NR1DATCreERT2 mice in which NMDARs are lacking in DA neurons have shown that NMDAR-dependent activation of DA neurons in the VTA is a prerequisite for robust cocaine seeking after prolonged withdrawal from long access cocaine self-administration and the associated synaptic incorporation of CP-AMPARs in the NAc (Mameli et al., 2009; see Section 2.2). Together, these findings suggest that cocaine-induced alterations in the VTA during early withdrawal are critical for the development of incubation and also modulate its magnitude. This is consistent with work in other models of addiction indicating a critical role for the VTA in initiating drug-induced plasticity and enabling subsequent adaptations in the NAc (Kalivas and Stewart, 1991; White and Kalivas, 1998; Wolf, 1998; Li et al., 1999; Vezina, 2004).
The next question that remains to be answered regarding CP-AMPAR accumulation is what underlying mechanisms would take so long to manifest. Hebbian plasticity does not seem a reasonable candidate, as it is hard to imagine a sudden change in excitatory transmission after weeks of withdrawal in the home cage. Homeostatic plasticity is a more reasonable candidate for mediating changes in AMPAR levels after cocaine exposure, as has been suggested previously (Boudreau and Wolf, 2005; Conrad et al., 2008; Wolf, 2010a; Huang et al., 2011). Considerable evidence indicates that cortical activity decreases during cocaine withdrawal (e.g. humans: Volkow et al., 1992; Goldstein and Volkow, 2002; primates: Beveridge et al., 2006; Porrino et al., 2007; rats: Hammer et al., 1993; Macey et al., 2004; Sun & Rebec, 2006). A resultant decrease in the activity of glutamate projections to the NAc could lead to a synaptic scaling-mediated increase in AMPAR levels on MSN (see Sun and Wolf, 2009 for discussion). However, while this seems reasonable for the CI-AMPAR upregulation that occurs during the first week of withdrawal from non-contingent cocaine injections (Wolf and Ferrario, 2010), the time-course of CP-AMPAR accumulation during incubation is considerably longer than that required to elicit known forms of homeostatic plasticity in NAc neurons (Ishikawa et al., 2009; Sun and Wolf, 2009). This could be explained in various ways. There could be a cascade of changes involving multiple brain regions, ultimately leading to a sufficient impact in the NAc. It is also possible that scaling first involves CI-AMPARS but that, as withdrawal progresses, CP-AMPARs are recruited in an attempt to further strengthen NAc synapses. Finally, it is possible that time-dependent morphological plasticity must occur to enable CP-AMPAR accumulation, raising the exciting possibility that the persistence of incubation involves the formation of novel synaptic connections in the NAc (see Wolf and Tseng, 2012 for more discussion).
Moving away from the circuit level, it is interesting to consider this problem from the standpoint of the biochemical state of NAc neurons. Since CP-AMPARs normally contribute only ~5–10% of evoked EPSC amplitude in NAc MSN of the adult rat (Conrad et al., 2008; Purgianto et al., 2013), there may be mechanisms operating in MSN to maintain these low levels. Perhaps the function of such mechanisms is altered during incubation – this could be part of or independent of homeostatic plasticity. Below we will consider several candidate mechanisms that may regulate CP-AMPAR plasticity in the NAc: mGluR1 mediated synaptic depression, phosphorylation of GluA1 at the PKA site, and time-dependent changes in BDNF levels.
3. mGluR1 negatively regulates CP-AMPAR transmission in the NAc of “incubated rats”
3.1 Group I mGluRs are linked to cocaine addiction
The group I mGluRs (mGluR1 and mGluR5) are postsynaptic receptors that couple to the Gq-like class of G-proteins. They are important in modulating neurotransmission and plasticity through their linkages with multiple signaling pathways as well as NMDA receptors (Lüscher and Huber, 2010). The NAc expresses mGluR1 and mGluR5 in similar abundance, mainly in extrasynaptic and perisynaptic regions (Testa et al., 1994; Mitrano and Smith, 2007; Mitrano et al., 2008; Mitrano et al., 2010). Interest in the role of group I mGluRs in drug addiction intensified after the report that mGluR5 knockout mice do not exhibit increased locomotor activity after cocaine injection nor learn to self-administer cocaine (Chiamulera et al., 2001). Subsequent studies demonstrated that mGluR5 negative allosteric modulators such as MPEP or the more selective agent MTEP prevented the development of cocaine conditioned place preference, reduced motivation to self-administer cocaine in progressive ratio experiments, and reduced reinstatement of cocaine seeking in animal models of relapse (Olive 2009, 2010). Although mGluR1 has been less thoroughly studied, several studies have reported similar “anti-addictive” effects in animal models after inhibiting mGluR1 transmission (Dravolina et al., 2006; Xie et al., 2010, 2012; Achat-Mendes et al., 2012; Yu et al., 2013). Together, these results suggest beneficial effects of decreasing group I mGluR activity. However, as we will discuss in Section 3.3, group I mGluR transmission is qualitatively altered during incubation, potentially shifting therapeutic strategies.
3.2 mGluR1 is a negative regulator of CP-AMPAR levels across brain regions
There are multiple forms of group I mGluR-dependent LTD (mGluR-LTD) and they have been implicated in many disease states, including fragile X syndrome and addiction (Gerdeman et al., 2003; Grueter et al., 2007; Lüscher and Huber, 2010). As we have reviewed recently (Loweth et al., 2013), when CP-AMPARs are present in synapses, stimulation of mGluR1 produces a form of mGluR-LTD that is mediated by CP-AMPAR removal. This form of plasticity has been well characterized at glutamatergic synapses on VTA DA neurons (Lüscher and Huber, 2010; Wolf and Tseng, 2012). Cocaine exposure in vivo leads to incorporation of CP-AMPARs into these synapses; once they are present, mGluR1 activation leads to CP-AMPAR removal coupled with insertion of lower conductance CI-AMPARs, resulting in a net decrease in synaptic strength (Bellone and Lüscher, 2005, 2006). This mGluR1-LTD requires activation of the phosphoinositide 3-kinase-Akt-mammalian target of Rapamycin (mTOR) pathway and is dependent on de novo protein synthesis of GluA2 (Mameli et al., 2007). A similar form of mGluR1-dependent LTD has been identified in cerebellar stellate cells, i.e., mGluR1 stimulation leads to removal of CP-AMPARs and their replacement with CI-AMPARs (Kelly et al., 2009). While this cerebellar mGluR1-LTD has also been shown to be protein synthesis-dependent (Kelly et al., 2009), it does not necessarily require de novo protein synthesis of GluA2; instead, it may involve lateral movement of CI-AMPARs from extrasynaptic to synaptic sites (Gardner et al., 2005). Finally, a similar form of mGluR1-LTD has been shown to occur in the lateral amygdala after fear conditioning leads to synaptic incorporation of CP-AMPARs (Clem and Huganir, 2010).
Based on these results in other brain regions, we wondered if mGluR1 might exert negative control over CP-AMPAR levels in the NAc of “incubated rats”. This is an exciting prospect because mGluRs, due to their potential to tune glutamate transmission up or down in disease states, have been of considerable interest as therapeutic targets (e.g., Olive 2009, 2010). Below, we discuss our recent studies investigating the role that mGluR1 plays in regulating CP-AMPAR mediated transmission in NAc synapses of “incubated rats.”
3.3 mGluR1 negatively regulates CP-AMPAR levels in the NAc and cue-induced cocaine craving
It is well established that MSN of the dorsal striatum and NAc exhibit an mGluR5-dependent, presynaptically-expressed form of LTD mediated by endocannabinoid release and CB1 receptor activation (Robbe et al., 2002; Lovinger, 2008). We have shown that, in the NAc of “incubated rats”, there is a profound shift in the mechanism of mGluR-LTD (McCutcheon et al., 2011b). After a withdrawal period sufficient for CP-AMPAR accumulation (>45 days), the presynaptically-expressed, mGluR5-dependent LTD normally observed in NAc MSNs is disabled. Instead, an mGluR1-dependent, postsynaptically-expressed form of mGluR-LTD emerges which is mediated by elimination of CP-AMPAR mediated transmission (McCutcheon et al., 2011b). Similar to what has been found in VTA DA neurons and cerebellar stellate cells (Section 3.2), mGluR1-mediated elimination of CP-AMPAR transmission is accompanied by enhancement of CI-AMPAR transmission (McCutcheon et al., 2011b). We will discuss the behavioral significance of the emergence of mGluR1-LTD in the next paragraph. However, it is interesting to note that the disabling of mGluR5-LTD may reflect postsynaptic uncoupling between mGluR5 and endocannabinoid signaling, since presynaptic CB1R signaling was intact in the NAc of “incubated rats” as revealed by the ability of the CB1R agonist WIN-55212-2 to attenuate EPSC amplitude. In fact, the magnitude of WIN-mediated synaptic inhibition was significantly greater in MSN from “incubated rats” compared to controls (McCutcheon et al., 2011b). This could suggest that uncoupling of mGluR5 led to decreased endocannabinoid tone, which in turn led to CB1R supersensitivity. More work will be required to understand the significance of altered CB1R signaling in the NAc during cocaine withdrawal.
