Abstract
Nuclear factor erythroid 2-related factor (Nrf2) is a key transcription factor that regulates antioxidant defense in cells. In this study, we investigated whether over-expression of Nrf2 can prevent ethanol-induced oxidative stress and apoptosis in neural crest cells (NCCs). We found that transfection of NCCs with pcDNA3.1-Nrf2 resulted in statistically significant increases in the Nrf2 protein levels in control and ethanol-exposed NCCs as compared to the cells transfected with control vector. Luciferase reporter gene assay revealed that over-expression of Nrf2 significantly increased the antioxidant response element (ARE) promoter activity in NCCs. Nrf2 over-expression also increased the protein expression and activities of Nrf2 target antioxidants in NCCs. In addition, over-expression of Nrf2 significantly decreased ROS generation and diminished apoptosis in ethanol-exposed NCCs. These results demonstrate that over-expression of Nrf2 can confer protection against ethanol-induced oxidative stress and apoptosis in NCCs by the induction of an antioxidant response.
Keywords: Nrf2, ethanol, apoptosis, neural crest cells, antioxidant
1. Introduction
The vulnerability of selected cell populations, including neural crest cells (NCCs), to ethanol-induced apoptosis is one of the mechanisms underlying the pathogenesis of Fetal Alcohol Spectrum Disorder (FASD), a leading known cause of mental retardation [1-5]. The NCC is a multipotent cell population that can give rise to a diversity of neural and nonneural cell types including neurons, glial cells, melanocytes, and mesenchymal cells that form craniofacial cartilages, bones, dermis, adipose tissue and vascular smooth muscle cells [6-8]. Studies have demonstrated that ethanol can diminish NCC population by the induction of apoptosis, which contributes heavily to subsequent abnormalities [1, 2, 4, 9, 10].
Studies from our laboratory and others have shown that oxidative stress is involved in ethanol-induced apoptosis and dysmorphology [9, 11-14]. Increases in ROS generation have been observed in NCCs [9, 12, 15] as well as in mouse embryos exposed to ethanol both in vitro and in vivo [11, 13]. Using a whole embryo culture system, we have shown that SOD can reduce ethanol-induced ROS generation, cell death and neural tube defects in mouse embryos [11]. Maternal administration of EUK-134, a SOD and catalase mimetic, has also been shown to diminish ethanol-induced apoptosis in the developing limb buds and reduce subsequent limb defects in mouse embryos [16]. In addition, nuclear factor erythroid 2-related factor 2 (Nrf2) signaling has been recently found to be involved in ethanol-induced apoptosis in NCCs and in mouse embryos [13, 15, 17].
Nrf2 is a transcription factor that is known to regulate a variety of antioxidant genes through the antioxidant response element (ARE) [18, 19]. Nrf2 signaling is activated by a range of oxidative and electrophilic stimuli, such as heavy metals, ROS and certain disease processes [20, 21]. Activation of Nrf2-ARE pathway results in the induction of a broad range of genes including phase 2 enzymes, such as heme oxygenase-1 (HO-1) and NAD(P)H:quinone oxidoreductase 1 (NQO1), and antioxidant proteins, such as SOD and catalase [18-20]. Nrf2-mediated endogenous antioxidant response is considered to be the major defense against a wide range of chemical toxicity, cancer and chronic diseases in which oxidative stress is involved [18, 21].
Induction of Nrf2-mediated transcription of antioxidant response has been observed in ethanol-exposed mouse embryos. Studies have shown that up-regulation of Nrf2 signaling by 1,2-dithiole-3-thiones (D3T), an Nrf2 inducer, can result in the induction of antioxidant response in mouse embryos and significantly decrease ROS generation and apoptosis in mouse embryos exposed to ethanol in vivo [13]. It has also been shown that treatment with another chemical inducer of Nrf2, tert-butylhydroquinone (tBHQ), can confer protection against ethanol-induced apoptosis in NCCs by induction of an antioxidant response [15]. In addition, it has been shown that Nrf2-dependent maintenance of glutathione (GSH) homeostasis is important for preventing ethanol-induced oxidative stress and apoptosis in cerebral cortical neurons [22]. Another recent study has also shown that resveratrol can protect the cerebellar granule neurons against ethanol-induced cell death in a rat model of FASD by the induction of Nrf2 [23]. These observations illustrate the critical role of Nrf2 signaling in conferring protection against ethanol-induced oxidative toxicity.
Growing evidence suggests that apoptosis in NCCs is a contributor for ethanol-induced teratogenesis and that ROS plays a critical role in ethanol-induced apoptosis in NCCs. This suggests that therapeutic strategies directed against apoptosis in NCCs by up-regulation of endogenous antioxidant activity in this cell population are particularly valuable for the prevention of FASD. Although our previous studies have found that activation of Nrf2 by its chemical inducers can result in an enhanced antioxidant response and diminish ethanol-induced apoptosis in NCCs, the evidence that direct gene transfer that results in over-expression of Nrf2 is sufficient to confer protection against ethanol-induced apoptosis in NCCs is lacking. In this study, using JoMa cell line as a model system, we found that over-expression of Nrf2 resulted in a significantly greater increase in the protein expression of SOD1, catalase, Gpx3 and NQO1 in control and ethanol-exposed NCCs. Nrf2 over-expression also significantly increased the activities of SOD and catalase in control and ethanol-exposed NCCs, indicating that over-expression of Nrf2 can induce an enhanced antioxidant response in control and ethanol-exposed NCCs. In addition, we found that Nrf2 over-expression significantly decreased ROS generation and apoptosis in ethanol-exposed NCCs. This study is the first to show that Nrf2 gene transfer can prevent ethanol-induced oxidative stress and apoptosis in NCCs. These results demonstrate that over-expression of Nrf2 can confer protection against ethanol-induced oxidative stress and apoptosis in NCCs by the induction of an antioxidant response and suggest that Nrf2 is a key molecular target for prevention of FASD.
2. Materials and methods
2. 1. Cell culture, transfection and ethanol treatment
NCCs (JoMa1.3 cells) were kindly provided by Dr. Schorle and cultured as described previously [24]. Briefly, cells were cultured on cell culture dishes coated with fibronectin and cultured in Dulbecco's modified Eagle's medium (DMEM): Ham's F12 (1:1) at 37°C in a humidified atmosphere of 5% CO2 and 95% air. For Nrf2 over-expression, NCCs were transfected with Nrf2 expression vector, pcDNA3.1-Nrf2 (Addgene, Cambridge, MA) using Lipofectamine 2000 reagent according to the manufacturer's instructions. The cells transfected with control vector or Nrf2-expressing construct were exposed to 100 mM ethanol for 24 hours. Stable ethanol levels were maintained by placing the cell culture plates in a plastic desiccator containing 100 mM ethanol in distilled water as described previously [15].
