Abstract
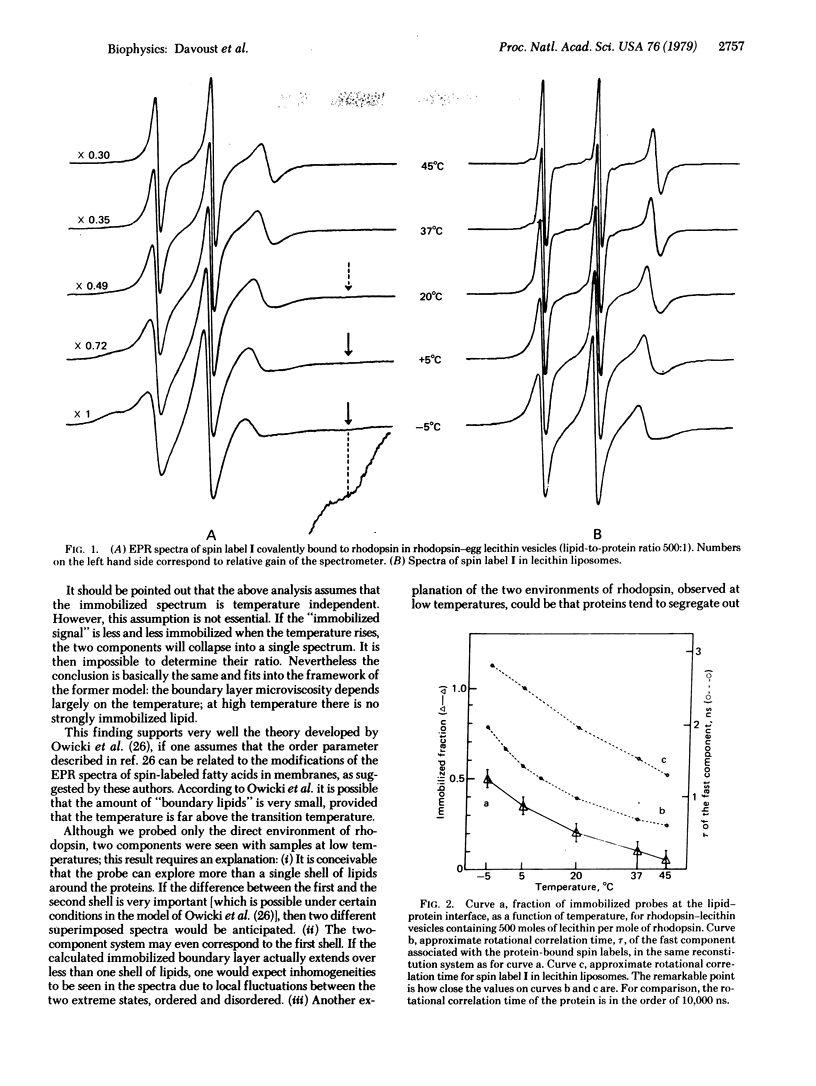
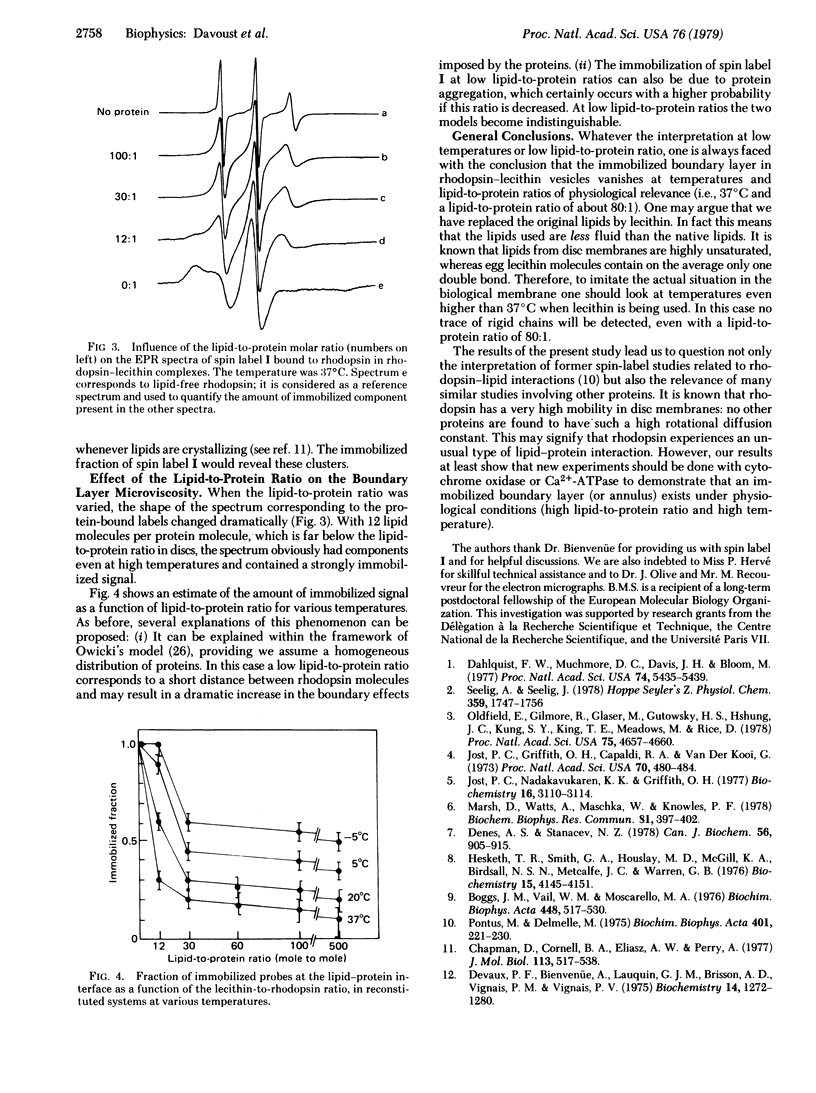
The microviscosity of rhodopsin boundary lipids was studied with a spin-labeled fatty acid covalently attached to rhodopsin, in rhodopsin-egg lecithin vesicles. When the lipid-to-protein ratio was high (500:1, mole to mole), only narrow peaks were visible in electron paramagnetic resonance spectrum at 37 degrees C. This enabled us to show that, under these conditions, not more than 10% of the probes have their motion strongly restricted by the proximity of the protein. When the temperature was reduced, a second component characteristic of strong immobilization appeared. It corresponds to 50% of the signal at -5 degrees C. At all temperatures reduction of the lipid-to-protein ratio also resulted in an increase of the amount of immobilized lipid. These results show that the rhodopsin boundary layer under physiological conditions is associated with low microviscosity. However, low temperatures, low lipid-to-protein ratios, or combinations of the two can induce dramatic modifications of the physical state of the boundary lipids, which under these conditions may no longer be representative of the functional biological system. These results are relevant to the general theory of lipid-protein interaction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bienvenüe A., Rousselet A., Kato G., Devaux P. F. Fluidity of the lipids next to the acetylcholine receptor protein of torpedo membrane fragments. Use of amphiphilic reversible spin-labels. Biochemistry. 1977 Mar 8;16(5):841–848. doi: 10.1021/bi00624a005. [DOI] [PubMed] [Google Scholar]
- Boggs J. M., Vail W. J., Moscarello M. A. Preparation and properties of vesicles of a purified myelin hydrophobic protein and phospholipid. A spin label study. Biochim Biophys Acta. 1976 Nov 2;448(4):517–530. doi: 10.1016/0005-2736(76)90107-3. [DOI] [PubMed] [Google Scholar]
- Brown M. F., Miljanich G. P., Dratz E. A. Proton spin-lattice relaxation of retinal rod outer segment membranes and liposomes of extracted phospholipids. Proc Natl Acad Sci U S A. 1977 May;74(5):1978–1982. doi: 10.1073/pnas.74.5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman D., Cornell B. A., Ellasz A. W., Perry A. Interactions of helical polypepetide segments which span the hydrocarbon region of lipid bilayers. Studies of the gramicidin A lipid-water system. J Mol Biol. 1977 Jul 5;113(3):517–538. doi: 10.1016/0022-2836(77)90236-4. [DOI] [PubMed] [Google Scholar]