Given that CP-AMPARs in the NAc mediate the expression of incubated cocaine craving and that acute mGluR1 activation removes CP-AMPARs from NAc synapses, we hypothesized that mGluR1 activation should reduce cue-induced craving in “incubated rats”. Our preliminary studies have supported this hypothesis by demonstrating that activation of mGluR1 in the NAc significantly reduces the expression of incubation (J.A. Loweth et al., abstract in Soc Neurosci Abstr 2012, 669.14). This is not necessarily at odds with the many studies indicating that mGluR1 or mGluR5 antagonists decrease cocaine-related behaviors (Olive, 2009; Section 3.1) because none of these prior studies were performed under the conditions (long access cocaine self-administration and prolonged withdrawal) that lead to CP-AMPAR accumulation. In other words, until CP-AMPARs accumulate, mGluR1 stimulation would not be expected to decrease cue-induced cocaine craving. Once they have accumulated, craving may be most effectively reduced by stimulating mGluR1 transmission rather than blocking it. Furthermore, although we have not tested this idea, the loss of mGluR5-mediated synaptic depression that we have observed in the NAc of “incubated rats” (McCutcheon et al., 2011b) may suggest that mGluR5 based pharmacotherapy will be less effective after incubation. In fact, Hao et al. (2010) found that mGluR5 antagonists were less effective at reducing cocaine self-administration on a progressive-ratio schedule of reinforcement in rats tested following long access cocaine self-administration compared to rats tested after a short access regimen. Future studies of mGluR5 function after incubation would help define potential therapeutic targets for maintaining abstinence after prolonged withdrawal.
Having shown that mGluR1 negatively regulates CP-AMPARs in the NAc of “incubated rats”, we hypothesized that a persistent decrease in mGluR1 signaling during cocaine withdrawal might contribute to synaptic accumulation of CP-AMPARs. We assessed this by measuring surface levels of NAc mGluR1 on withdrawal days that “bracketed” the increase in CP-AMPAR transmission as reflected by the rectification index (Wolf and Tseng, 2012). Our preliminary results suggest that mGluR1 surface expression in the NAc decreases just before CP-AMPARs are first detected (J.A. Loweth et al., abstract in Soc Neurosci Abstr 2012, 669.14). Together with prior results demonstrating that mGluR1 stimulation decreases CP-AMPAR transmission in MSNs from “incubated rats” (McCutcheon et al., 2011b), this suggests that mGluR1 tone in the NAc normally exerts a braking effect on CP-AMPAR accumulation and that this braking effect may be reduced during withdrawal, allowing CP-AMPAR levels to increase. Restoring mGluR1 tone during this critical period, using mGluR1 positive allosteric modulators (PAMs), prevents incubation and CP-AMPAR accumulation for ~3 days after the last PAM injection (J.A. Loweth et al., abstract in Soc Neurosci Abstr 2012, 669.14). These results are exciting because they suggest that abstinent addicts could use an mGluR1 PAM to avoid relapse when they anticipated exposure to cues that might elicit craving. Interestingly, CP-AMPAR levels in the NAc are also regulated by the level of tone at mGluR1 receptors in the VTA of cocaine-treated mice (Mameli et al., 2009), suggesting that mGluR1 PAMs could exert beneficial effects through actions in both the VTA and the NAc.
4. Role of GluA1 phosphorylation in CP-AMPAR accumulation
4.1 GluA1 phosphorylation sites
Phosphorylation of the GluA1 subunit is critical for regulating AMPAR channel function and trafficking during synaptic plasticity. GluA1 is phosphorylated on serine 845 by PKA, on serine 831 by Ca2+/calmodulin-dependent kinase II (CaMKII) and protein kinase C (PKC), and on serine 818 by PKC (Boehm et al., 2006; Derkach et al., 2007; Shepherd and Huganir, 2007). The possible relevance of serine 845 and serine 831 for CP-AMPAR plasticity during incubation will be considered in Sections 4.2 and 4.3, respectively. Section 4.2 will also touch on the potential role of transmembrane AMPA receptor regulatory proteins (TARPs), auxiliary AMPAR subunits which modulate AMPAR trafficking and conductance (Jackson and Nicoll, 2011). Serine 818 phosphorylation will not be considered here; although it is critical for activity-dependent AMPAR synaptic incorporation in cultured slices (Boehm et al., 2006), it has not been studied after cocaine exposure. In the sections that follow, we will mention results from other animal models or experimental systems when relevant to the incubation model. However, we are not aiming to comprehensively review all studies that have measured GluA1 phosphorylation after cocaine exposure.
4.2. Phosphorylation of GluA1 on serine 845 may prime extrasynaptic CP-AMPARs for synaptic insertion, contributing to their accumulation during incubation
Section 3 presented evidence that CP-AMPAR accumulation in the NAc during incubation results in part from impairment of an mGluR1 “braking mechanism” that normally restricts synaptic CP-AMPAR levels. A second contributing factor may involve altered regulation of GluA1 phosphorylation at serine 845. Phosphorylation at this residue increases channel open probability and promotes AMPAR insertion into extrasynaptic sites that supply the PSD (e.g., during LTP), whereas dephosphorylation of serine 845 is important for AMPAR endocytosis during LTD (Derkach et al., 2007; Shepherd and Huganir, 2007). Two lines of work in NAc MSNs support the hypothesis that increased serine 845 phosphorylation contributes to CP-AMPAR accumulation: 1) studies in primary cultures consisting of NAc neurons co-cultured with PFC neurons (NAc/PFC co-cultures; the PFC neurons provide excitatory inputs onto the NAc MSNs), and 2) subcellular fractionation studies in NAc tissue obtained from “incubated rats”.
Our studies in NAc/PFC co-cultures have shown that synaptic incorporation of GluA1-containing AMPARs occurs in two steps: 1) AMPARs from intracellular pools are inserted into extrasynaptic regions of the plasma membrane, and 2) extrasynaptic AMPARs are then translocated into the synapse in an NMDAR- and CaMKII-dependent manner. The first step (extrasynaptic insertion) can be accelerated by increasing PKA activity with a D1 receptor agonist or a PKA activator (SpcAMPS), presumably via phosphorylation of GluA1 on serine 845. This increases the size of the extrasynaptic AMPAR pool and thus facilitates AMPAR synaptic incorporation in response to subsequent NMDAR stimulation (Sun et al., 2008; Wolf, 2010b). Results in other brain regions similarly indicate that PKA phosphorylation of GluA1 primes AMPARs for synaptic insertion (Esteban et al., 2003; Sun et al., 2005; Oh et al., 2006; Gao et al., 2006; Man et al., 2007) and that extrasynaptic surface AMPARs are important for supplying the synapse (Passafaro et al., 2001; Sun et al., 2005; Gao et al., 2006; Yudowski et al., 2007; Heine et al., 2008; Yang et al., 2008; Lin et al., 2009; Makino and Malinow, 2009). In particular, perisynaptic CP-AMPARs are important for increasing synaptic strength during some forms of LTP (Guire et al., 2008; Yang et al., 2010).
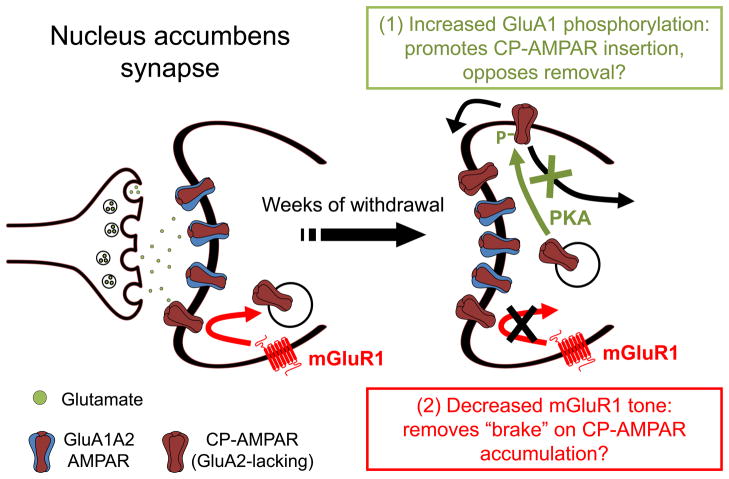
To determine if CP-AMPARs contribute to extrasynaptic AMPAR pools in the NAc of adult rats, we used subcellular fractionation to prepare a fraction enriched for extrasynaptic membranes. We then used an antibody recognizing both GluA2 and GluA3 (GluA2/3 antibody) to deplete the extrasynaptic membrane fraction of AMPARs that contained GluA2 or GluA3, leaving homomeric GluA1 receptors in the unbound fraction. In drug-naïve rats, this immunodepletion strategy revealed that ~35% of the GluA1 protein in the extrasynaptic membrane fraction was present in homomeric GluA1 receptors (Ferrario et al., 2011a). Compared to the 7% value obtained in a crude membrane fraction (Reimers et al., 2011), this suggests a substantial enrichment of homomeric GluA1 receptors in extrasynaptic membranes. When we then prepared extrasynaptic membranes from “incubated rats” and controls, we found that a greater portion of the extrasynaptic GluA1 was phosphorylated at serine 845 in the NAc of “incubated rats” (Ferrario et al., 2011b). Thus, through serine 845 phosphorylation, the extrasynaptic pool of CP-AMPARs may be primed for synaptic insertion, increasing the likelihood that they will enter the synapse. Serine 845 phosphorylation may also slow removal of CP-AMPARs from synapses, further promoting their synaptic accumulation. This idea is based on work in hippocampal neurons showing that serine 845 phosphorylation stabilizes perisynaptic GluA1-containing CP-AMPARs by preventing their degradation (He et al., 2009), whereas calcineurin, when anchored to A-kinase anchoring protein (AKAP), opposes PKA phosphorylation of GluA1 and thus limits synaptic levels of CP-AMPARs (Sanderson et al., 2012). AKAPs are scaffolding proteins that position PKA and calcineurin in proximity to GluA1 and other synaptic substrates, and thereby indirectly regulate AMPAR trafficking (Wong and Scott, 2004; Sanderson and Dell’Acqua, 2011). Together, these findings indicate that enhanced serine 845 phosphorylation of extrasynaptic GluA1 in the NAc of “incubated rats” may promote CP-AMPAR synaptic accumulation through multiple mechanisms. In combination with impairment of the mGluR1 mechanism that normally removes CP-AMPARs from NAc synapses, this could account for the persistent synaptic accumulation of CP-AMPARs that we have detected in electrophysiological studies on WD30-70 (Ferrario et al. 2011b; McCutcheon et al., 2011a,b; Wolf and Tseng, 2012; Purgianto et al. 2013). The proposed interaction between decreased mGluR1 tone and increased serine 845 phosphorylation is depicted in Figure 2.