2.2. Luciferase reporter gene assays
NCCs were plated in 24-well culture plates one day before transfection. Cells were then co-transfected with pNQO1 ARE-luciferase plasmid (a generous gift from Dr. John D. Hayes) [25] and the pRL-TK Renilla luciferase control plasmid (Promega, Madison, WI) using Lipofectamine 2000 reagent (Life technologies Inc., Gaithersburg, MD) according to the manufacturer's protocol. Transfected cells were treated as described above and were lysed. Luciferase activities were measured with the Dual-Lucifease Reporter Assay System (Promega, Madison, WI) using a Lumat LB 9507 ultra-sensitive tube luminometer (Berthold Technologies). The firefly luciferase activities were normalized to the internal Renilla luciferase control.
2.3. Quantitative real-time PCR
For quantitative real-time PCR analysis, total RNA was isolated from NCCs using the QIAGEN RNeasy mini Kit (QIAGEN, Valencia, CA) according to the manufacturer's instructions. Quantitative RT-PCR was performed on a Rotor-Gene 6000 Real-Time system (Corbett LifeScience, Sydney, Australia) with the FastStart Universal SYBR Green Master qPCR kit (Roche Diagnostics, Mannheim, Germany). Primer pairs used for this study were designed using Primer3 and synthesized by Integrated DNA Technologies, Inc. (IDT, Coralville, IA, USA). The following primer pairs were used for this analysis: SOD1: forward: 5’-TAACTGAAGGCCAGCATGGG-3’; reverse: 5’-CATGGACCACCATTGTACGG-3’; catalase: forward: 5’-CACTCAGGTGCGGACATTCT-3’; reverse: 5’-TCCGGAGTGGGAGAATCCAT-3’; NQO1: forward: 5’-AGCTTTAGGGTCGTCTTGGC -3’; reverse: 5’-ACCACTGCAATGGGAACTGA -3’; glutathione peroxidase-3 (GPx-3): forward: 5’-TGGATGGCTTGAAATGTGGC-3’; reverse: 5’-CCATGGCAGTCTGTCTTAGACTT -3’; β-actin: forward: 5’-AGCCTTCCTTCTTGGGTATGGAATC-3’; reverse: 5’-GGAGCAATGATCTTGATCTTCATGG-3’. All real-time PCR assays were conducted in triplicate. Relative quantitative analysis was performed by comparing threshold cycle number of target genes and a reference β-actin mRNA.
2.4. Western blotting
NCCs were lysed in pre-cold RIPA lysis buffer (Cell Signaling, Beverly, MA) with 1 mM fresh-prepared PMSF (Sigma-Aldrich, St Louis, MO) and protease cocktail inhibitors (Roche Applied, Indianapolis, IN). The lysates were then centrifuged at 12000 ×g for 10 min at 4°C and the protein concentration was determined using BCA protein assay kit (Pierce, Rockford, IL) following the manufacturer's instructions. Western blotting was performed as described previously [26]. Proteins were probed with the following antibodies: rabbit polyclonal anti-Nrf2 antibody (Santa Cruz, Santa Cruz, CA), rabbit polyclonal anti-superoxide dismutase (SOD) antibody (Abcam, Cambridge, MA), rabbit polyclonal anti-catalase antibody (Abcam, Cambridge, MA), rabbit polyclonal anti-glutathione peroxidase-3 (GPx-3) antibody (Santa Cruz, Santa Cruz, CA), goat polyclonal anti- NAD(P)H:quinone oxidoreductase 1 (NQO1) antibody (Santa Cruz, Santa Cruz, CA), and rabbit monoclonal anti-cleaved caspase-3 antibody (Cell Signaling, Beverly, MA). The membranes were developed on a Kodak X-OMAT 2000A imaging system (Kodak, Rochester, NY). The intensity of the protein band was analyzed using the Adobe Photoshop CS software (Adobe Systems, San Jose, CA). All western blot analyses were performed in triplicate.
2.5. Determination of antioxidant enzyme activities
The activities of SOD and catalase were determined as described previously [15]. Total SOD and catalase activities were determined using an SOD Assay Kit (Dojindo Molecular Technologies, Inc., Gaithersburg, MD) and an Amplex Red Catalase Assay Kit (Molecular Probes, Eugene, OR), respectively, according to the manufacturer's protocol and were measured using a SpectraMax M5 Microplate Reader (Molecular Devices, Sunnyvale, CA).
2.6. Detection of intracellular ROS generation
Levels of ROS were measured using a 2’, 7’-dichlorodihydrofluoroscein diacetate (DCHFDA) assay as described by Ling et al [27]. Briefly, after washing with Hanks’ balanced salt solution buffer (HBSS) without phenol red, control and treated NCCs were incubated with 10 μM DCHFDA (Molecular Probes, Eugene, OR) at 37 °C for 30 min. Fluorescence intensity was determined at excitation 485 nm/emission 538 nm with a SpectraMax M5 Microplate Reader. The ROS levels in experimental groups were expressed as fold change over control.
2.7. Analysis of cell viability and apoptosis
Cell viability was measured using an MTS (3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl) -2H-tetrazolium salt) assay kit (Promega, Madison, WI) following the manufacturer's protocol. Apoptosis was determined by the analysis of caspase-3 cleavage and activity as well as TUNEL assay. Caspase-3 cleavage was determined by Western blot as described previously [13]. Caspase-3 activity was determined by using homogeneous luminescent Caspase-Glo™ 3/7 Assay (Promega, Madison, WI), following the manufacturer's protocols. TUNEL assay was conducted by using a TiterTACS In Situ Detection Kit (Trevigen, MD, USA), according to the manufacturer's protocol.
2.8. Statistical analysis
Statistical analyses were performed using GraphPad Prism software (GraphPad Software, San Diego, CA). All data are expressed as means ± SEM of three separate experiments. Comparisons between groups were analyzed by a One-way ANOVA. Multiple comparison post-tests between groups were conducted by using Bonferroni's test. Differences between groups were considered significant at p < 0.05.