- Dahlquist F. W., Muchmore D. C., Davis J. H., Bloom M. Deuterium magnetic resonance studies of the interaction of lipids with membrane proteins. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5435–5439. doi: 10.1073/pnas.74.12.5435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Grip W. J., Bonting S. L., Daemen F. J. Biochemical aspects of the visual process. XXVIII. Classification of sulfhydryl groups in phodopsin and other photoreceptor membrane proteins. Biochim Biophys Acta. 1975 Jul 8;396(1):104–115. doi: 10.1016/0005-2728(75)90193-0. [DOI] [PubMed] [Google Scholar]
- Denes A. S., Stanacev N. Z. Spin-label study of the relation between enzymatic activity and lipid-protein organization in reconstituted cytochrome c oxidase. Can J Biochem. 1978 Sep;56(9):905–915. doi: 10.1139/o78-140. [DOI] [PubMed] [Google Scholar]
- Devaux P. F., Bienvenüe A., Lauquin G., Brisson A. D., Vignais P. M., Vignais P. V. Interaction between spin-labeled acyl-coenzyme A and the mitochondrial adenosine diphosphate carrier. Biochemistry. 1975 Mar 25;14(6):1272–1280. doi: 10.1021/bi00677a028. [DOI] [PubMed] [Google Scholar]
- Favre E., Baroin A., Bienvenue A., Devaux P. F. Spin-label studies of lipid-protein interactions in retinal rod outer segment membranes. Fluidity of the boundary layer. Biochemistry. 1979 Apr 3;18(7):1156–1162. doi: 10.1021/bi00574a006. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hubbell W. L. Organization of rhodopsin in photoreceptor membranes. 1. Proteolysis of bovine rhodopsin in native membranes and the distribution of sulfhydryl groups in the fragments. Biochemistry. 1978 Oct 17;17(21):4396–4402. doi: 10.1021/bi00614a007. [DOI] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Houslay M. D., McGill K. A., Birdsall N. J., Metcalfe J. C., Warren G. B. Annular lipids determine the ATPase activity of a calcium transport protein complexed with dipalmitoyllecithin. Biochemistry. 1976 Sep 21;15(19):4145–4151. doi: 10.1021/bi00664a002. [DOI] [PubMed] [Google Scholar]
- Jost P. C., Griffith O. H., Capaldi R. A., Vanderkooi G. Evidence for boundary lipid in membranes. Proc Natl Acad Sci U S A. 1973 Feb;70(2):480–484. doi: 10.1073/pnas.70.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jost P. C., Nadakavukaren K. K., Griffith O. H. Phosphatidylcholine exchange between the boundary lipid and bilayer domains in cytochrome oxidase containing membranes. Biochemistry. 1977 Jul 12;16(14):3110–3114. doi: 10.1021/bi00633a011. [DOI] [PubMed] [Google Scholar]
- Keith A., Bulfield G., Snipes W. Spin-labeled Neurospora mitochondria. Biophys J. 1970 Jul;10(7):618–629. doi: 10.1016/S0006-3495(70)86324-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauquin G. J., Devaux P. F., Bienvenüe A., Villiers C., Vignais P. V. Spin-labeled acyl atractyloside as a probe of the mitochondrial adenosine diphosphate carrier. Asymmetry of the carrier and direct lipid environment. Biochemistry. 1977 Mar 22;16(6):1202–1208. doi: 10.1021/bi00625a027. [DOI] [PubMed] [Google Scholar]