Figure 2. Increased GluA1 phosphorylation and decreased mGluR1 tone may work in concert to enable CP-AMPAR accumulation in the NAc during withdrawal from long access cocaine self-administration.
Synaptic incorporation of GluA1-containing AMPARs in MSNs occurs in two steps: 1) AMPARs from intracellular pools are inserted into extrasynaptic regions of the plasma membrane, and 2) AMPARs are then translocated into the synapse in an NMDAR- and CaMKII-dependent manner. The synapse on the left depicts the situation in drug-naïve rats or in early withdrawal, when GluA2-containing AMPARs (mainly GluA1A2) are responsible for 90–95% of AMPAR transmission. After ~1 month of withdrawal, CP-AMPARs accumulate to the extent that they account for ~30% of AMPAR transmission. We propose that this results from two adaptations that work in concert: 1) Increased phosphorylation of GluA1 on serine 845, and 2) decreased mGluR1 surface expression. Increased phosphorylation of serine 845 is expected to accelerate insertion of GluA1-containing CP-AMPARs from intracellular pools into extrasynaptic portions of the plasma membrane, increasing the size of extrasynaptic AMPAR pools that supply the synapse and thereby increasing the likelihood that CP-AMPARs will enter the synapse. Phosphorylation of serine 845 may also stabilize CP-AMPARs by preventing their internalization and degradation. See Section 4 for more discussion and citations. As in other brain regions, mGluR1 negatively regulates synaptic levels of CP-AMPARs in the NAc. A withdrawal-dependent decrease in mGluR1 surface expression in the NAc, by removing this “braking effect”, may enable CP-AMPARs to accumulate. See Section 3 for more discussion and citations. PKA, protein kinase A.
As discussed in Section 2.4, the question remains as to why weeks of withdrawal are required for these processes to be set into motion and lead to a new equilibrium level of synaptic CP-AMPARs. The first step in answering this question is to determine the time course of underlying adaptations, as described for mGluR1 surface expression in Section 3.3. Serine 845 phosphorylation has been measured only on WD45 (Ferrario et al., 2011b). However, Lu et al. (2003) measured PKA enzymatic activity and adenylate cyclase activity at three time-points during incubation. They found that cocaine-exposed rats exhibited increased PKA enzymatic activity, relative to sucrose self-administering controls, on WD1 and WD30, but not WD90. Sucrose and cocaine groups did not differ in measures of adenylate cyclase activity, and sucrose controls did not differ from drug-naïve rats in either measure (Lu et al., 2003). Thus PKA activation occurs much earlier than the appearance of elevated CP-AMPAR levels (Figure 1). There are many ways to interpret this. For example, even if PKA activation leads to GluA1 phosphorylation as soon as cocaine self-administration is discontinued, this may not be sufficient to enable significant CP-AMPAR accumulation until mGluR1 surface expression decreases (~WD25; see Section 3). On the other hand, it is important to keep in mind that the level of serine 845 phosphorylation is a function of many factors besides PKA enzymatic activity, including activity of phosphatases such as calcineurin and the AKAP proteins that regulate the balance between GluA1 phosphorylation and dephosphorylation (see previous paragraph). It is possible that incubation is associated with time-dependent changes in calcineurin and AKAP function, as well as PKA activity, and that the sum of these changes helps explain delayed accumulation of CP-AMPARs. Determining the time-course of changes in serine 845 phosphorylation during incubation would help direct future investigations.
Interestingly, studies conducted during withdrawal from non-contingent cocaine injections suggest that cocaine-induced changes in both PKA and phosphatase activity are occurring in the NAc. Increases in PKA enzymatic activity were found on WD1 and WD7 but not WD21 after discontinuing cocaine injections (Terwilliger et al., 1991; Hope et al., 2005; but see Crawford et al., 2004). However, we obtained different results using an antibody that recognizes phosphorylated PKA substrates and thereby provides a read-out reflecting the sum of all processes that influence their phosphorylation state. This analysis showed that overall PKA substrate phosphorylation in the NAc of cocaine-sensitized rats increased gradually during withdrawal, from levels significantly less than saline controls on WD1 to significantly greater than controls on WD21 (Boudreau et al., 2009). Because PKA enzymatic activity was not altered on WD21 from a similar regimen (Hope et al. 2005), we suggested that a withdrawal-dependent decrease in phosphatase activity might account for increased PKA substrate phosphorylation in our study (Boudreau et al., 2009). In fact, calcineurin levels are decreased in the NAc after non-contingent cocaine treatment (Hu et al., 2005). AKAP signaling was not investigated in these studies. However, Reissner et al. (2011) found that intra-NAc injection of a peptide interfering with the AKAP-PKA interaction reduced GluA1 surface expression and impaired cocaine-primed reinstatement of cocaine seeking.
To summarize these results, phosphorylation of GluA1 at the PKA site (serine 845) is increased in an extrasynaptic membrane fraction obtained from the NAc of “incubated rats” (Ferrario et al., 2011b). Based on work in cultured NAc neurons (Sun et al., 2008) and other systems (He et al., 2009; Sanderson et al., 2012), we hypothesize that this primes CP-AMPARs for synaptic insertion and impairs their removal, contributing to their accumulation in the NAc synapses of “incubated rats”. An interesting parallel has been reported in the hippocampus, where the synaptic incorporation of GluA1-containing CP-AMPARs, 12 h after discontinuing repeated morphine exposure, is accompanied by an increase in PKA phosphorylation of GluA1 (Billa et al., 2010). Of course, PKA activation may contribute to cocaine-induced plasticity through substrates other than GluA1 and through mechanisms unrelated to CP-AMPAR accumulation, but a review of such possibilities is beyond the scope of this article.
While this section focused on GluA1 phosphorylation, interactions of CP-AMPARs with TARPs may also be important for AMPAR trafficking between synaptic and extrasynaptic pools during incubation. In the NAc of drug-naïve animals, the TARP γ4 is mainly found in an extrasynaptic membrane-enriched fraction, while γ2 (stargazin) is enriched in synaptic membranes (Ferrario et al., 2011a). Analysis of TARPs and GluA1 after long access cocaine self-administration (WD35-45) suggests that γ4 is associated with CP-AMPARs added to extrasynaptic membranes during incubation, whereas synaptic CP-AMPARs may be associated with γ2 (Ferrario et al., 2011b). More work is required to characterize the roles of these TARPs in normal synaptic transmission in the NAc as well as after cocaine exposure.
4.3 GluA1 phosphorylation on serine 831
CaMKII plays two roles in synaptic strengthening during LTP: 1) CAMKII-mediated phosphorylation of GluA1 on serine 831 leads to an increase in AMPAR conductance, and 2) CaMKII activity is required for the delivery of additional AMPARs to the synapse; in this case, the relevant substrate is not GluA1 itself but is probably a TARP (Lisman et al., 2012). The CaMKII-mediated regulation of AMPAR conductance was originally thought to be important only for homomeric GluA1 receptors, since the presence of edited GluA2 subunits in heteromeric receptors can abolish the ability of serine 831 phosphorylation to alter AMPAR conductance (Derkach, 2007). It was later shown that this effect of GluA2 is offset by the presence of certain TARPs (Kristensen et al., 2011; Derkach, 2011). Thus, phosphorylation of serine 831 has the potential to enhance the conductance of CI-AMPARs as well as CP-AMPARs. While GluA1 phosphorylation on serine 831 has not been assessed after incubation, we found increased activation of CaMKII (based on threonine 286 phosphorylation) in PSD fractions from the NAc of “incubated rats” killed on WD45 (Ferrario et al., 2011b). Since these rats had no “challenge”, this suggests an increase in basal CaMKII activation during prolonged withdrawal. This could simply be a consequence of increased Ca2+ entry through CP-AMPARs. Alternatively, CaMKII activation could play a causal role in maintaining increased synaptic strength during incubation, by promoting synaptic insertion of CP-AMPARs and/or by enhancing the conductance of synaptic AMPARs via serine 831 phosphorylation. In addition to the withdrawal-dependent increase in CaMKII activation observed during incubation, a rapid increase in CaMKII-dependent phosphorylation of serine 831 and GluA1 surface expression was observed during cocaine-primed reinstatement of cocaine seeking following short access cocaine self-administration and extinction training (Anderson et al., 2008; see Pierce and Wolf, 2013 for more discussion). It will be important for future studies to investigate the regulation of serine 831 phosphorylation during incubation.
