Abstract
Dialysis remains the predominant form of renal replacement therapy in the U.S., but the optimal timing for the initiation of dialysis remains poorly defined. Not only clinical factors such as signs/symptoms of uremia, co-existing cardiovascular disease, and presence of diabetes, but also key demographic characteristics including age, gender, race/ethnicity and socioeconomics have all been considered as potential modifying factors in the decision for the timing of dialysis initiation. The 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease (CKD) suggests that dialysis be initiated when signs/symptoms attributable to kidney failure such as serositis, acid-base or electrolyte abnormalities, pruritus, poorly controlled volume status or blood pressure, deteriorating nutritional status despite dietary intervention, or cognitive impairment are visible or noted. These signs/symptoms typically occur when the glomerular filtration rate (GFR) is in the range of 5 to 10 ml/min/1.73 m2, although they may occur at higher levels of GFR. We review recent data on the timing of dialysis initiation, their implications for managing patients with late stage CKD, and the important role of considering key demographics in making patient-centered decisions for the timing of dialysis initiation.
Keywords: Timing dialysis initiation, race, ethnicity, demographics
Over the last 15 years there has been a consistent rise in the level of residual renal function at which patients with end-stage renal disease (ESRD) initiate renal replacement therapy (RRT) with dialysis. Early dialysis initiation (or early RRT) is typically defined as dialysis start at an estimated glomerular filtration rate (eGFR) ≥10 ml/min/1.73m2 using the 4 variable Modification of Diet in Renal Disease (MDRD) derived equation, while late dialysis initiation is defined as an eGFR <10 ml/min/1.73m2.(1) However, in different studies that will be described below the authors have used varying definitions of early versus late dialysis start. While in 1996 less than 20% of patients began dialysis therapy with an eGFR > 10 ml/min/1.73m2, by 2005 45% of dialysis patients began dialysis therapy with an eGFR > 10 ml/min/1.73m2.(2)
It has been posited that patients with advanced chronic kidney disease (CKD) who begin dialysis early may have lower morbidity and mortality compared with patients who begin dialysis at a more traditional or later dialysis initiation. This hypothesis is based on the premise that patients with decreased GFR are at an increased risk for malnutrition, and that malnourishment at the start of dialysis is associated with worse clinical outcomes.(3, 4) Thus, it has been suggested that early RRT could improve nutritional status, which could further improve clinical outcomes prompting the early initiation of dialytic therapy at higher levels of eGFR.
The perceived survival advantage of early start of dialysis has been questioned by a series of recent observational studies suggesting early RRT is not associated with a survival advantage and may be associated with an excess rate of adverse clinical outcomes.(5–10) Thus, several major renal organizations have reassessed the risks and benefits of the practice of initiating early RRT and the guidelines for initiating dialytic therapy for patients with advanced CKD. As American medicine moves toward patient-centered care, identifying demographic factors, such as gender, age, and race/ethnicity (often as a surrogate for sociocultural differences and economic status), which can have a significant influence on the relationship of timing of dialysis initiation and subsequent clinical outcomes has become essential. This review will explore not only recent data on the timing of dialysis initiation, but the implications of key demographics for managing patients with late stage CKD.
STUDIES OF THE LATE INITIATION OF DIALYSIS
Several studies have aimed to assess differences in clinical outcomes between the early and timely initiation of dialysis, but appear to focus more on the risk of late initiation of dialysis. In 2001 the Netherlands Cooperative Study on the Adequacy of Dialysis Study Group (NECOSAD) reported on 3-year survival rates for 253 patients with new ESRD of whom 94 (37%) started dialysis at GFR 4.9 ± 1.7ml/min/1.73 m2 and 159 started at GFR 7.1±2.4ml/min/1.73 m2. GFR was calculated as the mean of creatinine and urea clearance. There was a trend toward an increase in 3-year survival for the higher eGFR group compared to the lower eGFR group that did not reach statistical significance (adjusted mortality hazard ratio (HR) for lower eGFR starters compared with higher eGFR starters was 1.66; CI: 0.95–2.89).(11) Gender had no influence on outcomes, whereas increasing age, increased comorbidity and the presence of diabetes increased the risk for mortality associated with lower eGFR dialysis initiation. However this report compared late initiation of dialysis with very late initiation of dialysis, and did not address the early initiation of dialysis.
Likewise, Kim and colleagues examined 1-year mortality rates in 210 patients with classified eGFR >5 mL/min/1.73 m2 or <5 mL/min/1.73 m2 using MDRD eGFR. The authors found no difference in the mortality rate in the overall cohort based on residual renal function at initiation. They also found that among hemodialysis patients a lower eGFR was associated with a higher mortality rate than those with a higher eGFR, while among peritoneal dialysis patients there was no difference.(12) Again, this report compared the late initiation of dialysis with very late initiation of dialysis and did not truly address the early initiation of dialysis.
By contrast, an analysis of 271 Canadian patients followed by a nephrologist in the CKD clinic prior to initiating hemodialysis found that patients with a GFR >5 ml/min/1.73 m2 had a similar unadjusted 1-year mortality rate but greater 2-year mortality rates compared with the patients with GFR <5 ml/min/1.73 m2. (13) Patients in the higher GFR group were older, less employed, had more cardiac and peripheral vascular disease, and were more likely to be taking medication for hypertension. The nutritional status of this group was also higher, as expressed by a higher serum albumin than late dialysis initiators. After adjusting for these factors mortality rates did not differ between groups, suggesting demographics and premorbid health condition are important mediators of survival in the later initiation of dialysis.
INITIAL OBSERVATIONAL STUDIES QUESTIONING THE PROJECTED BENEFIT OF EARLY INITIATION OF DIALYSIS
A 2002 report found no benefit in survival in 685 study participants starting dialysis early (median creatinine clearance - CrCl - 10.4 ml/min) in comparison to 116 study participants starting dialysis late (median CrCl - 6.7 ml/min) after eliminating the effect of lead-time bias (occurring when survival is measured from start of dialysis rather than from a time point before dialysis, when patients have the same renal function).(14) Gender and age along with clinical factors and comorbidity such as presence of diabetes were independent predictors of mortality in their analysis. The authors found an 11% greater adjusted HR for mortality with each 1 ml/min increase in CrCl at start of dialysis (HR 1.11; 95% CI 1.01–1.21; P=0.024) which retained significance after adjustment for all confounders. They concluded that there was no benefit from the early initiation of dialysis.
