Abstract
Objectives
This report describes one, two, and three-year outcomes of a combined psychosocial skills training and preventive health care intervention (Helping Older People Experience Success – HOPES) for older persons with serious mental illness.
Design
A randomized controlled trial compared HOPES to treatment as usual (TAU) for n=183 older adults (age≥50) with serious mental illness (mean age=60.2; 28% schizophrenia, 28% schizoaffective disorder, 20% bipolar disorder, 24% major depression).
Setting
Two community mental health centers in Boston, MA and one in Nashua, NH.
Intervention
Twelve months of weekly skills training classes, twice-monthly community practice trips, and monthly nurse preventive health care visits, followed by a 1-year maintenance phase of monthly sessions.
Measurements
Blinded evaluations of functioning, symptoms, and service use were conducted at baseline, one-year (end of the intensive phase), two-year (end of the maintenance phase), and three-year (12 months after the intervention) follow-up.
Results
HOPES compared to TAU was associated with improved community living skills and functioning, greater self-efficacy, lower overall psychiatric and negative symptoms, greater acquisition of preventive health care (more frequent eye exams, visual acuity, hearing tests, mammograms, and PAP smears) and nearly twice the rate of completed advance directives. No differences were found for medical severity, number of medical conditions, subjective health status, or acute service use at 3-year follow-up.
Conclusions
Skills training and nurse facilitated preventive health care for older adults with serious mental illness was associated with sustained long-term improvement in functioning, symptoms, self-efficacy, preventive health care screening, and advance care planning.
Keywords: older adults, serious mental illness, psychosocial skills training, healthcare management, preventive health care, integrated care
The aging of the baby boomer population will dramatically impact the number of middle aged and older adults with serious mental illness (SMI) over the coming decades, foreshadowing an unprecedented challenge to a public mental health system unprepared to address the special needs of this emerging demographic. Adults with SMI constituted over four percent of those age 55 and older or 3.4 million adults in 2010,1, 2 and are projected to nearly double by 2050.3 In contrast to an array of evidence-based interventions and implementation guides targeting younger adults,4, 5 few models of care are specifically designed for older adults with SMI. Among available interventions, those that have emerged as effective include combined cognitive behavioral therapy and social skills training (CBSST),6-8 group-based psychosocial support,9 and functional adaptation skills training (FAST).10, 11 Interventions such as these are necessary towards addressing the complex psychosocial and health care needs of this rapidly growing subgroup with the highest per person Medicare and Medicaid costs,12 rates of institutionalization over three times those of other Medicaid beneficiaries,13 and greater use of emergency care than older adults without SMI.14
Several factors—lack of independent living skills, poor social skills, and medical comorbidity—are strongly associated with high cost service use and differentiate older adults with SMI living in nursing homes from those in the community.12 In addition, persons with SMI, when compared to those without, are known to be at risk for receiving preventive health care services at a lower rate.15 To address these needs, we developed and pilot-tested an intervention combining community living and social skills training with integrated preventive health care.16, 17 The HOPES program (Helping Older People Experience Success) is designed to improve independent functioning and community tenure by teaching social skills, community living skills, and healthy living skills to older persons with SMI living in the community with nurse coordination of preventive health care as an integrated component. In a series of studies we have reported that HOPES is associated with improved psychosocial outcomes following one year of weekly skills training and a second year of monthly maintenance sessions,18 as well as improved executive functioning at 1, 2, and 3 years of follow-up.19
The purpose of this final report of primary study outcomes is to address the following two remaining study questions: 1) Does HOPES result in long-term improved psychosocial functioning that persists at three-year follow-up after withdrawing maintenance sessions and nurse health management? 2) Is Hopes associated with improved preventive health care and reduced acute service use?
To address these questions we evaluated three-year psychosocial, preventive health care and service use outcomes of a randomized, controlled trial comparing HOPES with treatment as usual (TAU) at one-year (end of the intensive phase of skills training), two-year (end of the maintenance phase), and three-year (12 months after intervention withdrawal) follow-up. We hypothesize that HOPES compared to treatment as usual at three-year follow-up is associated with greater long-term improvement in independent living skills, social skills, self-efficacy, and psychiatric symptom severity, as well as greater quality of preventive health care and lower acute service use.
Methods
A randomized controlled trial compared outcomes for HOPES and TAU at 1-, 2-, and 3-years. Written informed consent was obtained through procedures approved by the Committee for the Protection of Human Subjects at Dartmouth College and by Institutional Review Boards specific to each site.
Study Participants
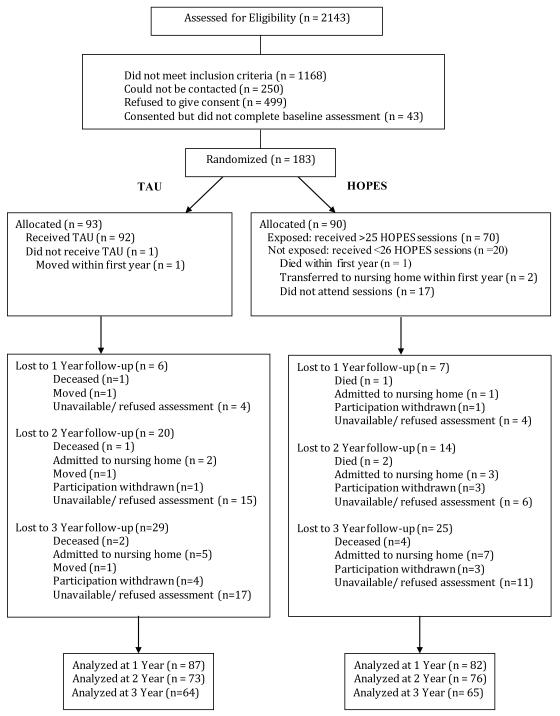
Community-dwelling adults with SMI age≥50 (n=183) were recruited from two community mental-health agencies in Boston, MA and one in Nashua, NH, and were randomized to HOPES (n=90) or TAU (n=93). Eligibility criteria included ability and willingness to provide informed consent and a DSM-IV Axis I disorder diagnosis of schizophrenia, schizoaffective disorder, bipolar disorder, or major depression based on the Structured Clinical Interview for DSM-IV20 in conjunction with documented persistent impairment in multiple areas of functioning. Exclusion criteria were residence in a nursing home or other institutional setting, primary diagnosis of dementia or significant cognitive impairment as indicated by a Mini Mental Status Exam score<20,21 physical illness expected to cause death within one year, or current substance dependence. Figure 1 summarizes the flow of participants in the study.