- Marsh D., Barrantes F. J. Immobilized lipid in acetylcholine receptor-rich membranes from Torpedo marmorata. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4329–4333. doi: 10.1073/pnas.75.9.4329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh D., Watts A., Maschke W., Knowles P. F. Protein--immobilized lipid in dimyristoylphosphatidylcholine-substituted cytochrome oxidase: evidence for both boundary and trapped-bilayer lipid. Biochem Biophys Res Commun. 1978 Mar 30;81(2):397–402. doi: 10.1016/0006-291x(78)91546-2. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Gilmore R., Glaser M., Gutowsky H. S., Hshung J. C., Kang S. Y., King T. E., Meadows M., Rice D. Deuterium nuclear magnetic resonance investigation of the effects of proteins and polypeptides on hydrocarbon chain order in model membrane systems. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4657–4660. doi: 10.1073/pnas.75.10.4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne H. B., Sardet C., Helenius A. Bovine rhodopsin: characterization of the complex formed with Triton X-100. Eur J Biochem. 1974 May 15;44(2):383–390. doi: 10.1111/j.1432-1033.1974.tb03495.x. [DOI] [PubMed] [Google Scholar]
- Owicki J. C., Springgate M. W., McConnell H. M. Theoretical study of protein--lipid interactions in bilayer membranes. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1616–1619. doi: 10.1073/pnas.75.4.1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontus M., Delmelle M. Fluid lipid fraction in rod outer segment membrane. Biochim Biophys Acta. 1975 Aug 20;401(2):221–230. doi: 10.1016/0005-2736(75)90306-5. [DOI] [PubMed] [Google Scholar]
- Rouser G., Fkeischer S., Yamamoto A. Two dimensional then layer chromatographic separation of polar lipids and determination of phospholipids by phosphorus analysis of spots. Lipids. 1970 May;5(5):494–496. doi: 10.1007/BF02531316. [DOI] [PubMed] [Google Scholar]
- SINGLETON W. S., GRAY M. S., BROWN M. L., WHITE J. L. CHROMATOGRAPHICALLY HOMOGENEOUS LECITHIN FROM EGG PHOSPHOLIPIDS. J Am Oil Chem Soc. 1965 Jan;42:53–56. doi: 10.1007/BF02558256. [DOI] [PubMed] [Google Scholar]
- Seelig A., Seelig J. Lipid-protein interaction in reconstituted cytochrome c oxidase/phospholipid membranes. Hoppe Seylers Z Physiol Chem. 1978 Dec;359(12):1747–1756. doi: 10.1515/bchm2.1978.359.2.1747. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