Another study stratified the study population into 3 groups: a “low-risk” subgroup without diabetes, heart failure, or atherosclerotic heart disease; an older patient group ≥ 67 years; and a general population group including the rest of the patients older than 18 years, and compared 4 levels of eGFR at dialysis initiation within these 3 groups. The study found that adjusting for available comorbidity data did not fully explain the increased risk for death for patients initiating dialysis therapy earlier (GFR > 10 ml/min/1.73 m2) vs. very late (< 5 ml/min/1.73 m2). These findings suggest that early dialysis initiation may have an independent effect on mortality risk.(15)
Using propensity scores to reduce imbalances in distribution of variables across treatment groups, Beddhu et al. analyzed baseline data and outcomes from over 4000 patients in the United States Renal Data System (USRDS) Dialysis Morbidity Mortality Study Wave II (USRDS DMMS II). They reported that for each 5-ml/min increase in the MDRD derived GFR at the initiation of dialysis, mortality risk increased with HR= 1.27; P <0.001. There was no significant difference for calculated CrCl (0.98; P = 0.81) (16). In a multivariable logistic regression male sex, black race, Medicare insurance, as well as diabetes, coronary artery disease, congestive heart failure, cerebrovascular disease, increased hematocrit, serum bicarbonate, body mass index (BMI) and serum albumin were associated with higher MDRD eGFR at initiation of dialysis. This analysis reinforced the potential risk of the early initiation of dialysis and placed significant emphasis on the influence of the methodology of GFR calculation as an important mediator of patient classification and as an important confounder in assessing dialysis timing and mortality risk. The adverse effects of early initiation of dialysis may be due to falsely elevated eGFR, as assessed by the MDRD formula, likely related to reduced muscle mass and lowered endogenous creatinine production.
By contrast, a 2007 report found an increased all cause 1-year mortality rate among those initiating peritoneal dialysis later.(17) The authors compared 151 subjects who were electively initiated on peritoneal dialysis when GFR rate reached 10 ml/min/1.73 m2 or below (‘elective starters’ - 50.3% male, mean+/−SD age=57.7+/−13.9 years, 39.7% diabetic) with 82 subjects who declined dialysis when offered at the eGFR level (initial refusers). The data are difficult to interpret since the group was self selected and not randomized and the reasons for the refusal were not reported.
MORE RECENT OBSERVATIONAL STUDIES ASSESSING THE BENEFIT OF EARLY VERSUS USUAL INITIATION OF DIALYSIS
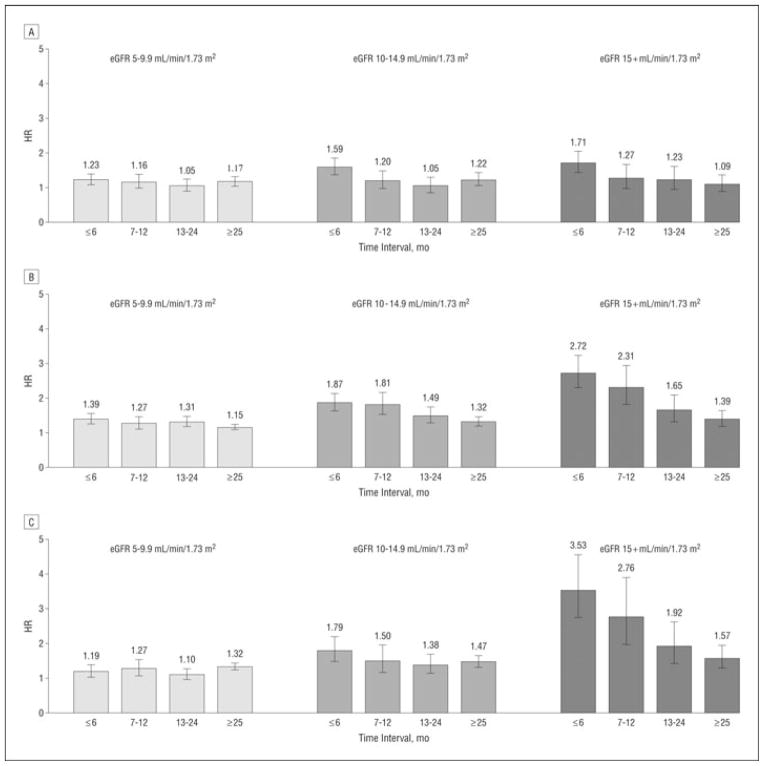
In 2011 a large observational study sought to examine the effect of early initiation of dialysis on survival in a ‘healthy’ group (albumin level ≥ 3.5 g/dL) of 81,176 subjects with ESRD. Subjects were aged 20–64 years, without diabetes, and with no comorbidity other than hypertension to minimize confounding issues that are highly prevalent in many of the studies examining the role of the early initiation of hemodialysis on survival. Overall the healthy group had a lower 1-year mortality (9.4%) when compared to the entire USRDS population (23.8%).(18) Within the healthy cohort, there was a graded increase in mortality rates with increased eGFR at dialysis initiation with mortality HRs of 1.27 (eGFR 5.0–9.9 ml/min/1.73 m2), 1.53 (eGFR 10.0–14.9 ml/min/1.73 m2), and 2.18 (eGFR ≥15.0 ml/min/1.73 m2) compared to the reference group (eGFR <5.0 ml/min/1.73 m2) (figure 1). Demographic factors such as increasing age, male sex, black race, had a negative effect while Asian race was protective on survival for the overall cohort. Higher levels of hemoglobin, later year of treatment, a BMI >25.0 primary etiology of polycystic kidney disease (PCKD) or glomerular disease had a positive effect on survival. The effects of demographics and clinical factors were similar in the healthy group to the USRDS population except for a survival advantage noted for black race. These findings suggest that in relatively healthy patients with advanced CKD the early start of dialysis may be harmful.
Figure 1. The mortality hazard ratios (HRs) using an extended Cox model with time-dependent effects of estimated glomerular filtration rates (eGFRs) and stratified by level of serum albumin.