3. Results
3.1. Transfection with Nrf2 expression vector significantly increased the Nrf2 protein expression in control and ethanol-exposed NCCs
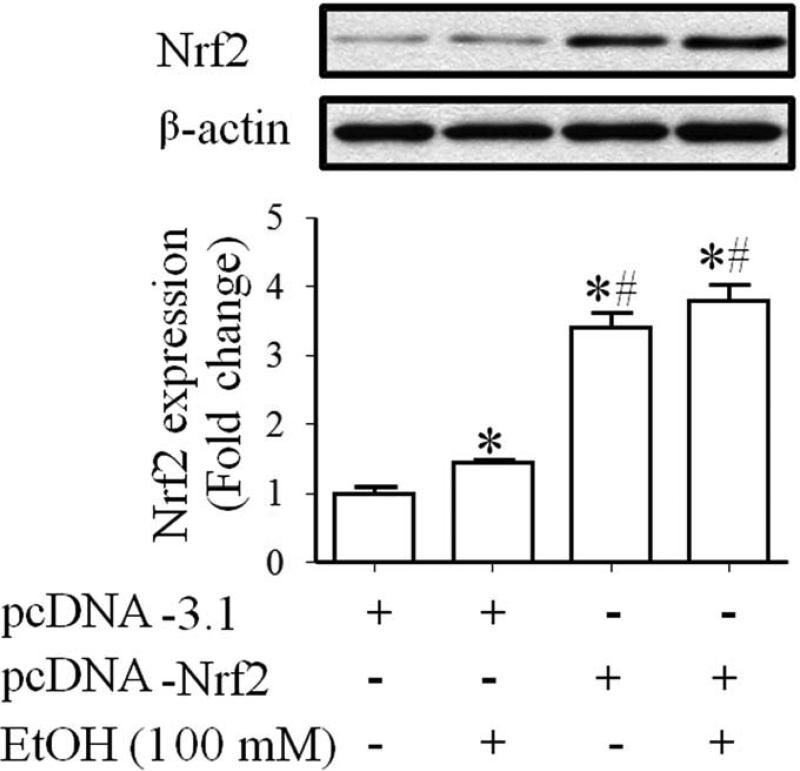
To confirm that transfection with Nrf2 expression vector can significantly increase Nrf2 protein expression in NCCs, the levels of Nrf2 protein were determined in control and ethanol-exposed JoMa 1.3 cells transfected with Nrf2 expression vector or control vector. JoMa 1.3 cells were used as a model for this study because they are NCCs derived from mouse embryos. This cell line expresses early NCC markers and can be instructed to differentiate into neurons, glia, melanocyte, chondrocytes and smooth muscle cells [28]. Recently, this cell line has been used as a model to study the mechanisms of NCC development [29, 30] and to elucidate the role of microRNAs in NCC development [31, 32]. As shown in Fig. 1, treatment with 100 mM ethanol resulted in a moderate increase in Nrf2 protein expression in NCCs transfected with control vector. NCCs transfected with Nrf2 expression vector displayed a significantly greater increase in the levels of Nrf2 protein expression in control and ethanol-exposed NCCs as compared to the cells transfected with control vector. These results indicate that transfection of NCCs with Nrf2 expression vector can significantly increase Nrf2 protein levels in NCCs.
Fig. 1.
Transfection with Nrf2 expression vector significantly increased the Nrf2 protein expression in control and ethanol-exposed NCCs. NCCs transfected with control or Nrf2 expression vector were treated with 100 mM ethanol for 24 hours. Protein expression of Nrf2 was determined by Western blot. Data are expressed as fold change over control and represent the mean ± SEM of three separate experiments. * p < 0.05 vs. control. # p < 0.05 vs. EtOH.
3.2. Nrf2 over-expression significantly increased the activity of the antioxidant response element (ARE) promoter in NCCs
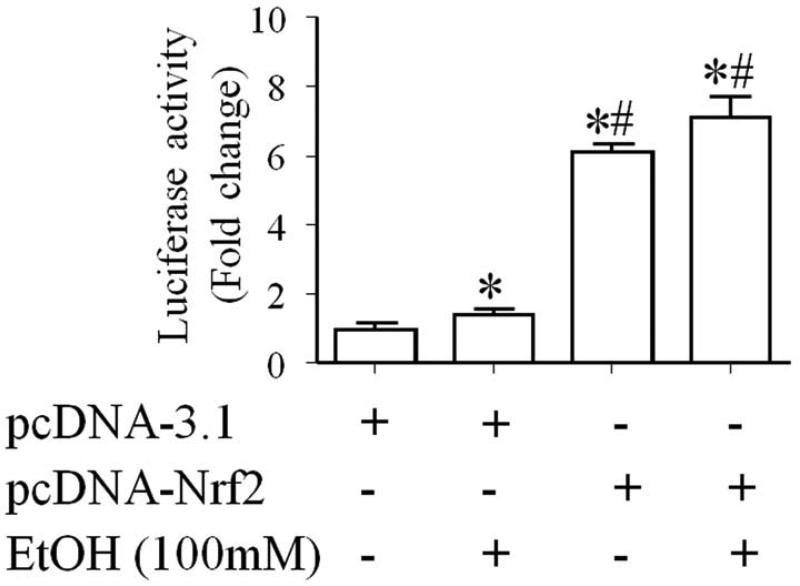
Nrf2 initiates the transcription of its target genes by binding to the ARE in the upstream promoter region of many antioxidant genes [19, 20, 33]. To determine whether over-expression of Nrf2 protein can result in increased activation of Nrf2-ARE pathway, we examined the NQO1-ARE promoter activity in control and ethanol-exposed NCCs transfected with Nrf2 expression vector. ARE-luciferase reporter assay showed an increased ARE promoter activity in ethanol-exposed NCCs that were transfected with control vector. Over-expression of Nrf2 resulted in a significantly greater increase in the ARE promoter activity in control and ethanol-exposed NCCs (Fig. 2), indicating that Nrf2 over-expression can significantly increase ARE promoter activity in NCCs.
Fig. 2.
Nrf2 over-expression significantly increased the ARE promoter activity in NCCs. NCCs transfected with control vector and pNQO1 ARE-luciferase plasmid or Nrf2 expression vector and pNQO1 ARE-luciferase plasmid were treated with 100 mM ethanol for 24 hours. The firefly luciferase activities were measured as described in the Methods. Data are expressed as fold change over control and represent the mean ± SEM of three separate experiments. * p < 0.05 vs. control. # p < 0.05 vs. EtOH.