5. Brain-derived neurotrophic factor: a potential regulator of CP-AMPAR accumulation during incubation?
5.1. BDNF regulates diverse types of plasticity, including CP-AMPAR plasticity
Brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, exerts its major biological functions through binding to tropomyosin receptor kinase B (TrkB). Activated receptors trigger a number of signal transduction cascades including the mitogen-activated protein kinase (MAPK), phosphoinositide 3-kinase (PI3-K) and phospholipase C-γ (PLC-γ) pathways (Reichardt, 2006). Through these pathways, BDNF is an important mediator of diverse types of synaptic plasticity. It exerts both presynaptic and postsynaptic effects, and contributes to both early-phase and late-phase long-term potentiation (LTP) (Bramham and Messaoudi, 2005; Waterhouse and Xu, 2009) and homeostatic plasticity (Rutherford et al., 1998). This section will focus on BDNF’s potential role in the incubation of cocaine craving, although it is important to point out that BDNF is important for many additional types of plasticity elicited by drugs of abuse (Russo et al., 2009; Ghitza et al., 2010; McGinty et al., 2010), some of which will be mentioned below.
We became interested in BDNF’s potential role in incubation for two reasons. First, as described below, BDNF levels increase in several brain regions during incubation (Grimm et al., 2003). Second, prior in vitro studies have demonstrated that BDNF can regulate the expression and synaptic delivery of AMPARs, especially CP-AMPARs. In cultured hippocampal neurons, application of BDNF increased the amount of GluA1 associated with the plasma membrane in a translation-dependent manner and facilitated synaptic delivery of homomeric GluA1 receptors into synapses in cultured hippocampal organotypic slices (Caldeira et al., 2007). Similarly, BDNF induced translocation of GluA1 to the postsynaptic membrane, an effect that was blocked by an inhibitor of exocytosis (Nakata and Nakamura, 2007). In pond turtle brainstem, bath application of BDNF increased synaptic delivery of GluA1 and GluA4 through a TrkB and ERK-mediated mechanism, reproducing cellular changes associated with a classical conditioning model (Li and Keifer, 2008, 2009). Moreover, mutant mice with lower BDNF levels showed reduced hippocampal expression of GluA1, but not GluA2 or GluA3 (Giralt et al., 2009). However, BDNF can also regulate GluA2 and GluA3 protein levels in cultured neurons (Narisawa-Saito et al., 1999, 2002; Caldeira et al., 2007).
It is well established that cocaine and other drugs of abuse can increase BDNF protein levels in the mesocorticolimbic system and that BDNF can modify addiction-related behaviors (McGinty et al., 2010). For example, elevation of BDNF levels in the NAc is associated with enhancement of many behavioral responses to cocaine, including locomotor sensitization, responding for conditioned reward, conditioned place preference, and cocaine-seeking after withdrawal (Horger et al., 1999; Grimm et al., 2003; Graham et al., 2007; Bahi et al., 2008), whereas decreasing BDNF-TrkB signaling in the NAc reduces cocaine conditioned place preference, self-administration and reinstatement (Graham et al., 2007; Bahi et al., 2008; Graham et al., 2009). BDNF was first linked to the incubation of cue-induced cocaine craving by the demonstration of time-dependent increases in BDNF levels in the rat NAc, as well as VTA and amygdala, which paralleled the development of incubation (Grimm et al., 2003). Given that CP-AMPAR accumulation in the NAc underlies incubation after prolonged withdrawal (Conrad et al., 2008) and that BDNF can promote synaptic delivery of CP-AMPARs (see previous paragraph), we hypothesized that elevated BDNF levels in the NAc may contribute to accumulation of CP-AMPARs in the incubation model.
5.2 BDNF can regulate CP-AMPAR levels in the NAc but is not responsible for CP-AMPAR accumulation during incubation
As a first step towards testing the hypothesis that BDNF mediates CP-AMPAR accumulation during incubation, we infused exogenous BDNF directly into the NAc core or shell of adult rats and analyzed AMPAR surface expression at post-injection times ranging from 30 min to 3 days. BDNF injection into the NAc core led to a protein synthesis- and ERK-dependent increase in cell surface GluA1 and a trend towards increased total GluA1 that was detected 30 min post-injection but not at longer time-points. GluA2 and GluA3 were not affected, indicating that BDNF increased surface expression of homomeric GluA1 CP-AMPARs. In contrast, surface GluA1 was not increased when BDNF was infused into the shell subregion of the NAc (Li and Wolf, 2011). These results led us to study the role of BDNF in the incubation of cue-induced cocaine craving over 3 months of withdrawal from the same regimen used for studies of CP-AMPARs, mGluR1-LTD, and PKA phosphorylation (Sections 2–4; 6 h/day for 10 days). Our results did not support the hypothesized causal role for elevated NAc BDNF in CP-AMPAR accumulation during incubation or in incubation itself, as will be discussed in turn below.
Two lines of evidence argue against the idea that elevated BDNF drives CP-AMPAR accumulation during withdrawal. First, immunohistochemical studies on WD45 and WD90, comparing saline controls to “incubated rats”, showed that BDNF elevation was detectable in the core on WD45 but was significantly more robust on WD90, whereas BDNF elevation in the shell was detected on WD90 but not on WD45 (Li et al., 2013). A prior immunoblotting study using whole NAc tissue revealed a trend towards increased BDNF protein on WD1 and a significant increase on WD30 in similarly treated cocaine rats compared to sucrose controls (Grimm et al., 2003). Together, these results leave open the possibility that BDNF levels in the NAc core increase in early withdrawal (between WD1 and WD30) and thus precede CP-AMPAR accumulation in the NAc core. However, CP-AMPAR accumulation in the core is stable from WD30-70 (Wolf and Tseng, 2012), a time when BDNF levels in the core are increasing dramatically (Li et al., 2013). In shell, BDNF levels are unchanged from saline controls on WD45 (Li et al., 2013), suggesting that the increase detected in whole NAc tissue on WD30 by Grimm et al. (2003) is likely attributable to increased BDNF in the core. Since elevated CP-AMPAR levels are detected in the shell on WD35-49 (McCutcheon et al., 2011a), BDNF elevation in the shell follows rather than precedes CP-AMPAR accumulation in the shell. Further arguing against a causal role for BDNF in CP-AMPAR accumulation, we found that long-term over-expression of BDNF in the NAc (in the absence of cocaine self-administration) was not sufficient to increase CP-AMPAR surface expression (Li et al., 2013), although the possibility remains that BDNF contributes to but is not sufficient for CP-AMPAR accumulation. An additional caveat is that viral-mediated BDNF over-expression does not reproduce the spatial or temporal pattern of the cocaine-induced elevation in endogenous BDNF during incubation.
To test the role of NAc BDNF in the incubation of cocaine craving per se (independent of the CP-AMPAR question), we used lentiviral vectors that expressed either TrkB siRNA or a truncated form of TrkB (TrkB.T1) that acts as a dominant negative. These viral vectors were injected into core or shell prior to cocaine self-administration training in order to produce persistent attenuation of BDNF-TrkB signaling in the NAc during withdrawal. Attenuation of BDNF-TrkB signaling in the core enhanced cue-induced cocaine seeking on WD1 compared to controls, whereas no effect was observed on WD30 or WD90. Attenuating BDNF-TrkB signaling in the shell did not affect cocaine seeking on WD1 or WD45 but significantly decreased cocaine seeking on WD90. These results suggest that levels of BDNF present in the NAc core on WD1 (before a significant elevation of BDNF is detected) suppress cocaine seeking in early withdrawal (WD1), whereas the late elevation of BDNF protein observed in the NAc shell of “incubated rats” promotes cocaine seeking in late withdrawal (WD90) (Li et al., 2013).
Overall, our results suggest that time-dependent increases in BDNF signaling do not cause incubation or trigger the accumulation of CP-AMPARs during incubation, although BDNF transmission in the shell may play a role in sustaining elevated cocaine craving after very prolonged withdrawal (Li et al., 2013). A role in long-term maintenance of cocaine craving is consistent with conclusions that can be derived from comparing incubation of cocaine seeking with incubation of sucrose seeking (Grimm et al., 2003; Lu et al., 2004a). Incubation of cocaine seeking is at peak levels between WD30 and WD90 and is accompanied by an elevation of NAc BDNF levels that persists at least through WD90. In contrast, incubation of sucrose seeking returns to basal levels by WD90 and is not accompanied by increased BDNF levels in the NAc (Grimm et al., 2003; Lu et al., 2004a).
An interesting aspect of our results pertains to core-shell differences. Thus, as described above, acute exogenous BDNF application increased CP-AMPAR levels in core but not shell (Li and Wolf, 2011), while endogenous BDNF levels in core and shell were elevated with different time-courses during incubation and had different consequences for cocaine seeking (Li et al., 2013). This is consistent with prior results showing that cocaine differently affects BDNF levels in core and shell subregions, and may explain some discrepancies in the literature regarding BDNF’s role in neuroadaptations in the NAc (see Li et al., 2013 for more discussion and McGinty et al., 2010 for a review of this literature).