Models were run separately for each of 3 serum albumin groups as surrogates of general health: less than 2.5 g/dL, 2.5 to 3.49 g/dL, and 3.5 g/dL or higher. Panel A: Mortality HRs for patients with predialysis serum albumin level of less than 2.5 g/dL (n=12,040). The confidence intervals for the HRs include 1 in many instances and the effect of early start, an eGFR higher than 10 mL/min /1.73 m2, is not as marked as in the higher albumin level groups. Panel B: Mortality HRs for patients with predialysis serum albumin levels of 2.5 to 3.49 g/dL (n=33,471). In this healthier, higher serum albumin group, greater initial eGFR is associated with a significant adverse effect on survival, especially with early starts, the group with an eGFR higher than 10 mL/min /1.73 m2. Panel C: Mortality HRs for patients with predialysis serum albumin levels of 3.5 g/dL or higher (n=35 665). In this healthiest of the healthy cohort studied (HG), the adverse effects of early start on survival are seen, especially in the group with an eGFR of 15 mL/min /1.73 m2 or higher. Error bars indicate 95% confidence intervals. To convert serum albumin to grams per liter, multiply by 10.(18)
Another adjusted retrospective analysis compared 99,231 patients with dialysis initiated at eGFR >15 ml/min/1.73 m2 with 113,510 with a dialysis initiated at eGFR ≤5 ml/min/1.73 m2 in patients entering USRDS from 1995 to 2006. It showed an incremental increase in mortality associated with earlier dialysis start, arguing against aggressive early dialysis initiation based primarily on eGFR alone.(5) Additionally, a study that examined mortality rates across quintiles of eGFR at hemodialysis initiation in over 25,000 patients found higher mortality rates among those initiating dialysis earlier, but that the excess mortality rate was largely explained by demographic and clinical conditions at dialysis.(6) Lassalle et al. found similar results in over 11,000 dialysis patients with age and patient condition strongly determining the decision to start dialysis and explaining much of the inverse association between eGFR at dialysis initiation and survival.(19)
A recent meta-analysis (15 studies; 1,285,747 patients) found the earlier start of dialysis (higher eGFR) was associated with increased risk of mortality (OR = 1.33, p < 0.001) and this was due in part to early starters being older (by 6.6 years), and more likely to have diabetes mellitus and severe comorbid diseases.(20) Moreover, another study also found a higher GFR at dialysis therapy initiation was associated with a higher pooled adjusted HR for all-cause mortality. Higher GFR in their study was associated with a lower mortality risk in cohorts that calculated GFR from average of creatinine and urea clearance from 24-hour urine collection (HR, 0.80; P = 0.003) in contrast to cohorts that estimated GFR by MDRD equation (HR, 1.04; P < 0.001) emphasizing again the importance of the method of determination of GFR. In this study, same as in the study of Kim et al (12) the authors reported a significant heterogeneity according to the type of dialysis with the highest mortality risk observed in hemodialysis cohorts and no association between GFR and mortality in peritoneal dialysis cohorts.(21)
A possible effect of demographic factors affecting dialysis mortality is reported in a study comparing survival and dialysis initiation among dialysis patients in British Columbia and Scotland.(8) A more recent analysis of over 25,000 Canadian adults compared risk of death among those with advanced CKD who initiated hemodialysis earlier (mean GFR 15.5 mL/min per 1.73 m2) and later (mean GFR 7.1 mL/min per 1.73 m2). The unadjusted HR for mortality with earlier relative to later initiation was 1.48 (95% CI 1.43–1.54), and remained significantly increased (aHR: 1.18, 95% CI 1.13–1.23) after adjustment for demographic characteristics, serum albumin, primary cause of ESRD, vascular access type, comorbidities, late referral and transplant status.(7) Similar to large observational studies in the U.S., in this Canadian study, initiation of dialysis with a higher eGFR was associated with an increased risk of death that was partially, but not fully explained by differences in baseline characteristics.
In summary numerous observational trials suggest that the early initiation of dialysis based on eGFR alone is unlikely to be associated with improved survival and should be limited to high-risk and symptomatic patients. Several factors such as lead-time bias, survivor bias, estimating GFR from serum creatinine in people with low muscle mass, fluid overload and/or comorbidities may influence interpretations of findings. In addition, the imprecision of renal function measurements and marked clinical variation in disease progression and complications, reinforces the need to pay close attention to patients with advanced CKD for the early onset of uremic signs/symptoms, which vary by patient and eGFR value. The role of age, race/ethnicity and gender remain to be defined as they may be surrogates for comorbid conditions, access to care, quality of care and socio-cultural factors which may influence late stage CKD and ESRD outcomes.
The Initiating Dialysis Early and Late (IDEAL) TRIAL
After a series of early reports and commentaries suggesting that early initiation of dialysis may be beneficial, more recently the preponderance of observational studies suggest that starting dialysis early may not be beneficial and in fact may be harmful. However, as noted above, many of these studies are limited by numerous biases such as method of GFR measurement, patient selection and referral time, lead-time bias, comorbidities assessed, prevailing practice guidelines, and differences in definition of early initiation of dialysis. Thus a randomized, controlled trial was performed to more firmly establish the optimal timing for the initiation of dialysis.
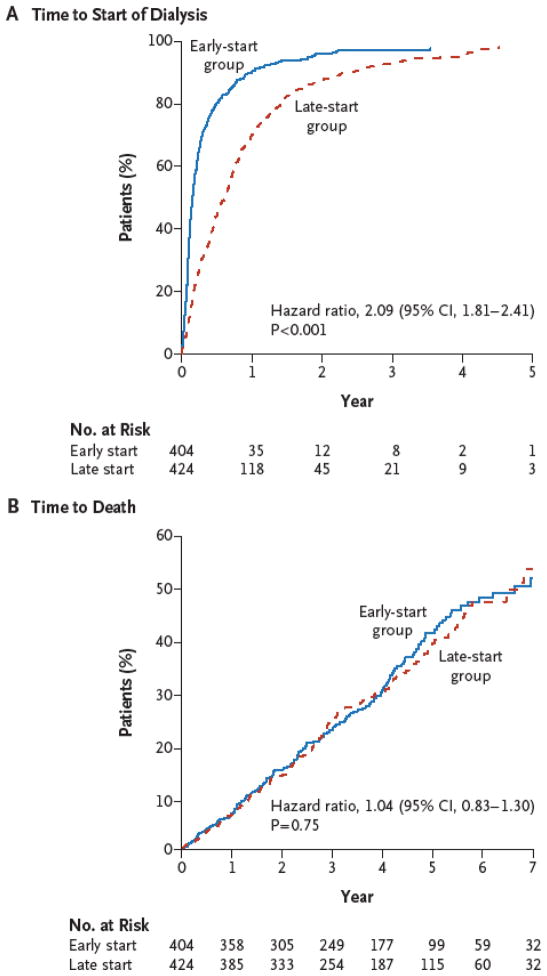
The IDEAL trial examined 828 patients with advanced CKD assigned to initiate dialysis at an eGFR of 10.0 to 14.0 ml/min/1.73 m2 (early start) or 5.0 to 7.0 ml/min/1.73 m2 (late start).(22) GFR assessment was based on the Cockcroft–Gault equation corrected for body surface area and a post hoc analysis of survival with baseline GFR estimated according to the MDRD equation revealed no significant between-group differences in outcomes based on GFR methodology. During a median follow-up period of 3.6 years the authors found no significant difference between the groups in mortality or the frequency of key adverse events (cardiovascular events, infections, or complications of dialysis), figure 2.