3.3. Over-expression of Nrf2 in NCCs resulted in significant increases in the mRNA and protein expression of antioxidant genes and their catalytic activity
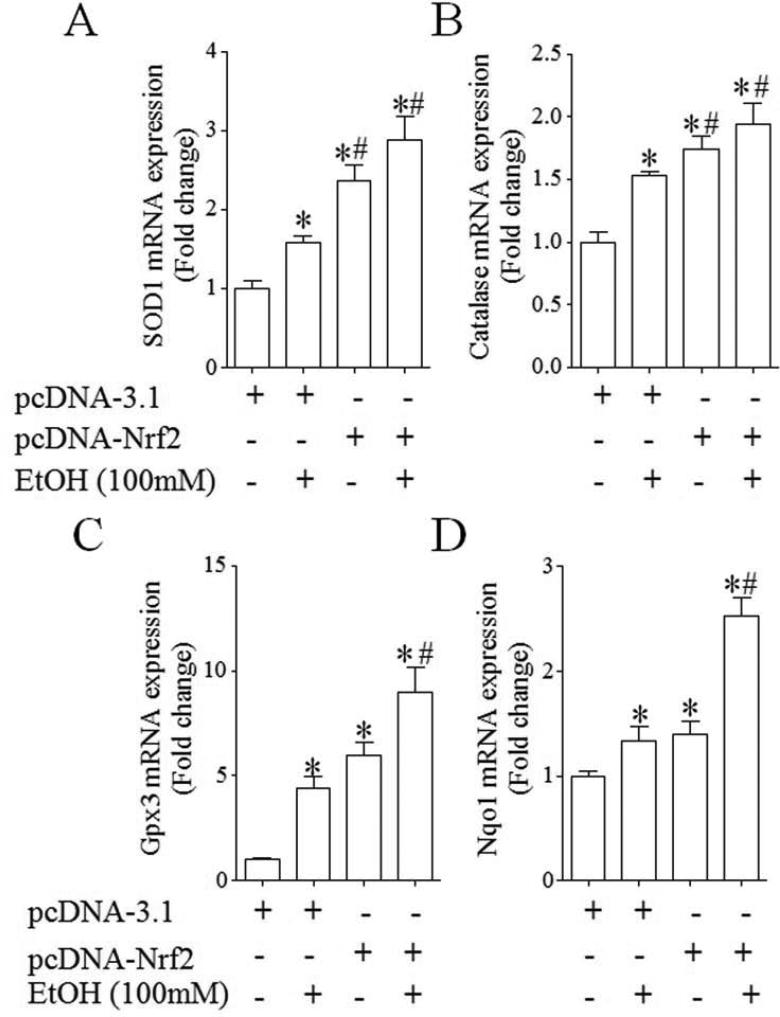
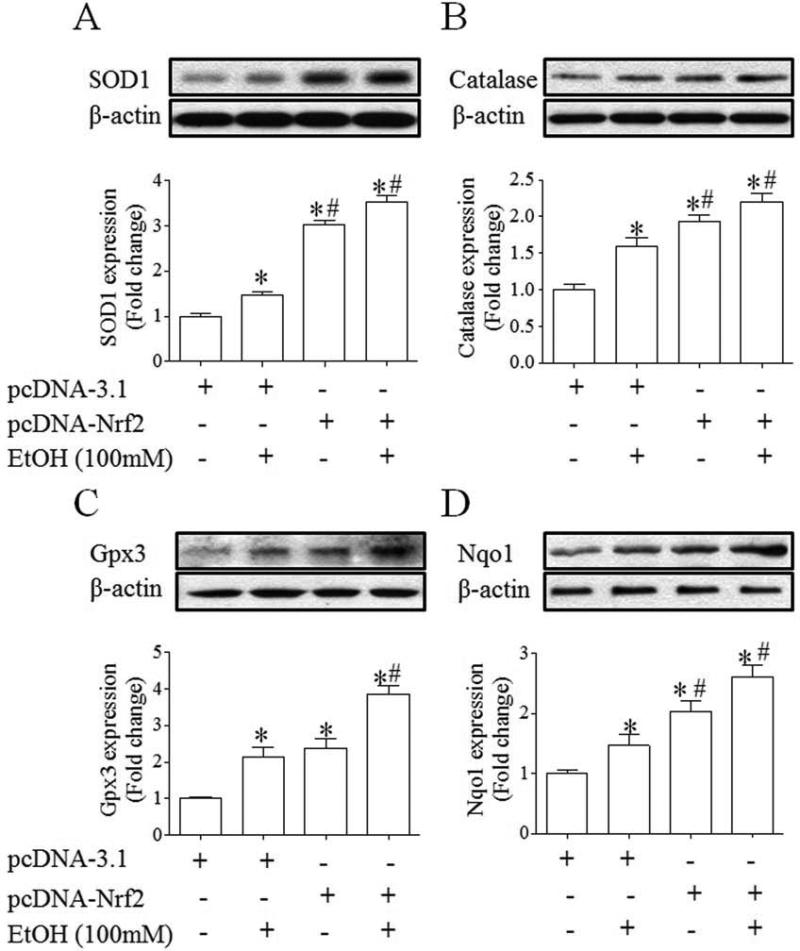
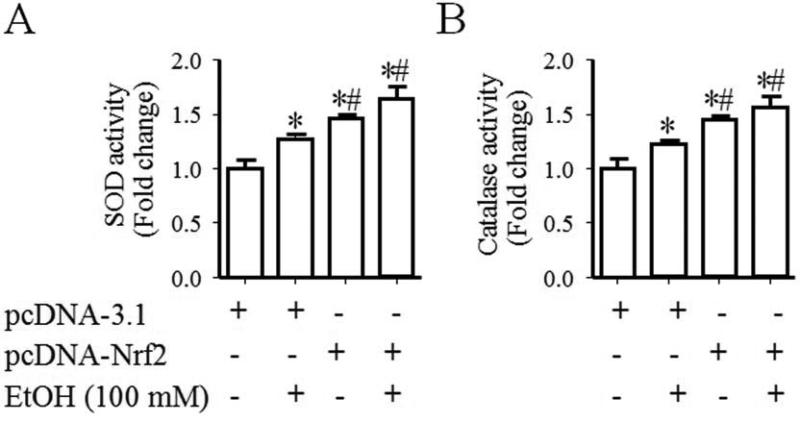
To examine whether increases in the activation of Nrf2-ARE pathway can result in an enhanced expression of Nrf2 target antioxidant genes, the mRNA and protein expression of antioxidant genes, SOD1, catalase, Gpx3 and NQO1, was determined in control and ethanol-exposed NCCs transfected with control or Nrf2 expression vector. As shown in Fig. 3, ethanol exposure of NCCs transfected with control vector resulted in a moderate increase in the mRNA expression of SOD1, catalase and NQO1 and a significant increase in the mRNA expression of Gpx3. Transfection with Nrf2 expression vector resulted in a greater increase in the mRNA expression of all tested genes in control and ethanol-exposed NCCs. Exposure of NCCs transfected with control vector to ethanol also resulted in a moderate increase in the protein expression of SOD1 and catalase, confirming the findings from our previous studies [15]. A moderate increase in the protein expression of Gpx3 and NQO1 was also observed in ethanol-exposed NCCs transfected with control vector. Over-expression of Nrf2 resulted in a significantly greater increase in the protein expression of SOD1, catalase, Gpx3 and NQO1 in control and ethanol-exposed NCCs (Fig. 4). In addition, we found that Nrf2 over-expression statistically significantly increased the activities of SOD and catalase in control and ethanol-exposed NCCs (Fig. 5), indicating that over-expression of Nrf2 can induce an enhanced antioxidant response in control and ethanol-exposed NCCs.
Fig. 3.
Over-expression of Nrf2 significantly increased the mRNA expression of antioxidant genes in control and ethanol-exposed NCCs. NCCs transfected with control or Nrf2 expression vector were treated with 100 mM ethanol for 24 hours. The mRNA expression of SOD1 (A), catalase (B), Gpx3 (C) and NQO1 (D) was determined as described in the Methods. Data are expressed as fold change over control and represent the mean ± SEM of three separate experiments. * p < 0.05 vs. control. # p < 0.05 vs. EtOH.