5.3 A novel hypothesis regarding the relationship between BDNF and incubation of cocaine craving
As described in Section 5.2, our original hypothesis that increased BDNF levels in the NAc underlie CP-AMPAR accumulation during incubation was not supported. Therefore, we formulated a novel hypothesis for the relationship between BDNF and CP-AMPARs during cocaine withdrawal. This hypothesis is based on studies of ampakines, positive allosteric modulators of AMPAR transmission that slow deactivation and desensitization of AMPARs (Lynch and Gall, 2006). Ampakines increase BDNF protein levels in cultured hippocampal neurons as well as rat hippocampus in vivo (Lauterborn et al., 2000; 2003; Rex et al., 2006; Lauterborn et al., 2009). Thus, rather than proposing that increased BDNF triggers CP-AMPAR accumulation during incubation, we wondered if enhanced AMPAR transmission after prolonged withdrawal (due to accumulation of high conductance CP-AMPARs) might trigger the time-dependent increase in BDNF levels in the NAc. This would explain the observation that CP-AMAPRs are detected in the shell on WD35-49 (McCutcheon et al., 2011a) whereas BDNF levels are not yet increased in the shell on WD45 (only on WD90; Li et al., 2013). Furthermore, the more rapid increase in BDNF levels in core (detectable on WD45 and WD90) might explain the observation that nearly all core MSN recorded on WD35-49 exhibit inward rectification whereas more variability is observed in shell MSN (McCutcheon et al., 2011a).
Testing the hypothesis that CP-AMPAR transmission triggers elevation of BDNF during incubation would require blocking AMPAR transmission in the NAc of intact rats for weeks, a manipulation likely to produce numerous confounds. However, as proof of principle, we showed that an ampakine regimen known to elevate BDNF protein in the hippocampus (Rex et al., 2006) also elevated BDNF protein in the rat NAc (Li et al., 2013). This supports the possibility that CP-AMPAR transmission triggers BDNF elevation, although there are certainly many other ways to explain increased BDNF levels in the NAc during incubation. For example, it could result from anterograde transport from other brain regions such as PFC or VTA (although BDNF was not elevated in the PFC at a withdrawal time when it was elevated in the NAc core; Li et al., 2013). Regardless of why BDNF levels increase in the NAc during incubation, results summarized in Section 5.2 suggest that the role of NAc BDNF in incubation is quite complex. On the other hand, there is strong evidence that neurotrophin transmission in the VTA plays a critical role in the development of incubation (see Section 2.4), and a role for neurotrophins in the mPFC is possible based on results in other animal models of cocaine addiction (e.g., Berglind et al., 2007, 2009; Lu et al., 2010; Sadri-Vakili et al., 2010).
6. Conclusions
CP-AMPARs accumulate in NAc synapses after ~1 month of withdrawal from long access cocaine self-administration and mediate the expression of incubation of cue-induced cocaine craving (Conrad et al., 2008). While many questions remain about the neuroadaptations that underlie the delayed accumulation of CP-AMPARs, two mechanisms have been identified that are likely to play a significant role. First, mGluR1 exerts a negative regulatory effect on CP-AMPAR transmission that may decline in a withdrawal-dependent manner, enabling CP-AMPAR accumulation (McCutcheon et al., 2011b; J.A. Loweth et al., abstract in Soc Neurosci Abstr 2012, 669.14). Second, an increase in phosphorylation of extrasynaptic GluA1 on serine 845 (Ferrario et al., 2011b) may promote CP-AMPAR synaptic insertion and oppose CP-AMPAR removal. Through these mechanisms, insertion of CP-AMPARs may be enhanced at the same time as their removal is slowed (Figure 2). It will be important in the future to explore potential interactions between mGluR1 transmission and GluA1 phosphorylation (e.g., Oh et al., 2013). On the other hand, despite the fact that BDNF accumulates in the NAc during incubation (Grimm et al., 2003) and regulates cocaine seeking through actions in the NAc (e.g., Graham et al., 2007), the relationship of NAc BDNF to incubation turns out to be very complex, although a role in maintaining incubation after months of withdrawal has been supported (Li et al., 2013).
Due to our focus on the specific mechanisms mentioned above, many important issues have not been considered in this review. We have only touched on incubation-related adaptations that occur outside of the NAc (Pickens et al., 2011) and we have not addressed the role of CP-AMPAR transmission in the VTA in incubation (Mameli et al., 2009; see Wolf and Tseng, 2012 for more discussion). More broadly, we have focused on postsynaptic adaptations but not attempted to integrate this perspective with information on presynaptic and structural plasticity. Unfortunately, little is known about the latter topic, although changes in the activity of glutamate inputs to the NAc no doubt play a critical role in triggering postsynaptic AMPAR plasticity during incubation. Likewise, strong evidence implicates changes in homeostasis of extracellular glutamate levels in the NAc in regulating cocaine seeking (Kalivas, 2009), but gaps exist which make it difficult to integrate this literature with findings on excitatory synaptic transmission during incubation (Wolf, 2010a). With regard to structural plasticity, regimens leading to incubation, like many other types of cocaine exposure, increase spine density in the NAc (Ferrario et al., 2005). Are the CP-AMPARs added to the new spines, pre-existing spines, or both? If they are added to new spines, perhaps these spines form synapses with new presynaptic terminals that have sprouted during withdrawal. This would represent a very profound rewiring of NAc circuits that could contribute to the persistence of incubation.
An important goal of research on incubation is to understand mechanisms underlying the protracted risk of cue-induced relapse for cocaine users who have achieved abstinence. The incubation model is particularly relevant to heavy cocaine users who undergo a period of forced abstinence, e.g., due to incarceration or hospitalization (Reichel and Bevins, 2009). If the rat provides a reasonable model, the 30 days of abstinence required for CP-AMPAR accumulation corresponds reasonably well with the abstinence period that might be imposed by incarceration or hospitalization. Thus, incubation of cue-induced craving and CP-AMPAR accumulation may be relatively common in human users and contribute to the risk of relapse when they return to normal life and encounter cues related to cocaine use. By understanding mechanisms underlying incubation, we may uncover novel therapeutic strategies – for example, positive allosteric modulation of mGluR1 (Loweth et al., 2013) – to help recovering users maintain abstinence.
Highlights.
Incubation of cocaine craving is relevant to cue-induced relapse in abstinent users
CP-AMPARs in the NAc mediate expression of incubation after prolonged withdrawal
Decreased mGluR1 negative regulatory tone may promote CP-AMPAR accumulation
CP-AMPAR synaptic insertion may be enhanced by increased GluA1 phosphorylation
NAc BDNF does not mediate incubation but may contribute to its maintenance
Acknowledgments
This work was supported by National Institutes of Health grants DA009621 (M.E.W. and K.Y.T.), DA0015835 (M.E.W.), Senior Scientist Research and Mentorship Award DA029099 (M.E.W.) and postdoctoral National Research Service Award F32 DA030844 (J.A.L.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achat-Mendes C, Platt DM, Spealman RD. Antagonism of metabotropic glutamate 1 receptors attenuates behavioral effects of cocaine and methamphetamine in squirrel monkeys. J Pharmacol Exp Ther. 2012;343:214–224. doi: 10.1124/jpet.112.196295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–353. doi: 10.1038/nn2054. [DOI] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Dreyer JL. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned-place preference, and reinstatement in rats. Psychopharmacology (Berl) 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y, de Wit H. Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol Psychiatry. 2011;69:708–711. doi: 10.1016/j.biopsych.2010.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. mGluRs induce a long-term depression in the ventral tegmental area that involves a switch of the subunit composition of AMPA receptors. Eur J Neurosci. 2005;21:1280–1288. doi: 10.1111/j.1460-9568.2005.03979.x. [DOI] [PubMed] [Google Scholar]
- Bellone C, Lüscher C. Cocaine triggered AMPA receptor redistribution is reversed in vivo by mGluR-dependent long-term depression. Nat Neurosci. 2006;9:636–641. doi: 10.1038/nn1682. [DOI] [PubMed] [Google Scholar]
- Berendse HW, Galis-de Graaf Y, Groenewegen HJ. Topographical organization and relationship with ventral striatal compartments of prefrontal corticostriatal projections in the rat. J Comp Neurol. 1992;316:314–347. doi: 10.1002/cne.903160305. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur J Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. Eur J Neurosci. 2006;23:3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Billa SK, Liu J, Bjorklund NL, Sinha N, Fu Y, Shinnick-Gallagher Morón JA. Increased insertion of glutamate receptor 2-lacking α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptors at hippocampal synapses upon repeated morphine administration. Mol Pharmacol. 2010;77:874–883. doi: 10.1124/mol.109.060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME. Signaling pathway adaptations and novel protein kinase A substrates related to behavioral sensitization to cocaine. J Neurochem. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Milovanovic M, Conrad KL, Nelson C, Ferrario CR, Wolf ME. A protein cross-linking assay for measuring cell surface expression of glutamate receptor subunits in the rodent brain after in vivo treatments. Curr Protocols Neurosci. 2012;Chapter 5(Units 5.30):1–19. doi: 10.1002/0471142301.ns0530s59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: the synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, Carvalho AL, Esteban JA, Duarte CB. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy- 5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J Biol Chem. 2007;282:12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Clem RL, Huganir RL. Calcium-permeable AMPA receptor dynamics mediate fear memory erasure. Science. 2010;330:1108–1112. doi: 10.1126/science.1195298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford CA, Choi FY, Kohutek JL, Yoshida ST, McDougall SA. Changes in PKA activity and Gs alpha and Golf alpha levels after amphetamine- and cocaine-induced behavioral sensitization. Synapse. 2004;51:241–248. doi: 10.1002/syn.10301. [DOI] [PubMed] [Google Scholar]