Figure 2. Kaplan–Meier Curves for Time to the Initiation of Dialysis and for Time to Death.
The data for time to the initiation of dialysis (Panel A) were censored at the time of death, transplantation, or withdrawal of consent or at the time a patient transferred to a nonparticipating hospital, emigrated, or could not be contacted. The curves for time to death (Panel B) are truncated at 7 years of follow-up and a cumulative hazard ratio of 60%.(22)
It should be noted however that 19% of early starters actually started late and 76% of the patients assigned to the late-start group initiated dialysis when the estimated GFR was above the target of 7.0 ml/min/1.73 m2, owing to the development of symptoms yielding a final difference of only 2.2 ml/min/1.73 m2 between the early and late groups. Thus, while the planned early initiation of dialysis in patients with advanced CKD was not associated with an improvement in survival or clinical outcomes, the planned timely or later initiation of dialysis (eGFR 5.0–7.0 ml/min/1.73 m2) was associated with a substantial greater percentage of patients developing uremic symptoms. The authors concluded that with careful clinical management, dialysis may be delayed until either the GFR drops below 7.0 ml/min/1.73 m2 or more traditional clinical indicators for the initiation of dialysis are present.
A subset (642 of the 828) of patients in the IDEAL Trial, also agreed to participate in a quality of life and cost-effectiveness study. While mean direct dialysis costs were significantly higher in the early-start group, total costs, including costs for resources used to manage adverse events did not differ.(23) Quality-adjusted survival between groups also did not differ. These findings suggest planned early initiation of dialysis therapy in patients with progressive CKD has higher dialysis costs with similar overall medical costs, but no difference in quality of life. This prompted the authors to question the benefit of initiating early dialysis given the paucity of existing clinical evidence to support a substantive impact on patients’ outcomes and dialysis resources in the absence of clinical symptoms related to reduced renal function.
Race, Demographics and the Timing of Initiating Dialysis
Because the relationship of onset of uremic signs/symptoms (a driving factor for dialysis initiation) and eGFR value vary by patient, making an “a priori” judgment for early or late dialysis is extremely difficult. Having an insight into key patient level characteristics that may help identify patients at greater or lesser risk for adverse outcomes should dialysis be initiated earlier can assist in that judgment process.
Historically, women, older patients, Hispanics and Asians (vs. Caucasians), and uninsured patients (vs. private insurance) have been more likely to be started on dialysis later.(24–27) This finding may further bias clinician recommendations to assign these patients to later dialysis start. Therefore, it may be necessary to pay additional attention to early uremic symptoms indicating the need for dialysis initiation. In addition, the presence of concomitant medical conditions such as diabetes, cardio-vascular diseases, and poor functional status are associated earlier start of dialysis and could be more frequent in US nonwhites or in socio-economic groups associated with later dialysis start.(19, 24–26, 28) The effect of timing of initiation of dialysis on mortality in blacks and Asians is a result of the combination of these factors. This is well illustrated in the study of Rosansky et al in which blacks had a 10% increase in mortality compared with other patients after adjustment for all available confounders. However, in a subgroup of patients with good nutritional status (albumin level>3.5g/L), blacks had 8% lower mortality than the rest of the group.(18)
Race and Early vs. Late Dialysis Initiation
The IDEAL trial that showed no survival benefit from early initiation was conducted in Australia. It included a 28.5% non-white population of the 828 patients, but did not include Hispanics nor blacks.(29) Beddhu’s study showed that higher eGFR was associated with higher death risk and included 2,920 US dialysis patients of which 28% were blacks and 8% were listed as ‘other’ but minorities were not studied separately.(16) The only study to include a Hispanic ethnicity population concluded that higher eGFR was associated with higher death risk adjusted for Hispanic ethnicity but did not report racial/ethnic subgroup analyses.(15) Two studies in Asian populations included patients from Japan and Taiwan, possibly dissimilar in regards to comorbid conditions and nutritional status compared to Asian-American patients.(6, 30) Two studies in U.S. dialysis patients included analyses of a larger percentage of non-white populations, but only demonstrated that the likelihood of early vs. late dialysis start were higher in non-white subgroups as compared to whites and did not report race and ethnicity subgroup survival analyses, nor did they report on Hispanic populations.(5, 18)
Racial Differences in eGFR at Initiation of Dialysis and in Dialysis Outcomes
African American CKD patients, due to the race adjusting factor in eGFR calculations, have a higher eGFR for the same creatinine level and therefore may be started on dialysis with some delay, at a higher creatinine level.(24) The clinical implication of the standard eGFR adjustment by race at very low eGFR is not well known. By contrast, poor quality of predialysis care and presence of comorbid conditions may lead to some African Americans being placed on dialysis earlier.(31–33) Since many of the factors associated with the differences in timing and mortality in racial/ethnic populations are not clearly identified, race/ethnicity specific propensity scores and other modeling strategies may be necessary to obtain less biased estimates and enhance our understanding of observed variations in dialysis timing and ESRD outcomes across different patient populations. Anemia is a co-morbid condition that has no racial ethnic differences in the association with either mortality or timing of initiation of dialysis. However, the high prevalence of hemoglobin variants and other hematologic factors in African Americans could affect the need and use of erythropoietin stimulating agents (ESA).(34) Consequently patients with hemoglobin variants will receive excessive doses of ESA, which in turn may result in a health risk itself.(34, 35)
Racial differences in loss of residual renal function
It is not known if the rate of loss of residual renal function differs by race/ethnicity. Holley et al(36) reported that race does not affect the rate of loss of renal function but the study included only 25 non-white patients. In addition to race/ethnicity and gender(37, 38), future studies should include as many key factors that may affect the rate of loss of residual renal function such as the type of renal replacement(39, 40), BMI(38, 41), arterial pressure (41–44), use of angiotensin converting enzyme inhibitors/angiotensin receptor blockers(37, 45, 46), diuresis (41, 47–49), protein intake(42, 50, 51), diabetes(37, 38, 50, 52–55), cardiovascular comorbidity(37, 38, 52, 54, 56), and others. Additional studies should also be pursued to determine the potential impact of reduced access to care and whether late initiation of dialysis results in adverse clinical and economic outcomes among different ESRD patient groups in the United States.