Fig. 4.
Over-expression of Nrf2 significantly increased the expression of antioxidant proteins in control and ethanol-exposed NCCs. NCCs transfected with control or Nrf2 expression vector were treated with 100 mM ethanol for 24 hours. The protein expression of SOD1 (A), catalase (B), Gpx3 (C) and NQO1 (D) was determined as described in the Methods. Data are expressed as fold change over control and represent the mean ± SEM of three separate experiments. * p < 0.05 vs. control. # p < 0.05 vs. EtOH.
Fig. 5.
Nrf2 over-expression resulted in significant increases in the catalytic activities of antioxidant proteins in control and ethanol-exposed NCCs. NCCs transfected with control or Nrf2 expression vector were treated with 100 mM ethanol for 24 hours. The catalytic activities of SOD (A) and catalase (B) were determined as described in the Methods. Data are expressed as fold change over control and represent the mean ± SEM of three separate experiments. * p < 0.05 vs. control. # p < 0.05 vs. EtOH.
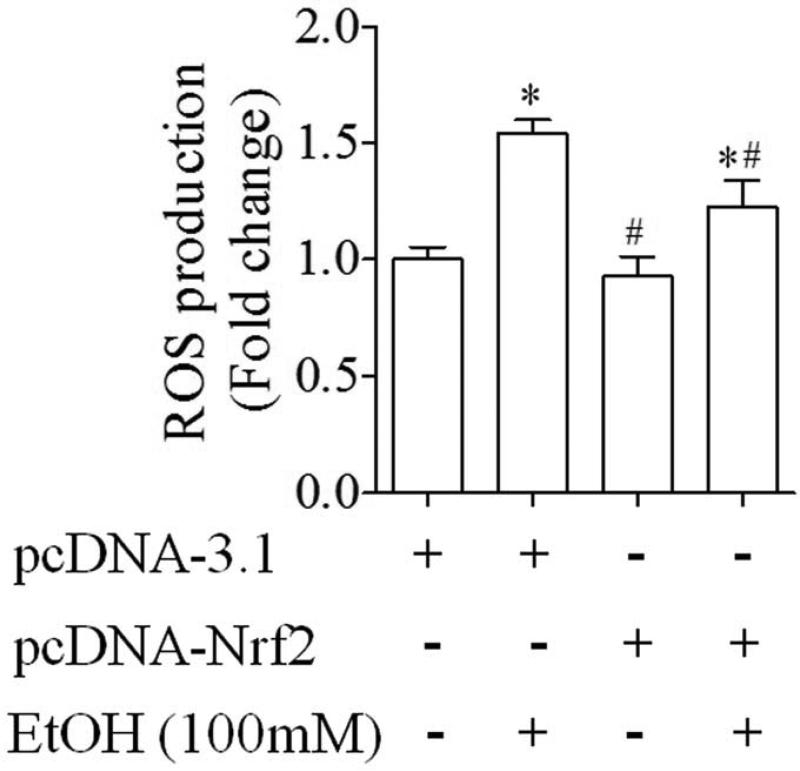
3.4. ROS generation was significantly decreased in ethanol-exposed NCCs transfected with Nrf2-expression vector
We next examined whether enhanced antioxidant response in NCCs transfected with Nrf2 expression vector can decrease ethanol-induced ROS generation in the cells. We found that exposure of NCCs transfected with control vector to ethanol resulted in a significant increase in ROS generation, consistent with the results from our previous study [15]. Over-expression of Nrf2 in NCCs significantly decreased ethanol-induced ROS generation (Fig. 6).
Fig. 6.
ROS generation was significantly decreased in ethanol-exposed NCCs transfected with Nrf2 expression vector. NCCs transfected with control or Nrf2 expression vector were treated with 100 mM ethanol for 24 hours. ROS generation was determined as described in the Methods. Data are expressed as fold change over control and represent the mean ± SEM of three separate experiments. * p < 0.05 vs. control. # p < 0.05 vs. EtOH.
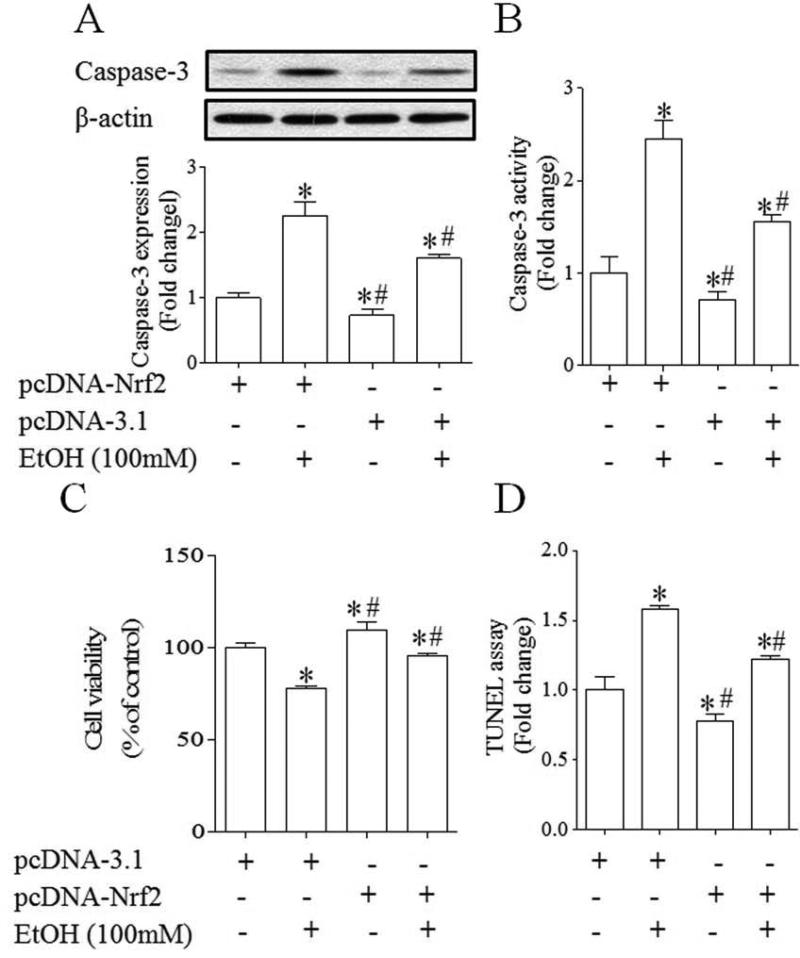
3.5. Over-expression of Nrf2 resulted in a significant decrease in ethanol-induced apoptosis in NCCs
Exposure of NCCs to ethanol resulted in significant increases in caspase-3 cleavage, as evidenced by an increase in the expression of its 17-kDa subunit. Ethanol exposure also increased caspase-3 activity in NCCs transfected with control vector. Both ethanol-induced caspase-3 cleavage and activity were significantly decreased in NCCs transfected with Nrf2 expression vector (Fig. 7, A, B). MTS analysis revealed a significant decrease in cell viability in NCCs exposed to 100 mM ethanol for 24 hours. Transfection with Nrf2 expression vector inhibited the reduction of cell viability in NCCs exposed to ethanol (Fig. 7. C). In addition, TUNEL analysis of apoptosis confirmed that ethanol exposure resulted in an increase in apoptosis in NCCs transfected with control vector and that ethanol-induced apoptosis in NCCs can be diminished by over-expression of Nrf2 (Fig. 7, D), demonstrating that over-expression of Nrf2 can prevent ethanol-induced apoptosis in NCCs.