- Cull-Candy S, Kelly L, Farrant M. Regulation of Ca2+-permeable AMPA receptors: synaptic plasticity and beyond. Curr Opin Neurobiol. 2006;16:288–297. doi: 10.1016/j.conb.2006.05.012. [DOI] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderlng TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat Rev Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Derkach V. Zooming in on AMPA receptor regulation by CaMKII. Nat Neurosci. 2011;14:674–675. doi: 10.1038/nn.2852. [DOI] [PubMed] [Google Scholar]
- Dravolina OA, Danysz W, Bespalov AY. Effects of group I metabotropic glutamate receptor antagonists on the behavioral sensitization to motor effects of cocaine in rats. Psychopharmacology. 2006;187:397–404. doi: 10.1007/s00213-006-0440-1. [DOI] [PubMed] [Google Scholar]
- Engblom D, Bilbao A, Sanchis-Segura C, Dahan L, Perreau-Lenz S, Balland B, Parkitna JR, Luján R, Halbout B, Mameli M, Parlato R, Sprengel R, Lüscher C, Schütz G, Spanagel R. Glutamate receptors on dopamine neurons control the persistence of cocaine seeking. Neuron. 2008;59:497–508. doi: 10.1016/j.neuron.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE. Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry. 2005;58:751–759. doi: 10.1016/j.biopsych.2005.04.046. [DOI] [PubMed] [Google Scholar]
- Ferrario CR, Li X, Wang X, Reimers JM, Uejima JL, Wolf ME. The role of glutamate receptor redistribution in locomotor sensitization to cocaine. Neuropsychopharmacology. 2010;35:818–833. doi: 10.1038/npp.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Wang X, Wolf ME. Distribution of AMPA receptor subunits and TARPs in synaptic and extrasynaptic membranes of the adult rat nucleus accumbens. Neurosci Lett. 2011a;490:180–184. doi: 10.1016/j.neulet.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Loweth JA, Milovanovic M, Ford KA, Galiñanes GL, Heng LJ, Tseng KY, Wolf ME. Alterations in AMPA receptor subunits and TARPs in the rat nucleus accumbens related to the formation of Ca2+-permeable AMPA receptors during the incubation of cocaine craving. Neuropharmacology. 2011b;61:1141–1151. doi: 10.1016/j.neuropharm.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao C, Sun X, Wolf ME. Activation of D1 dopamine receptors increases surface expression of AMPA receptors and facilitates their synaptic incorporation in cultured hippocampal neurons. J Neurochem. 2006;98:1664–1677. doi: 10.1111/j.1471-4159.2006.03999.x. [DOI] [PubMed] [Google Scholar]
- Gardner SM, Takamiya K, Xia J, Sug JG, Johnson R, Yu S, Huganir RL. Calcium-permeable AMPA receptor plasticity is mediated by subunit-specific interactions with PICK1 and NSF. Neuron. 2005;45:903–915. doi: 10.1016/j.neuron.2005.02.026. [DOI] [PubMed] [Google Scholar]
- Gawin FH, Kleber HD. Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry. 1986;43:107–113. doi: 10.1001/archpsyc.1986.01800020013003. [DOI] [PubMed] [Google Scholar]
- Gerdeman GL, Partridge JG, Lupica CR, Lovinger DM. It could be habit forming: drugs of abuse and striatal synaptic plasticity. Trends Neurosci. 2003;26:184–192. doi: 10.1016/S0166-2236(03)00065-1. [DOI] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA, Kalivas PW. Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron. 2013;77:867–872. doi: 10.1016/j.neuron.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, Rodrigo T, Martín ED, Gonzalez JR, Milà M, Ceña V, Dierssen M, Canals JM, Alberch J. Brain-derived neurotrophic factor modulates the severity of cognitive alterations induced by mutant huntingtin: involvement of phospholipaseCgamma activity and glutamate receptor expression. Neuroscience. 2009;158:1234–1250. doi: 10.1016/j.neuroscience.2008.11.024. [DOI] [PubMed] [Google Scholar]
- Ghasemzadeh MB, Vasudevan P, Mueller C, Seubert C, Mantsch JR. Region specific alterations in glutamate receptor expression and subcellular distribution following extinction of cocaine self-administration. Brain Research. 2009;1267:89–102. doi: 10.1016/j.brainres.2009.01.047. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Zhai H, Wu P, Airavaara M, Shaham Y, Lu L. Role of BDNF and GDNF in drug reward and relapse: a review. Neurosci Biobehav Rev. 2010;35:157–171. doi: 10.1016/j.neubiorev.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, Simmons D, Gent LM, Berton O, Bolanos CA, DiLeone RJ, Parada LF, Nestler EJ, Self DW. Tropomyosin-related kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol Psychiatry. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Hope BT, Wise RA, Shaham Y. Incubation of cocaine craving after withdrawal. Nature. 2001;412:141–142. doi: 10.1038/35084134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueter BA, McElligott ZA, Winder DG. Group I mGluRs and long-term depression: potential roles in addiction? Mol Neurobiol. 2007;36:232–244. doi: 10.1007/s12035-007-0037-7. [DOI] [PubMed] [Google Scholar]
- Grueter BA, Rothwell PE, Malenka RC. Integrating synaptic plasticity and striatal circuit function in addiction. Curr Opin Neurobiol. 2012;22:545–551. doi: 10.1016/j.conb.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guire ES, Oh MC, Soderling TR, Derkach VA. Recruitment of calcium-permeable AMPA receptors during synaptic potentiation is regulated by CaM-kinase I. J Neurosci. 2008;28:6000–6009. doi: 10.1523/JNEUROSCI.0384-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer RP, Jr, Pires WS, Markou A, Koob GF. Withdrawal following cocaine self-administration decreases regional cerebral metabolic rate in critical brain reward regions. Synapse. 1993;14:73–80. doi: 10.1002/syn.890140110. [DOI] [PubMed] [Google Scholar]
- Hao Y, Martin-Fardon R, Weiss F. Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: Factor in the transition to dependence. Biol Psychiatry. 2010;68:240–248. doi: 10.1016/j.biopsych.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He K, Song L, Cummings LW, Goldman J, Huganir RL, Lee HK. Stabilization of Ca2+-permeable AMPA receptors at perisynaptic sites by GluR1-S845 phosphorylation. Proc. Natl. Acad. Sci. USA. 2009;106:20033–20038. doi: 10.1073/pnas.0910338106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Béïque JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface motility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Abstinence from cocaine self-administration heightens neural encoding of goal-directed behaviors in the accumbens. Neuropsychopharmacology. 2005;30:1464–74. doi: 10.1038/sj.npp.1300748. [DOI] [PubMed] [Google Scholar]
- Hollander JA, Carelli RM. Cocaine-associated stimuli increase cocaine seeking and activate accumbens core neurons after abstinence. J Neurosci. 2007;27:3535–3539. doi: 10.1523/JNEUROSCI.3667-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hope BT, Crombag HS, Jedynak JP, Wise RA. Neuroadaptations of total levels of adenylate cyclase, protein kinase A, tyrosine hydroxylase, cdk5 and neurofilaments in the nucleus accumbens and ventral tegmental area do not correlate with expression of sensitized or tolerant locomotor responses to cocaine. J Neurochem. 2005;92:536–545. doi: 10.1111/j.1471-4159.2004.02891.x. [DOI] [PubMed] [Google Scholar]
- Horger BA, Lyasere CA, Berhow MT, Messer CJ, Nestler EJ, Taylor JR. Enhancement of locomotor activity and conditioned reward to cocaine by brain-derived neurotrophic factor. J Neurosci. 1999;19:4110–4122. doi: 10.1523/JNEUROSCI.19-10-04110.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X-T, Ford K, White FJ. Repeated cocaine administration decreases calcineurin (PP2B) but enhances DARPP-32 modulation of sodium currents in rat nucleus accumbens neurons. Neuropsychopharmacology. 2005;30:916–926. doi: 10.1038/sj.npp.1300654. [DOI] [PubMed] [Google Scholar]
- Huang YH, Schlüter OM, Dong Y. Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons. Behav Brain Res. 2011;216(1):9–18. doi: 10.1016/j.bbr.2010.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt WA, Barnett LW, Branch LG. Relapse rates in addiction programs. J Clin Psychol. 1971;27:455–456. doi: 10.1002/1097-4679(197110)27:4<455::aid-jclp2270270412>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Isaac JT, Ashby MC, McBain CJ. The role of the GluR2 subunit in AMPA receptor function and synaptic plasticity. Neuron. 2007;54:859–871. doi: 10.1016/j.neuron.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schlüter OM, Dong Y. Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons. J Neurosci. 2009;29:5820–5831. doi: 10.1523/JNEUROSCI.5703-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Nicoll RA. The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron. 2011;70:178–199. doi: 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16:223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kalivas PW. The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci. 2009;10:561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- Kelly L, Farrant M, Cull-Candy SG. Synaptic mGluR activation drives plasticity of calcium-permeable AMPA receptors. Nat Neurosci. 2009;12:593–601. doi: 10.1038/nn.2309. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56 (Suppl 1):177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knackstedt LA, Moussawi K, Lalumiere R, Schwendt M, Klugmann M, Kalivas PW. Extinction training after cocaine self-administration induces glutamatergic plasticity to inhibit cocaine seeking. J Neurosci. 2010;30:7984–7992. doi: 10.1523/JNEUROSCI.1244-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC. Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nat Neurosci. 2012;15:816–818. doi: 10.1038/nn.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen AS, Jenkins MA, Banke TG, Schousboe A, Makino Y, Johnson RC, Huganir R, Traynelis SF. Mechanism of Ca2+/calmodulin-dependent kinase II regulation of AMPA receptor gating. Nat Neurosci. 2011;14:727–735. doi: 10.1038/nn.2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Lynch G, Vanderklish P, Arai A, Gall CM. Positive modulation of AMPA receptors increases neurotrophic expression by hippocampal and cortical neurons. J Neurosci. 2000;20:8–21. doi: 10.1523/JNEUROSCI.20-01-00008.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Pineda E, Chen LY, Ramirez EA, Lynch G, Gall CM. Ampakines cause sustained increases in brain-derived neurotrophic factor signaling at excitatory synapses without changes in AMPA receptor subunit expression. Neuroscience. 2009;159:283–295. doi: 10.1016/j.neuroscience.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterborn JC, Truong GS, Baudry M, Bi X, Lynch G, Gall CM. Chronic elevation of brain-derived neurotrophic factor by ampakines. J Phamacol Exp Ther. 2003;307:297–305. doi: 10.1124/jpet.103.053694. [DOI] [PubMed] [Google Scholar]