The Way Forward
Emerging data highlight the need to focus on not only the level of GFR, but the assessment of signs/symptoms as part of monitoring of patients with advanced CKD (eGFR 15–30 ml/min/1.73 m2). Individual assessment and availability of resources will dictate specific timing of therapies. Following the results of the IDEAL trial, a working group of the European Renal Best Practice advisory board released an updated position statement on dialysis initiation.(57) In summary, the board recommends patients with advanced CKD be prepared for dialysis, kidney transplant or conservative care before their CKD becomes symptomatic (eGFR > 15 ml/min/1.73 m2) and dialysis be considered when there are signs/symptoms of uremia, inability to control hydration status or blood pressure or a progressive deterioration in nutritional status, usually noted at an eGFR in the range 9–6 ml/min/1.73 m2 (Table 1). The new Kidney Disease: Improving Global Outcomes (KDIGO) guidelines were very careful to highlight the need to weigh the risks and benefits of RRT and recommended that clinicians: 1) consider living donor preemptive renal transplantation in adults when eGFR is <20 ml/min/1.73 m2, and 2) address clinical signs/symptoms rather than instituting dialysis therapy based on an arbitrary number (e.g. eGFR, CrCl) representing the estimated degree of residual renal function.(58)
Table 1.
Adapted from the European Renal Best Practice advisory board 2011 updated position statement on initiation of Dialysis.(57)
| Recommendation | Evidence |
|---|---|
| Dialysis preparation in patients with advanced CKD should include preparing for dialysis, kidney transplant or conservative care before their CKD becomes symptomatic. This process, including advanced preparation of appropriate access, careful observation for signs and symptoms of uremia, should be started while eGFR is >15 mL/min/1.73m2. Supervision in a dedicated clinic for advanced CKD patients is recommended. | Strong recommendation based on low-quality evidence |
| Dialysis initiation in patients with eGFR <15 mL/min/1.73m2 should be considered when there are signs/symptoms of uremia, inability to control hydration status or blood pressure and/or a progressive deterioration in nutritional status. It should be noted that the majority of patients will become symptomatic and require dialysis as eGFR falls to 9–6 mL/min/1.73m2. | Strong recommendation based on high-quality evidence |
| High-risk patients such as those with diabetes and renal function deteriorating more rapidly than 4 mL/min/year require particularly close supervision. In patients whose uremic symptoms may be difficult to detect and/or close supervision is not feasible a planned start to dialysis while still asymptomatic may be preferred. | Strong recommendation based on low-quality evidence |
| Asymptomatic patients presenting with advanced CKD may benefit from a delay in starting dialysis in order to allow preparation, planning and permanent access creation rather than using temporary access. | Weak recommendation based on low-quality evidence |
Chronic kidney disease – CKD; estimated glomerular filtration rate - eGFR
Slinin et al. reported a graded and progressively lower adjusted first-year mortality rate among 192,307 incident hemodialysis patients meeting a greater number of three key National Kidney Foundation Kidney Disease Outcomes Quality Initiative (KDOQI) guideline goals at dialysis initiation including use of arteriovenous fistula or graft at initiation, hemoglobin >11 g/dl; and serum albumin at goal. Unfortunately, 59% of patients met zero goals and 31% met only one goal.(59) Thus, their analysis suggests there remains a great opportunity for improving first-year mortality rate by ensuring more patients with advanced CKD meet one or more of the above KDOQI dialysis initiation goals.
In summary there appears to be no benefit of the early initiation of dialytic therapy (eGFR 10–15 ml/min/1.73 m2) in asymptomatic patients with advanced CKD. By contrast the late initiation of dialysis (eGFR is <5 ml/min/1.73 m2) may increase the risk for decreased survival over time and may increase the risk of developing adverse clinical signs and/or symptoms related to advanced CKD. As noted in the IDEAL Trial, patients starting dialysis at a later time were more likely to have uremic like symptoms at dialysis initiation. Clinicians should be aware of the impact of proper timing of dialysis start on quality of life. Also, it is important to achieve a hemoglobin >11 g/dl to have serum albumin at goal and to have arteriovenous fistula or graft placed prior to dialysis initiation.(59) In view of the low prevalence of achieving these goals, particularity in minorities, high priority should be given to their implementation. In absence of uremic signs/symptoms dialysis should be initiated at an eGFR of 6–9 ml/min/1.73 m2 In US non-whites, timing of dialysis initiation appears to be delayed as compared whites. Conversely, diabetes and other comorbid conditions may be more prevalent in these groups, leading to need for earlier start of dialysis. In view of this, establishing a timing of dialysis initiation other than by clinical status has not been attempted for these patients. Therefore, particularly in these high risk groups, astute clinical monitoring is essential.
Acknowledgments
Support was provided in part by NIH grants U54MD007598 (KN, SBN), MD000182 (KN), UL1TR000124 (KN), and Hubrecht and Burnham (SBN).
Footnotes
Disclosures
ES has no disclosures. SB Nicholas declared association with ConCERT and Janssen. KC Norris has declared associations with the following companies: Abbott, Davita, and Amgen,.
Relevant Potential Conflict of Interest:
None declared.