Fig. 7.
Over-expression of Nrf2 resulted in a significant decrease in ethanol-induced apoptosis in NCCs. NCCs transfected with control or Nrf2 expression vector were treated with 100 mM ethanol for 24 hours. Ethanol-induced apoptosis was determined by analysis of the cleavage and activity of caspase-3 (A, B) and TUNEL assay (D). Cell viability was determined by MTS assay (C). Data are expressed as fold change over control (A, B, D) or percentage of control (C) and represent the mean ± SEM of three separate experiments. * p < 0.05 vs. control. # p < 0.05 vs. EtOH.
4. Discussion
Nrf2 is the master regulator of the antioxidant response that modulates the expression of many genes, including the antioxidant genes. Our previous studies have shown that in vivo treatment with ethanol resulted in a moderate increase in the protein expression of Nrf2 and its downstream antioxidant and detoxifying genes. Up-regulation of Nrf2 signaling by maternal pretreatment with D3T, an Nrf2 inducer, significantly diminished ROS generation and apoptosis in mouse embryo exposed to ethanol in vivo [13], suggesting that chemical inducers of Nrf2 may be able to prevent ethanol-induced oxidative stress and malformations. In this study, we investigated whether gene transfer aimed at increasing Nrf2 expression can prevent ethanol-induced apoptosis in NCCs, an ethanol-sensitive cell population that contributes heavily to the ethanol-induced abnormalities [1, 2, 10]. Consistent with our previous in vivo and in vitro studies [13, 15], we found that ethanol can induce only a moderate increase in Nrf2 activation and antioxidant response in NCCs transfected with control or Nrf2 expression vector, indicating that ethanol-induced Nrf2 activation and antioxidant response in NCCs is an adaptive response that is insufficient to prevent ethanol-induced apoptosis. We also found that over-expression of the Nrf2 by transfecting with Nrf2 expression vector significantly increased the levels of Nrf2 protein in NCCs. Over-expression of Nrf2 in NCCs also resulted in the activation of Nrf2-ARE signaling pathway. This is supported by the findings that ARE promoter activity was significantly increased in NCCs transfected with Nrf2 expression vector.
Nrf2 can induce antioxidant responses that function to alleviate oxidative stress and protect cells from a variety stresses [18, 34-36]. Studies have shown that loss of Nrf2 function resulted in an increased ROS generation and apoptosis in mouse embryonic fibroblast cells exposed to chromium (VI) and cadmium [37, 38]. It was also reported that down-regulation of Nrf2 increased the susceptibility to inhalation particles, chemical carcinogenesis, toxicants, and autoimmune disease [39-43]. In contrast, it has been shown that activation of Nrf2 prevented cell death caused by ischemia/reperfusion [44]. Nrf2 over-expression has also been found to protect cells from Fas-induced apoptosis [45]. Consistent with our previous studies using Nrf2 inducers [13, 15], we found that over-expression of Nrf2 resulted in significantly greater increases in the protein expression of SOD and catalase and their catalytic activity in control and ethanol-exposed NCCs. In addition, significant increases in the mRNA and protein expression of two additional Nrf2 downstream target genes, Gpx3 and NQO1, were observed in control and ethanol-exposed NCCs transfected with Nrf2 expression vector, providing further confirmation that over-expression of Nrf2 can induce an enhanced antioxidant response in control and ethanol-exposed NCCs. We have also found that over-expression of Nrf2 significantly decreased ROS generation in ethanol-exposed NCCs. Moreover, ethanol-induced apoptosis was significantly diminished in NCCs transfected with Nrf2 expression vector, as indicated by significant decreases in caspase-3 cleavage and activity and confirmed by TUNEL analysis. These results demonstrate that over-expression of Nrf2 can confer protection against ethanol-induced oxidative stress and apoptosis in NCCs by the induction of an antioxidant response.
Because Nrf2 plays an important role in the induction of various endogenous antioxidants, it is not surprising that significant attention has been focused on identifying small chemical inducers of the Nrf2 pathway. A wide variety of chemically diverse molecules are potent Nrf2 inducers. Among of them are D3T, tBHQ, sulforaphane (SFN) from broccoli sprouts, and curcumin from turmeric [46-50]. Several Nrf2 potent inducers, such SFN, bardoxolone methyl (CDDO-me), are currently undergoing clinical trials for a variety of diseases and disorders. Our previous studies have also shown that activation of Nrf2-mediated antioxidant response by D3T prevented apoptosis in mouse embryos exposed to ethanol in vivo [13]. Ethanol-induced oxidative stress and apoptosis in NCCs can also be diminished through the activation of Nrf2 signaling by Nrf2 inducer tBHQ [51]. The potency of these Nrf2 inducers in inducing Nrf2 activation and preventing ethanol-induced oxidative stress in early mouse embryos and NCCs make them promising therapeutic agents for prevention of FASD.
Although all of these chemically diverse Nrf2 inducers have been demonstrated to induce antioxidant response by activation of Nrf2 signaling pathway, most of these chemicals are not exclusively an Nrf2 inducer. In addition to activation of Nrf2 pathway, these inducers may also produce additional effects, which might be responsible for unwanted or adverse effects. In attempting to maximize the therapeutic effects of Nrf2 activation while minimizing potential detrimental side effects of Nrf2 inducers, in this study, we adopted a gene transfer strategy to test a novel approach for modulation of Nrf2-mediated antioxidant defense system in embryonic cells. The results of this study demonstrate that over-expression of Nrf2 is able to induce an antioxidant response in NCCs. In addition, Nrf2 over-expression can protect NCCs from ethanol-induced oxidative stress and apoptosis. The demonstrated anti-apoptotic effects of Nrf2 gene transfer provide key insight into the role of Nrf2 signaling in ethanol-induced apoptosis in NCCs and suggest that Nrf2 gene transfer may be a promising approach for the prevention of FASD.
Highlights.