- Lee H-K. Ca2+-permeable AMPA receptors in homeostatic synaptic plasticity. Front Mol Neurosci. 2012;5:17. doi: 10.3389/fnmol.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Keifer J. Coordinate action of pre- and postsynaptic brain-derived neurotrophic factor is required for AMPAR trafficking and acquisition of in vitro classical conditioning. Neuroscience. 2008;155:686–697. doi: 10.1016/j.neuroscience.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Keifer J. BDNF-induced synaptic delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK. Neurobiol Learn Mem. 2009;91:243–249. doi: 10.1016/j.nlm.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME. Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens. Eur J Neurosci. 2011;34:190–198. doi: 10.1111/j.1460-9568.2011.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Ford KA, Ferrario CR, Loweth JA, Wolf ME. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-Induced cocaine craving and its long-term maintenance. J Neurosci. 2013;33:1130–1142. doi: 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hu XT, Berney TG, Vartanian AJ, Stine C, Wolf ME, White FJ. Both glutamate receptor antagonists and prefrontal cortex lesions prevent the induction of cocaine sensitization and associated neuroadaptations. Synapse. 1999;34:169–180. doi: 10.1002/(SICI)1098-2396(19991201)34:3<169::AID-SYN1>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Lin D-T, Makino Y, Sharma K, Kayashi T, Neve R, Takamiya K, Huganir RL. Regulation of AMPA receptor extrasynaptic insertion by 4.1N, phosphorylation and palmitoylation. Nat Neurosci. 2009;12:879–887. doi: 10.1038/nn.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Yasuda R, Raghavachari S. Mechanisms of CaMKII action in long-term potentiation. Nat Rev Neurosci. 2012;13:169–182. doi: 10.1038/nrn3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SJ, Zukin RS. Ca2+-permeable AMPA receptors in synaptic plasticity and neuronal death. Trends Neurosci. 2007;30:126–134. doi: 10.1016/j.tins.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Lobo MK, Covington HE, III, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, Dietz DM, Zaman S, Koo JW, Kennedy PJ, Mouzon E, Mogri M, Neve RL, Deisseroth K, Han MH, Nestler EJ. Cell type–specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM. Presynaptic modulation by endocannabinoids. Handb Exp Pharmacol. 2008;184:435–477. doi: 10.1007/978-3-540-74805-2_14. [DOI] [PubMed] [Google Scholar]
- Loweth J, Tseng KY, Wolf ME. Using metabotropic glutamate receptors to modulate cocaine’s synaptic and behavioral effects: mGluR1 finds a niche. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.01.009. Epub Feb. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Cheng PL, Lim BK, Khoshnevisrad N, Poo MM. Elevated BDNF after cocaine withdrawal facilitates LTP in medial prefrontal cortex by suppressing GABA inhibition. Neuron. 2010;67:821–833. doi: 10.1016/j.neuron.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Shaham Y, Hope BT. Molecular neuroadaptations in the accumbens and ventral tegmental area during the first 90 days of forced abstinence from cocaine self-administration in rats. J Neurochem. 2003;85:1604–1613. doi: 10.1046/j.1471-4159.2003.01824.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Hope BT, Shaham Y. Incubation of cocaine craving after withdrawal: a review of preclinical data. Neuropharmacology. 2004a;47(Suppl 1):214–226. doi: 10.1016/j.neuropharm.2004.06.027. [DOI] [PubMed] [Google Scholar]
- Lu L, Grimm JW, Dempsey J, Shaham Y. Cocaine seeking over extended withdrawal periods in rats: different time courses of responding induced by cocaine cues versus cocaine priming over the first 6 months. Psychopharmacology. 2004b;176:101–108. doi: 10.1007/s00213-004-1860-4. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Shirley YL, Bossert JM, Shaham Y. A single infusion of brain-derived neurotrophic factor into the ventral tegmental area induces long-lasting potentiation of cocaine seeking after withdrawal. J Neurosci. 2004c;24:1604–1611. doi: 10.1523/JNEUROSCI.5124-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Hope BT, Dempsey J, Liu SY, Bossert JM, Shaham Y. Central amygdala ERK signaling pathway is critical to incubation of cocaine craving. Nat Neurosci. 2005a;8:212–219. doi: 10.1038/nn1383. [DOI] [PubMed] [Google Scholar]
- Lu L, Dempsey J, Shaham Y, Hope BT. Differential long-term neuroadaptations of glutamate receptors in the basolateral and central amygdala after withdrawal from cocaine self-administration in rats. J Neurochem. 2005b;94:161–168. doi: 10.1111/j.1471-4159.2005.03178.x. [DOI] [PubMed] [Google Scholar]
- Lu L, Uejima JL, Gray SM, Bossert JM, Shaham Y. Systemic and central amygdala injections of the mGluR(2/3) agonist LY379268 attenuate the expression of incubation of cocaine craving. Biol Psychiatry. 2007;61:591–598. doi: 10.1016/j.biopsych.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Lu L, Wang X, Wu P, Xu C, Zhao M, Morales M, Harvey BK, Hoffer BJ, Shaham Y. Role of ventral tegmental area glial cell line-derived neurotrophic factor in incubation of cocaine craving. Biol Psychiatry. 2009;66:137–145. doi: 10.1016/j.biopsych.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu XY, Ghasemzadeh MB, Kalivas PW. Expression of D1 receptor, D2 receptor, substance P and enkephalin messenger RNAs in the neurons projecting from the nucleus accumbens. Neurosci. 1998;82:767–780. doi: 10.1016/s0306-4522(97)00327-8. [DOI] [PubMed] [Google Scholar]
- Lynch G, Gall CM. Ampakines and the threefold path to cognitive enhancement. Trends Neurosci. 2006;29:554–562. doi: 10.1016/j.tins.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lüscher C, Huber KM. Group 1 mGluR-dependent synaptic long-term depression: mechanisms and implications for circuitry and disease. Neuron. 2010;65:445–459. doi: 10.1016/j.neuron.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey DJ, Rice WN, Freedland CS, Whitlow CT, Porrino LJ. Patterns of functional activity associated with cocaine self-administration in the rat change over time. Psychopharmacology. 2004;172:384–392. doi: 10.1007/s00213-003-1676-7. [DOI] [PubMed] [Google Scholar]
- Makino H, Malinow R. AMPA receptor incorporation into synapses during LTP: The role of lateral movement and exocytosis. Neuron. 2009;64:381–390. doi: 10.1016/j.neuron.2009.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mameli M, Balland B, Luján R, Lüscher C. Rapid synthesis and synaptic insertion of GluR2 for mGluR-LTD in the ventral tegmental area. Science. 2007;317:530–533. doi: 10.1126/science.1142365. [DOI] [PubMed] [Google Scholar]
- Mameli M, Halbout B, Creton C, Engblom D, Parkitna JR, Spanagel R, Lüscher C. Cocaine-evoked synaptic plasticity: persistence in the VTA triggers adaptations in the NAc. Nat Neurosci. 2009;12:1036–1041. doi: 10.1038/nn.2367. [DOI] [PubMed] [Google Scholar]
- Mameli M, Bellone C, Brown MT, Lüscher C. Cocaine inverts rules for synaptic plasticity of glutamate transmission in the ventral tegmental area. Nat Neurosci. 2011;14:414–416. doi: 10.1038/nn.2763. [DOI] [PubMed] [Google Scholar]
- Man HY, Sekine-Aizawa Y, Huganir RL. Regulation of {alpha}-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor trafficking through PKA phosphorylation of the Glu receptor 1 subunit. Proc Natl Acad Sci USA. 2007;104:3579–3584. doi: 10.1073/pnas.0611698104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Marinelli M. Age matters. Eur J Neurosci. 2009;29:997–1014. doi: 10.1111/j.1460-9568.2009.06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME, Marinelli M. Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J Neurosci. 2011a;31:5737–5743. doi: 10.1523/JNEUROSCI.0350-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Loweth JA, Ford KA, Marinelli M, Wolf ME, Tseng KY. Group I mGluR activation reverses cocaine-induced accumulation of calcium-permeable AMPA receptors in nucleus accumbens synapses via a protein kinase C-dependent mechanism. J Neurosci. 2011b;31:14536–14541. doi: 10.1523/JNEUROSCI.3625-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendelson JH, Mello NK. Management of cocaine abuse and dependence. The New England Journal of Medicine. 1996;334:965–972. doi: 10.1056/NEJM199604113341507. [DOI] [PubMed] [Google Scholar]