References
- 1.Rosansky S, Glassock RJ, Clark WF. Early start of dialysis: a critical review. Clin J Am Soc Nephrol. 2011;6:1222–1228. doi: 10.2215/CJN.09301010. [DOI] [PubMed] [Google Scholar]
- 2.Rosansky SJ, Clark WF, Eggers P, Glassock RJ. Initiation of dialysis at higher GFRs: is the apparent rising tide of early dialysis harmful or helpful? Kidney Int. 2009;76:257–261. doi: 10.1038/ki.2009.161. [DOI] [PubMed] [Google Scholar]
- 3.Obrador GT, Pereira BJ. Early referral to the nephrologist and timely initiation of renal replacement therapy: a paradigm shift in the management of patients with chronic renal failure. Am J Kidney Dis. 1998;31:398–417. doi: 10.1053/ajkd.1998.v31.pm9506677. [DOI] [PubMed] [Google Scholar]
- 4.Liu H, Peng Y, Liu F, Xiao H, Chen X, Huang A, Liu Y. Renal function and serum albumin at the start of dialysis in 514 Chinese ESRD in-patients. Ren Fail. 2008;30:685–690. doi: 10.1080/08860220802212619. [DOI] [PubMed] [Google Scholar]
- 5.Wright S, Klausner D, Baird B, Williams ME, Steinman T, Tang H, Ragasa R, Goldfarb-Rumyantzev AS. Timing of dialysis initiation and survival in ESRD. Clin J Am Soc Nephrol. 2010;5:1828–1835. doi: 10.2215/CJN.06230909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hwang SJ, Yang WC, Lin MY, Mau LW, Chen HC. Impact of the clinical conditions at dialysis initiation on mortality in incident haemodialysis patients: a national cohort study in Taiwan. Nephrol Dial Transplant. 2010:2616–2624. doi: 10.1093/ndt/gfq308. [DOI] [PubMed] [Google Scholar]
- 7.Clark WF, Na Y, Rosansky SJ, Sontrop JM, Macnab JJ, Glassock RJ, Eggers PW, Jackson K, Moist L. Association between estimated glomerular filtration rate at initiation of dialysis and mortality. CMAJ: Canadian Medical Association journal = journal de l’Association medicale canadienne. 2011;183:47–53. doi: 10.1503/cmaj.100349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sawhney S, Djurdjev O, Simpson K, Macleod A, Levin A. Survival and dialysis initiation: comparing British Columbia and Scotland registries. Nephrol Dial Transplant. 2009;24:3186–3192. doi: 10.1093/ndt/gfp189. [DOI] [PubMed] [Google Scholar]
- 9.Shiao CC, Huang JW, Chien KL, Chuang HF, Chen YM, Wu KD. Early initiation of dialysis and late implantation of catheters adversely affect outcomes of patients on chronic peritoneal dialysis. Perit Dial Int. 2008;28:73–81. [PubMed] [Google Scholar]
- 10.Stel VS, Dekker FW, Ansell D, Augustijn H, Casino FG, Collart F, Finne P, Ioannidis GA, Salomone M, Traynor JP, Zurriaga O, Verrina E, Jager KJ. Residual renal function at the start of dialysis and clinical outcomes. Nephrol Dial Transplant. 2009;24:3175–3182. doi: 10.1093/ndt/gfp264. [DOI] [PubMed] [Google Scholar]
- 11.Korevaar JC, Jansen MA, Dekker FW, Jager KJ, Boeschoten EW, Krediet RT, Bossuyt PM. When to initiate dialysis: effect of proposed US guidelines on survival. Lancet. 2001;358:1046–1050. doi: 10.1016/S0140-6736(01)06180-3. [DOI] [PubMed] [Google Scholar]
- 12.Kim SG, Kim NH. The effect of residual renal function at the initiation of dialysis on patient survival. Korean J Intern Med. 2009;24:55–62. doi: 10.3904/kjim.2009.24.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilson B, Harwood L, Locking-Cusolito H, Chen SJ, Heidenheim P, Craik D, Clark WF. Optimal timing of initiation of chronic hemodialysis? Hemodial Int. 2007;11:263–269. doi: 10.1111/j.1542-4758.2007.00178.x. [DOI] [PubMed] [Google Scholar]
- 14.Traynor JP, Simpson K, Geddes CC, Deighan CJ, Fox JG. Early initiation of dialysis fails to prolong survival in patients with end-stage renal failure. J Am Soc Nephrol. 2002;13:2125–2132. doi: 10.1097/01.asn.0000025294.40179.e8. [DOI] [PubMed] [Google Scholar]
- 15.Kazmi WH, Gilbertson DT, Obrador GT, Guo H, Pereira BJ, Collins AJ, Kausz AT. Effect of comorbidity on the increased mortality associated with early initiation of dialysis. Am J Kidney Dis. 2005;46:887–896. doi: 10.1053/j.ajkd.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Beddhu S, Samore MH, Roberts MS, Stoddard GJ, Ramkumar N, Pappas LM, Cheung AK. Impact of timing of initiation of dialysis on mortality. J Am Soc Nephrol. 2003;14:2305–2312. doi: 10.1097/01.asn.0000080184.67406.11. [DOI] [PubMed] [Google Scholar]
- 17.Tang SC, Ho YW, Tang AW, Cheng YY, Chiu FH, Lo WK, Lai KN. Delaying initiation of dialysis till symptomatic uraemia--is it too late? Nephrol Dial Transplant. 2007;22:1926–1932. doi: 10.1093/ndt/gfm109. [DOI] [PubMed] [Google Scholar]
- 18.Rosansky SJ, Eggers P, Jackson K, Glassock R, Clark WF. Early start of hemodialysis may be harmful. Arch Intern Med. 2011;171:396–403. doi: 10.1001/archinternmed.2010.415. [DOI] [PubMed] [Google Scholar]
- 19.Lassalle M, Labeeuw M, Frimat L, Villar E, Joyeux V, Couchoud C, Stengel B. Age and comorbidity may explain the paradoxical association of an early dialysis start with poor survival. Kidney Int. 2010;77:700–707. doi: 10.1038/ki.2010.14. [DOI] [PubMed] [Google Scholar]
- 20.Pan Y, Xu XD, Guo LL, Cai LL, Jin HM. Association of early versus late initiation of dialysis with mortality: systematic review and meta-analysis. Nephron Clin Pract. 2012;120:c121–131. doi: 10.1159/000337572. [DOI] [PubMed] [Google Scholar]