Nrf2 gene transfer significantly increased the Nrf2 protein levels in NCCs
Over-expression of Nrf2 resulted in a significant increase in the ARE activity
Nrf2 overexpression increased the antioxidant response in ethanol-exposed NCCs
Over-expression of Nrf2 significantly decreased ethanol-induced oxidative stress
Nrf2 gene transfer significantly diminished ethanol-induced apoptosis in NCCs
Acknowledgments
We thank Drs. Hubert Schorle and Jochen Maurer (University of Bonn Medical School, Germany) for providing JoMa neural crest cell line. We also thank Dr. John D. Hayes (University of Dundee, U.K.) for providing pNQO1 ARE luciferase reporter. This work was supported by the National Institute of Health grants AA017446 and AA020265 (S.-Y.C) from the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kotch LE, Sulik KK. Experimental fetal alcohol syndrome: proposed pathogenic basis for a variety of associated facial and brain anomalies. Am J Med Genet. 1992;44:168–76. doi: 10.1002/ajmg.1320440210. [DOI] [PubMed] [Google Scholar]
- 2.Cartwright MM, Smith SM. Increased cell death and reduced neural crest cell numbers in ethanol-exposed embryos: partial basis for the fetal alcohol syndrome phenotype. Alcohol Clin Exp Res. 1995;19:378–86. doi: 10.1111/j.1530-0277.1995.tb01519.x. [DOI] [PubMed] [Google Scholar]
- 3.Dunty WC, Jr., Chen SY, Zucker RM, Dehart DB, Sulik KK. Selective vulnerability of embryonic cell populations to ethanol-induced apoptosis: implications for alcohol-related birth defects and neurodevelopmental disorder. Alcohol Clin Exp Res. 2001;25:1523–35. [PubMed] [Google Scholar]
- 4.Smith SM. Alcohol-induced cell death in the embryo. Alcohol Health Res World. 1997;21:287–97. [PMC free article] [PubMed] [Google Scholar]
- 5.West JR, Chen WJ, Pantazis NJ. Fetal alcohol syndrome: the vulnerability of the developing brain and possible mechanisms of damage. Metab Brain Dis. 1994;9:291–322. doi: 10.1007/BF02098878. [DOI] [PubMed] [Google Scholar]
- 6.Teng L, Labosky PA. Neural crest stem cells. Adv Exp Med Biol. 2006;589:206–12. doi: 10.1007/978-0-387-46954-6_13. [DOI] [PubMed] [Google Scholar]
- 7.Delfino-Machin M, Chipperfield TR, Rodrigues FS, Kelsh RN. The proliferating field of neural crest stem cells. Dev Dyn. 2007;236:3242–54. doi: 10.1002/dvdy.21314. [DOI] [PubMed] [Google Scholar]
- 8.Hall BK. The neural crest and neural crest cells: discovery and significance for theories of embryonic organization. J Biosci. 2008;33:781–93. doi: 10.1007/s12038-008-0098-4. [DOI] [PubMed] [Google Scholar]
- 9.Chen SY, Sulik KK. Free radicals and ethanol-induced cytotoxicity in neural crest cells. Alcohol Clin Exp Res. 1996;20:1071–6. doi: 10.1111/j.1530-0277.1996.tb01948.x. [DOI] [PubMed] [Google Scholar]
- 10.Sulik KK, Johnston MC, Webb MA. Fetal alcohol syndrome: embryogenesis in a mouse model. Science. 1981;214:936–8. doi: 10.1126/science.6795717. [DOI] [PubMed] [Google Scholar]
- 11.Kotch LE, Chen SY, Sulik KK. Ethanol-induced teratogenesis: free radical damage as a possible mechanism. Teratology. 1995;52:128–36. doi: 10.1002/tera.1420520304. [DOI] [PubMed] [Google Scholar]
- 12.Chen SY, Sulik KK. Iron-mediated free radical injury in ethanol-exposed mouse neural crest cells. J Pharmacol Exp Ther. 2000;294:134–40. [PubMed] [Google Scholar]
- 13.Dong J, Sulik KK, Chen SY. Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal. 2008;10:2023–33. doi: 10.1089/ars.2007.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson GI, Chen JJ, Schenker S. Ethanol, oxidative stress, reactive aldehydes, and the fetus. Front Biosci. 1999;4:D541–50. doi: 10.2741/henderson. [DOI] [PubMed] [Google Scholar]
- 15.Yan D, Dong J, Sulik KK, Chen SY. Induction of the Nrf2-driven antioxidant response by tert-butylhydroquinone prevents ethanol-induced apoptosis in cranial neural crest cells. Biochem Pharmacol. 2010;80:144–9. doi: 10.1016/j.bcp.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen SY, Dehart DB, Sulik KK. Protection from ethanol-induced limb malformations by the superoxide dismutase/catalase mimetic, EUK-134. FASEB J. 2004;18:1234–6. doi: 10.1096/fj.03-0850fje. [DOI] [PubMed] [Google Scholar]
- 17.Chen SY. Analysis of Nrf2-mediated transcriptional induction of antioxidant response in early embryos. Methods Mol Biol. 2012;889:277–90. doi: 10.1007/978-1-61779-867-2_17. [DOI] [PubMed] [Google Scholar]
- 18.Kensler TW, Wakabayashi N, Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu Rev Pharmacol Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 19.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol. 2013;53:401–26. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen T, Nioi P, Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–5. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang DD. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- 22.Narasimhan M, Mahimainathan L, Rathinam ML, Riar AK, Henderson GI. Overexpression of Nrf2 protects cerebral cortical neurons from ethanol-induced apoptotic death. Mol Pharmacol. 2011;80:988–99. doi: 10.1124/mol.111.073262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Singh CK, Lavoie HA, Dipette DJ, Singh US. Resveratrol restores Nrf2 level and prevents ethanol-induced toxic effects in the cerebellum of a rodent model of fetal alcohol spectrum disorders. Mol Pharmacol. 2011;80:446–57. doi: 10.1124/mol.111.071126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen X, Liu J, Chen SY. Sulforaphane protects against ethanol-induced oxidative stress and apoptosis in neural crest cells by the induction of Nrf2-mediated antioxidant response. Br J Pharmacol. 2013;169:437–48. doi: 10.1111/bph.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nioi P, McMahon M, Itoh K, Yamamoto M, Hayes JD. Identification of a novel Nrf2-regulated antioxidant response element (ARE) in the mouse NAD(P)H:quinone oxidoreductase 1 gene: reassessment of the ARE consensus sequence. Biochem J. 2003;374:337–48. doi: 10.1042/BJ20030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong J, Yan D, Chen SY. Stabilization of Nrf2 protein by D3T provides protection against ethanol-induced apoptosis in PC12 cells. PLoS One. 2011;6:e16845. doi: 10.1371/journal.pone.0016845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ling LU, Tan KB, Lin H, Chiu GN. The role of reactive oxygen species and autophagy in safingol-induced cell death. Cell Death Dis. 2011;2:e129. doi: 10.1038/cddis.2011.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maurer J, Fuchs S, Jager R, Kurz B, Sommer L, Schorle H. Establishment and controlled differentiation of neural crest stem cell lines using conditional transgenesis. Differentiation. 2007;75:580–91. doi: 10.1111/j.1432-0436.2007.00164.x. [DOI] [PubMed] [Google Scholar]