- Milton AL, Everitt BJ. The persistence of maladaptive memory: Addiction, drug memories and anti-relapse treatments. Neurosci Biobehav Rev. 2012;36:1119–1139. doi: 10.1016/j.neubiorev.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Mitrano DA, Arnold C, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the nucleus accumbens of cocaine-treated rats. Neuroscience. 2008;154:653–666. doi: 10.1016/j.neuroscience.2008.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Pare J-F, Smith Y. Ultrastructural relationships between cortical, thalamic, and amygdala glutamatergic inputs and group I metabotropic glutamate receptors in the rat accumbens. J Comp Neurol. 2010;518:1315–1329. doi: 10.1002/cne.22277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Nakata H, Nakamura S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007;581:2047–2054. doi: 10.1016/j.febslet.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Brain-derived neurotrophic factor regulates the expression of AMPA receptor proteins in neocortical neurons. Neuroscience. 1999;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Iwakura Y, Kawamura M, Araki K, Kozaki S, Takei N, Nawa H. Brain-derived neurotrophic factor regulates surface expression of alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors by enhancing the N-ethylmaleimide-sensitive factor/GluR2 interaction in developing neocortical neurons. J Biol Chem. 2002;277:40901–40910. doi: 10.1074/jbc.M202158200. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Oh JH, Yang JH, Ahn SM, Youn BH, Choi B-T, Wang JQ, Choe ES. Activation of protein kinase C is required for AMPA receptor GluR1 phosphorylation at serine 845 in the dorsal striatum following repeated cocaine administration. Psychopharmacol. 2013 doi: 10.1007/s00213-013-2968-1. [DOI] [PubMed] [Google Scholar]
- Olive MF. Metabotropic glutamate receptor ligands as potential therapeutics for addiction. Curr Drug Abuse Rev. 2009;2:83–98. doi: 10.2174/1874473710902010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olive MF. Cognitive effects of Group I metabotropic glutamate receptor ligands in the context of drug addiction. Eur J Pharmacol. 2010;639:47–58. doi: 10.1016/j.ejphar.2010.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortinski PI, Vassoler FM, Carlson GC, Pierce RC. Temporally dependent changes in cocaine-induced synaptic plasticity in the nucleus accumbens shell are reversed by D1-like dopamine receptor stimulation. Neuropsychopharmacology. 2012;37:1671–1682. doi: 10.1038/npp.2012.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Pitkänen A, Savander V, LeDoux JE. Organization of intra-amygdaloid circuitries in the rat: an emerging framework for understanding functions of the amygdala. Trends Neurosci. 1997;20:517–523. doi: 10.1016/s0166-2236(97)01125-9. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Wolf ME. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. In: Addiction RC, Pierce PJ, Kenny, editors. Cold Spring Harbor Perspectives in Medicine. 2. Vol. 3. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, New York: 2013. Feb 1, pp. 121–134.pp. a012021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrino LJ, Smith HR, Nader MA, Beveridge TJ. The effects of cocaine: a shifting target over the course of addiction. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1593–1600. doi: 10.1016/j.pnpbp.2007.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purgianto A, Scheyer AF, Loweth JA, Ford KA, Tseng KY, Wolf ME. Different adaptations in AMPA receptor transmission in the nucleus accumbens after short versus long access cocaine self-administration regimens. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.78. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361:1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichel CM, Bevins RA. Forced abstinence model of relapse to study pharmacological treatments of substance use disorder. Curr Drug Abuse Rev. 2009;2:184–194. doi: 10.2174/1874473710902020184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissner KJ, Uys JD, Schwacke JH, Comte-Walters S, Rutherford-Bethard JL, Dunn TE, Blumer JB, Schey KL, Kalivas PW. AKAP signaling in reinstated cocaine seeking revealed by iTRAQ proteomic analysis. J Neurosci. 2011;31:5648–5658. doi: 10.1523/JNEUROSCI.3452-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lauterborn J, Lin CY, Kramár EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbe D, Kopf M, Remaury A, Bockaert J, Manzoni OJ. Endogenous cannabinoids mediate long-term synaptic depression in the nucleus accumbens. Proc Natl Acad Sci. 2002;99:8384–8388. doi: 10.1073/pnas.122149199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56:73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Sadri-Vakili G, Kumaresan V, Schmidt HD, Famous KR, Chawla P, Vassoler FM, Overland RP, Xia E, Bass CE, Terwiliger EF, Pierce RC, Cha JH. Cocaine-induced chromatin remodeling increases brain-derived neurotrophic factor transcription in the rat medial prefrontal cortex, which alters the reinforcing effects of cocaine. J Neurosci. 2010;30:11735–11744. doi: 10.1523/JNEUROSCI.2328-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JL, Dell’Acqua ML. AKAP signaling complexes in regulation of excitatory synaptic plasticity. Neuroscientist. 2011;17:321–336. doi: 10.1177/1073858410384740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JL, Gorski JA, Gibson ES, Lam P, Freund RK, Chick WS, Dell’Acqua ML. AKAP150-anchored calcineurin regulates synaptic plasticitiy by limiting synaptic incorporation of Ca2+-permeable AMPA receptors. J Neurosci. 2012;32:15036–15052. doi: 10.1523/JNEUROSCI.3326-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann J, Yaka R. Prolonged withdrawal from repeated noncontingent cocaine exposure increases NMDA receptor expression and ERK activity in the nucleus accumbens. J Neurosci. 2009;29:6955–6963. doi: 10.1523/JNEUROSCI.1329-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacol. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Sun W, Rebec GV. Repeated cocaine self-administration alters processing of cocaine-related information in rat prefrontal cortex. J Neurosci. 2006;26:8004–8008. doi: 10.1523/JNEUROSCI.1413-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wolf ME. Nucleus accumbens neurons exhibit synaptic scaling that is occluded by repeated dopamine pre-exposure. Eur J Neurosci. 2009;30:539–550. doi: 10.1111/j.1460-9568.2009.06852.x. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwilliger RZ, Beitner-Johnson D, Sevarino KA, Crain SM, Nestler EJ. A general role for adaptations in G-proteins and the cyclic AMP system in mediating the chronic actions of morphine and cocaine on neuronal function. Brain Res. 1991;548:100–110. doi: 10.1016/0006-8993(91)91111-d. [DOI] [PubMed] [Google Scholar]
- Testa CM, Standaert DG, Young AB, Penney JB. Metabotropic glutamate receptor mRNA expression in the basal ganglia of the rat. J Neurosci. 1994;14:3005–3018. doi: 10.1523/JNEUROSCI.14-05-03005.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci Biobehav Rev. 2004;27:827–839. doi: 10.1016/j.neubiorev.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Voorn P, Vanderschuren LJ, Groenewegen HJ, Robbins TW, Pennartz CM. Putting a spin on the dorsal-ventral divide of the striatum. Trends Neurosci. 2004;27:468–474. doi: 10.1016/j.tins.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L. Long-term frontal brain metabolic changes in cocaine abusers. Synapse. 1992;11:184–190. doi: 10.1002/syn.890110303. [DOI] [PubMed] [Google Scholar]
- Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol Cell Neurosci. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug and Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda Triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010a;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME. Regulation of AMPA receptor trafficking in the nucleus accumbens by dopamine and cocaine. Neurotoxicol Res. 2010b;18:393–409. doi: 10.1007/s12640-010-9176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Ferrario CR. AMPA receptor plasticity in the nucleus accumbens after repeated exposure to cocaine. Neurosci Biobehav Rev. 2010;35:185–211. doi: 10.1016/j.neubiorev.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf ME, Tseng KY. Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how and why? Front. Mol Neurosci. 2012;5:72. doi: 10.3389/fnmol.2012.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong W, Scott JD. AKAP signalling complexes: focal points in space the time. Nat Rev Mol Cell Biol. 2004;5:959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- Xie X, Lasseter HC, Ramirez DR, Ponds KL, Wells AM, Fuchs RA. Subregion-specific role of glutamate receptors in the nucleus accumbens on drug context-induced reinstatement of cocaine-seeking behavior in rats. Addict Biol. 2012;17:287–299. doi: 10.1111/j.1369-1600.2011.00325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Ramirez DR, Lasseter HC, Fuchs RA. Effects of mGluR1 antagonism in the dorsal hippocampus on drug context-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology. 2010;208:1–11. doi: 10.1007/s00213-009-1700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Frerking M, Zhou Q. Delivery of AMPA receptors to perisynaptic sites precedes the full expression of long-term potentiation. Proc Natl Acad Sci USA. 2008;105:11388–11393. doi: 10.1073/pnas.0802978105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Wang XB, Zhou Q. Perisynaptic GluA2-lacking AMPA receptors control the reversibility of synaptic and spines modifications. Proc Natl Acad Sci USA. 2010;107:11999–12004. doi: 10.1073/pnas.0913004107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F, Zhong P, Xiu X, Sun D, Gao H-Q, Liu Q-S. Metabotropic glutamate receptor 1 (mGluR1) antagonism impairs cocaine-induced conditioned place preference via inhibition of protein synthesis. Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.29. Epub Jan. 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudowski GA, Puthenveedu MA, Leonoudakis D, Panicker S, Thorn KS, Beattie EC, von Zastrow M. Real-time imaging of discrete exocytic events mediating surface delivery of AMPA receptors. J Neurosci. 2007;27:11112–111121. doi: 10.1523/JNEUROSCI.2465-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]