- 21.Susantitaphong P, Altamimi S, Ashkar M, Balk EM, Stel VS, Wright S, Jaber BL. GFR at initiation of dialysis and mortality in CKD: a meta-analysis. Am J Kidney Dis, Inc Published by Elsevier Inc. 2012:829–840. doi: 10.1053/j.ajkd.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Fraenkel MB, Harris A, Johnson DW, Kesselhut J, Li JJ, Luxton G, Pilmore A, Tiller DJ, Harris DC, Pollock CA. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363:609–619. doi: 10.1056/NEJMoa1000552. [DOI] [PubMed] [Google Scholar]
- 23.Harris A, Cooper BA, Li JJ, Bulfone L, Branley P, Collins JF, Craig JC, Fraenkel MB, Johnson DW, Kesselhut J, Luxton G, Pilmore A, Rosevear M, Tiller DJ, Pollock CA, Harris DC. Cost-effectiveness of initiating dialysis early: a randomized controlled trial. Am J Kidney Dis. 2011;57:707–715. doi: 10.1053/j.ajkd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 24.Kausz AT, Obrador GT, Arora P, Ruthazer R, Levey AS, Pereira BJ. Late initiation of dialysis among women and ethnic minorities in the United States. J Am Soc Nephrol. 2000;11:2351–2357. doi: 10.1681/ASN.V11122351. [DOI] [PubMed] [Google Scholar]
- 25.Kinchen KS, Sadler J, Fink N, Brookmeyer R, Klag MJ, Levey AS, Powe NR. The timing of specialist evaluation in chronic kidney disease and mortality. Ann Intern Med. 2002;137:479–486. doi: 10.7326/0003-4819-137-6-200209170-00007. [DOI] [PubMed] [Google Scholar]
- 26.Navaneethan SD, Aloudat S, Singh S. A systematic review of patient and health system characteristics associated with late referral in chronic kidney disease. BMC Nephrol. 2008;9:3. doi: 10.1186/1471-2369-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Obrador GT, Arora P, Kausz AT, Ruthazer R, Pereira BJ, Levey AS. Level of renal function at the initiation of dialysis in the U.S. end-stage renal disease population. Kidney Int. 1999:2227–2235. doi: 10.1038/sj.ki.4491163. [DOI] [PubMed] [Google Scholar]
- 28.van de Luijtgaarden MW, Noordzij M, Tomson C, Couchoud C, Cancarini G, Ansell D, Bos WJ, Dekker FW, Gorriz JL, Iatrou C, Garneata L, Wanner C, Cala S, Stojceva-Taneva O, Finne P, Stel VS, van Biesen W, Jager KJ. Factors influencing the decision to start renal replacement therapy: results of a survey among European nephrologists. Am J Kidney Dis. 2012;60:940–948. doi: 10.1053/j.ajkd.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 29.Cooper BA, Branley P, Bulfone L, Collins JF, Craig JC, Dempster J, Fraenkel MB, Harris A, Harris DC, Johnson DW, Kesselhut J, Luxton G, Pilmore A, Pollock CA, Tiller DJ. The Initiating Dialysis Early and Late (IDEAL) study: study rationale and design. Perit Dial Int. 2004;24:176–181. [PubMed] [Google Scholar]
- 30.Yamagata K, Nakai S, Masakane I, Hanafusa N, Iseki K, Tsubakihara Y. Ideal timing and predialysis nephrology care duration for dialysis initiation: from analysis of Japanese dialysis initiation survey. Ther Apher Dial. 2012;16:54–62. doi: 10.1111/j.1744-9987.2011.01005.x. [DOI] [PubMed] [Google Scholar]
- 31.Gadegbeku C, Freeman M, Agodoa L. Racial disparities in renal replacement therapy. J Natl Med Assoc. 2002;94:45S–54S. [PMC free article] [PubMed] [Google Scholar]
- 32.Ifudu O, Dawood M, Iofel Y, Valcourt JS, Friedman EA. Delayed referral of black, Hispanic, and older patients with chronic renal failure. Am J Kidney Dis. 1999:728–733. doi: 10.1016/s0272-6386(99)70226-x. [DOI] [PubMed] [Google Scholar]
- 33.Winkelmayer WC, Glynn RJ, Levin R, Owen WF, Jr, Avorn J. Determinants of delayed nephrologist referral in patients with chronic kidney disease. Am J Kidney Dis. 2001:1178–1184. doi: 10.1053/ajkd.2001.29207. [DOI] [PubMed] [Google Scholar]
- 34.Derebail VK, Nachman PH, Key NS, Ansede H, Falk RJ, Rosamond WD, Kshirsagar AV. Variant hemoglobin phenotypes may account for differential erythropoiesis-stimulating agent dosing in African-American hemodialysis patients. Kidney Int. 2011:992–999. doi: 10.1038/ki.2011.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lea JP, Norris K, Agodoa L. The role of anemia management in improving outcomes for African-Americans with chronic kidney disease. Am J Nephrol. 2008;28:732–743. doi: 10.1159/000127981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holley JL, Aslam N, Bernardini J, Fried L, Piraino B. The influence of demographic factors and modality on loss of residual renal function in incident peritoneal dialysis patients. Perit Dial Int. 2001;21:302–305. [PubMed] [Google Scholar]
- 37.Moist LM, Port FK, Orzol SM, Young EW, Ostbye T, Wolfe RA, Hulbert-Shearon T, Jones CA, Bloembergen WE. Predictors of loss of residual renal function among new dialysis patients. J Am Soc Nephrol. 2000;11:556–564. doi: 10.1681/ASN.V113556. [DOI] [PubMed] [Google Scholar]
- 38.Singhal MK, Bhaskaran S, Vidgen E, Bargman JM, Vas SI, Oreopoulos DG. Rate of decline of residual renal function in patients on continuous peritoneal dialysis and factors affecting it. Perit Dial Int. 2000;20:429–438. [PubMed] [Google Scholar]
- 39.Misra M, Vonesh E, Van Stone JC, Moore HL, Prowant B, Nolph KD. Effect of cause and time of dropout on the residual GFR: a comparative analysis of the decline of GFR on dialysis. Kidney Int. 2001:754–763. doi: 10.1046/j.1523-1755.2001.059002754.x. [DOI] [PubMed] [Google Scholar]
- 40.Tam P. Peritoneal dialysis and preservation of residual renal function. Perit Dial Int. 2009:S108–110. [PubMed] [Google Scholar]