- 29.Murphy DA, Diaz B, Bromann PA, Tsai JH, Kawakami Y, Maurer J, et al. A Src-Tks5 pathway is required for neural crest cell migration during embryonic development. PLoS One. 2011;6:e22499. doi: 10.1371/journal.pone.0022499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Makeyev AV, Enkhmandakh B, Hong SH, Joshi P, Shin DG, Bayarsaihan D. Diversity and complexity in chromatin recognition by TFII-I transcription factors in pluripotent embryonic stem cells and embryonic tissues. PLoS One. 2012;7:e44443. doi: 10.1371/journal.pone.0044443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheehy NT, Cordes KR, White MP, Ivey KN, Srivastava D. The neural crest-enriched microRNA miR-452 regulates epithelial-mesenchymal signaling in the first pharyngeal arch. Development. 2010;137:4307–16. doi: 10.1242/dev.052647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cordes KR, Sheehy NT, White MP, Berry EC, Morton SU, Muth AN, et al. miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature. 2009;460:705–10. doi: 10.1038/nature08195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ohtsuji M, Katsuoka F, Kobayashi A, Aburatani H, Hayes JD, Yamamoto M. Nrf1 and Nrf2 play distinct roles in activation of antioxidant response element-dependent genes. J Biol Chem. 2008;283:33554–62. doi: 10.1074/jbc.M804597200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jin M, Kumar A, Kumar S. Ethanol-mediated regulation of cytochrome P450 2A6 expression in monocytes: role of oxidative stress-mediated PKC/MEK/Nrf2 pathway. PLoS One. 2012;7:e35505. doi: 10.1371/journal.pone.0035505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu Y, Zhang XH, Cederbaum AI. Ethanol induction of CYP2A5: role of CYP2E1-ROS-Nrf2 pathway. Toxicol Sci. 2012;128:427–38. doi: 10.1093/toxsci/kfs164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu KC, Liu J, Klaassen CD. Role of Nrf2 in preventing ethanol-induced oxidative stress and lipid accumulation. Toxicol Appl Pharmacol. 2012;262:321–9. doi: 10.1016/j.taap.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 37.He X, Chen MG, Ma Q. Activation of Nrf2 in defense against cadmium-induced oxidative stress. Chem Res Toxicol. 2008;21:1375–83. doi: 10.1021/tx800019a. [DOI] [PubMed] [Google Scholar]
- 38.He X, Lin GX, Chen MG, Zhang JX, Ma Q. Protection against chromium (VI)-induced oxidative stress and apoptosis by Nrf2. Recruiting Nrf2 into the nucleus and disrupting the nuclear Nrf2/Keap1 association. Toxicol Sci. 2007;98:298–309. doi: 10.1093/toxsci/kfm081. [DOI] [PubMed] [Google Scholar]
- 39.Chan K, Han XD, Kan YW. An important function of Nrf2 in combating oxidative stress: detoxification of acetaminophen. Proc Natl Acad Sci U S A. 2001;98:4611–6. doi: 10.1073/pnas.081082098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu R, Xu C, Shen G, Jain MR, Khor TO, Gopalkrishnan A, et al. Gene expression profiles induced by cancer chemopreventive isothiocyanate sulforaphane in the liver of C57BL/6J mice and C57BL/6J/Nrf2 (−/−) mice. Cancer Lett. 2006;243:170–92. doi: 10.1016/j.canlet.2005.11.050. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Stein TD, Johnson JA. Genetic dissection of systemic autoimmune disease in Nrf2-deficient mice. Physiol Genomics. 2004;18:261–72. doi: 10.1152/physiolgenomics.00209.2003. [DOI] [PubMed] [Google Scholar]
- 42.Ma Q, Battelli L, Hubbs AF. Multiorgan autoimmune inflammation, enhanced lymphoproliferation, and impaired homeostasis of reactive oxygen species in mice lacking the antioxidant-activated transcription factor Nrf2. Am J Pathol. 2006;168:1960–74. doi: 10.2353/ajpath.2006.051113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ramos-Gomez M, Kwak MK, Dolan PM, Itoh K, Yamamoto M, Talalay P, et al. Sensitivity to carcinogenesis is increased and chemoprotective efficacy of enzyme inducers is lost in nrf2 transcription factor-deficient mice. Proc Natl Acad Sci U S A. 2001;98:3410–5. doi: 10.1073/pnas.051618798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Danilov CA, Chandrasekaran K, Racz J, Soane L, Zielke C, Fiskum G. Sulforaphane protects astrocytes against oxidative stress and delayed death caused by oxygen and glucose deprivation. Glia. 2009;57:645–56. doi: 10.1002/glia.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kotlo KU, Yehiely F, Efimova E, Harasty H, Hesabi B, Shchors K, et al. Nrf2 is an inhibitor of the Fas pathway as identified by Achilles’ Heel Method, a new function-based approach to gene identification in human cells. Oncogene. 2003;22:797–806. doi: 10.1038/sj.onc.1206077. [DOI] [PubMed] [Google Scholar]
- 46.Dinkova-Kostova AT, Holtzclaw WD, Cole RN, Itoh K, Wakabayashi N, Katoh Y, et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc Natl Acad Sci U S A. 2002;99:11908–13. doi: 10.1073/pnas.172398899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131:3027S–33S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 48.Munday R, Munday CM. Induction of phase II enzymes by 3H-1,2-dithiole-3-thione: dose-response study in rats. Carcinogenesis. 2004;25:1721–5. doi: 10.1093/carcin/bgh162. [DOI] [PubMed] [Google Scholar]
- 49.Koh K, Cha Y, Kim S, Kim J. tBHQ inhibits LPS-induced microglial activation via Nrf2-mediated suppression of p38 phosphorylation. Biochem Biophys Res Commun. 2009;380:449–53. doi: 10.1016/j.bbrc.2009.01.082. [DOI] [PubMed] [Google Scholar]
- 50.Tapia E, Soto V, Ortiz-Vega KM, Zarco-Marquez G, Molina-Jijon E, Cristobal-Garcia M, et al. Curcumin induces Nrf2 nuclear translocation and prevents glomerular hypertension, hyperfiltration, oxidant stress, and the decrease in antioxidant enzymes in 5/6 nephrectomized rats. Oxid Med Cell Longev. 2012;2012:269039. doi: 10.1155/2012/269039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong J, Sulik KK, Chen SY. The role of NOX enzymes in ethanol-induced oxidative stress and apoptosis in mouse embryos. Toxicol Lett. 2010;193:94–100. doi: 10.1016/j.toxlet.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]