- 41.Drechsler C, de Mutsert R, Grootendorst DC, Boeschoten EW, Krediet RT, le Cessie S, Wanner C, Dekker FW. Association of body mass index with decline in residual kidney function after initiation of dialysis. Am J Kidney Dis. 2009:1014–1023. doi: 10.1053/j.ajkd.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 42.Hidaka H, Nakao T. Preservation of residual renal function and factors affecting its decline in patients on peritoneal dialysis. Nephrology (Carlton) 2003:184–191. doi: 10.1046/j.1440-1797.2003.00156.x. [DOI] [PubMed] [Google Scholar]
- 43.Jansen MA, Hart AA, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002:1046–1053. doi: 10.1046/j.1523-1755.2002.00505.x. [DOI] [PubMed] [Google Scholar]
- 44.Sung SA, Hwang YH, Kim S, Kim SG, Oh J, Chung W, Lee SY, Ahn C, Oh KH. Loss of residual renal function was not associated with glycemic control in patients on peritoneal dialysis. Perit Dial Int. 2011:154–159. doi: 10.3747/pdi.2009.00208. [DOI] [PubMed] [Google Scholar]
- 45.Akbari A, Knoll G, Ferguson D, McCormick B, Davis A, Biyani M. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in peritoneal dialysis: systematic review and meta-analysis of randomized controlled trials. Perit Dial Int. 2009:554–561. [PubMed] [Google Scholar]
- 46.Herget-Rosenthal S, von Ostrowski M, Kribben A. Definition and risk factors of rapidly declining residual renal function in peritoneal dialysis: an observational study. Kidney Blood Press Res, Basel. 2012:233–241. doi: 10.1159/000332887. [DOI] [PubMed] [Google Scholar]
- 47.Liao CT, Shiao CC, Huang JW, Hung KY, Chuang HF, Chen YM, Wu KD, Tsai TJ. Predictors of faster decline of residual renal function in Taiwanese peritoneal dialysis patients. Perit Dial Int. 2008:S191–195. [PubMed] [Google Scholar]
- 48.Bragg-Gresham JL, Fissell RB, Mason NA, Bailie GR, Gillespie BW, Wizemann V, Cruz JM, Akiba T, Kurokawa K, Ramirez S, Young EW. Diuretic use, residual renal function, and mortality among hemodialysis patients in the Dialysis Outcomes and Practice Pattern Study (DOPPS) Am J Kidney Dis. 2007:426–431. doi: 10.1053/j.ajkd.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 49.Medcalf JF, Harris KP, Walls J. Role of diuretics in the preservation of residual renal function in patients on continuous ambulatory peritoneal dialysis. Kidney Int. 2001:1128–1133. doi: 10.1046/j.1523-1755.2001.0590031128.x. [DOI] [PubMed] [Google Scholar]
- 50.Johnson DW, Mudge DW, Sturtevant JM, Hawley CM, Campbell SB, Isbel NM, Hollett P. Predictors of decline of residual renal function in new peritoneal dialysis patients. Perit Dial Int. 2003;23:276–283. [PubMed] [Google Scholar]
- 51.Jiang N, Qian J, Sun W, Lin A, Cao L, Wang Q, Ni Z, Wan Y, Linholm B, Axelsson J, Yao Q. Better preservation of residual renal function in peritoneal dialysis patients treated with a low-protein diet supplemented with keto acids: a prospective, randomized trial. Nephrol Dial Transplant. 2009:2551–2558. doi: 10.1093/ndt/gfp085. [DOI] [PubMed] [Google Scholar]
- 52.Kim SJ, Oh HJ, Yoo DE, Shin DH, Lee MJ, Park JT, Han SH, Yoo TH, Choi KH, Kang SW. Left atrial enlargement is associated with a rapid decline in residual renal function in ESRD patients on peritoneal dialysis. J Am Soc Echocardiogr, Inc. 2012:421–427. doi: 10.1016/j.echo.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 53.Liao CT, Chen YM, Shiao CC, Hu FC, Huang JW, Kao TW, Chuang HF, Hung KY, Wu KD, Tsai TJ. Rate of decline of residual renal function is associated with all-cause mortality and technique failure in patients on long-term peritoneal dialysis. Nephrol Dial Transplant. 2009:2909–2914. doi: 10.1093/ndt/gfp056. [DOI] [PubMed] [Google Scholar]
- 54.Liu JH, Wang SM, Chen CC, Hsieh CL, Lin SY, Chou CY, Liu YL, Lin HH, Huang CC. Relation of ankle-brachial index to the rate of decline of residual renal function in peritoneal dialysis patients. Nephrology (Carlton) 2011;16:187–193. doi: 10.1111/j.1440-1797.2010.01378.x. [DOI] [PubMed] [Google Scholar]
- 55.Park JT, Kim DK, Chang TI, Kim HW, Chang JH, Park SY, Kim E, Kang SW, Han DS, Yoo TH. Uric acid is associated with the rate of residual renal function decline in peritoneal dialysis patients. Nephrol Dial Transplant. 2009:3520–3525. doi: 10.1093/ndt/gfp272. [DOI] [PubMed] [Google Scholar]
- 56.Tian SL, Tian XK, Han QF, Axelsson J, Wang T. Presence of peripheral arterial disease predicts loss of residual renal function in incident CAPD patients. Perit Dial Int. 2012:67–72. doi: 10.3747/pdi.2010.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tattersall J, Dekker F, Heimburger O, Jager KJ, Lameire N, Lindley E, Van Biesen W, Vanholder R, Zoccali C. When to start dialysis: updated guidance following publication of the Initiating Dialysis Early and Late (IDEAL) study. Nephrol Dial Transplant. 2011;26:2082–2086. doi: 10.1093/ndt/gfr168. [DOI] [PubMed] [Google Scholar]
- 58.[1] KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Chapter 5 - Referral to specialists and models of care. 2013;3:112–119. doi: 10.1038/kisup.2012.68. doi:110.1038/kisup.2012.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Slinin Y, Guo H, Gilbertson DT, Mau LW, Ensrud K, Rector T, Collins AJ, Ishani A. Meeting KDOQI guideline goals at hemodialysis initiation and survival during the first year. Clin J Am Soc Nephrol. 2010;5:1574–1581. doi: 10.2215/CJN.01320210. [DOI] [PMC free article] [PubMed] [Google Scholar]