Figure 1.
Consort Diagram
Interventions
HOPES
HOPES combines psychosocial and preventive health care.17 The psychosocial component consists of weekly skills training classes delivered over one year, followed by a one-year maintenance phase with monthly booster sessions. The HOPES social rehabilitation curriculum, based on social skills training,22 is manualized and organized into seven modules: Communicating Effectively, Making and Keeping Friends, Making the Most of Leisure Time, Healthy Living, Using Medications Effectively, and Making the Most of a Health Care Visit. A complete list of topics covered is found in our report of interim outcomes.17 Sessions were video recorded and evaluated for fidelity by co-authors Mueser and Pratt. HOPES training sessions consisted of 8-10 participants and were delivered in mental health centers and senior housing settings in the community. To minimize transportation challenges, participants attended two sessions on the same day consisting of a 90-minute morning session focused on a specific skill and a 60-minute afternoon review session to consolidate the selected skill using role-play exercises, with a lunch break in between encouraging socialization. In addition, twice monthly community trips were organized so that participants could practice skills related to the current module topics in the community (e.g., bus station to practice using public transportation), and participants were encouraged to practice new skills with a family member or friend. Attendance across sites was approximately 75% in year 1 and 70% in year 2.
The preventive health care component consists of monthly meetings with a nurse embedded in the mental health setting who evaluates participants’ health care needs focusing on facilitating preventive screening, advance care planning, and coordination of primary health care visits. Goals were collaboratively set by the nurse and each participant based on a list of recommended preventive health screenings.17 Skills training leaders and nurses met weekly to coordinate the psychosocial and preventive health care components. Participants attended an average of 66% of the nurse visits across sites.
TAU
Participants in both groups continued to receive the same services they had been receiving prior to the study. Routine mental health services at all sites included: pharmacotherapy, case management or outreach by non-nurse clinicians, individual therapy, and access to rehabilitation services, such as groups and psychoeducation.
Measures
Community living skills were assessed from three perspectives: participant self-report, case manager ratings of observed functioning in the community, and performance-based assessments of simulated tasks. The Independent Living Skills Survey (ILSS)23 is a participant self-report measure of functioning assessing ten areas of community living activities. The Multnomah Community Ability Scale24 is a 17-item measure of observed community functioning completed by interviewing each individual’s case manager. The UCSD Performance-based Skills Assessment (UPSA) evaluates basic living skills using simulated tasks and role play in five areas: communication, trip planning, transportation, finances, and shopping.25 The Social Behavior Schedule (SBS)26 is a 23-item measure of social functioning in individuals with SMI. The Revised Self-Efficacy Scale (RSES) consists of 57 statements rating perceived self-efficacy in social functioning and in managing symptoms.27
The Brief Psychiatric Rating Scale (BPRS)28 and the Scale for the Assessment of Negative Symptoms (SANS)29 were used to assess psychiatric symptom severity and negative symptoms over the prior 2 weeks. Depressive symptom severity was assessed with the Center for Epidemiologic Studies Depression Scale (CES-D).30 Health status was assessed with the SF-3631 and an interview-based version of the Charlson Comorbidity Index.32 Self-report and medical record reviews were used to determine acute service use (e.g., hospitalizations, emergency room visits) and to quantify the proportion of preventive health care indicators from recommended screening examinations by the US Preventive Services Task Force.33 A detailed description of procedures and measures are provided in a prior report.18
Study Procedures
After obtaining informed consent, participants completed baseline assessments and were randomized to HOPES or TAU. Assessors were blind to treatment group and participants were reminded not to reveal information about their treatment to the interviewer. Randomization was conducted at the individual-level and stratified by diagnosis (schizophrenia-spectrum or mood disorder) and gender. A block randomization approach was used to ensure that no more than four participants could be randomized to the same treatment group in a row. Participants were paid for completing assessments, but not for participating in HOPES.
Statistical Analyses
Sample size was determined by computing statistical power to detect effect sizes based on our pilot study of the HOPES program.16 Two tailed t-tests and χ2 analyses were used to compare HOPES and TAU on demographic characteristics, psychiatric history, and outcome measures at baseline. Treatment effects were evaluated by conducting intent-to-treat analyses on the full sample of randomized study participants, regardless of their exposure to treatment. A mixed-effects linear model was used for analysis, which does not drop subjects with missing data as the statistical inference model assumes data missing at random. A doubly multivariate repeated measures analysis of variance (MANOVA), simultaneously analyzing all three dependent measures of community functioning, guarded against potential inflation of alpha with multiple dependent variables. Thereafter, an analysis of covariance (ANCOVA) approach controlling for gender and diagnosis was used to test for treatment effects on each of the dependent variables separately. Because there were no significant differences between HOPES and TAU at baseline, rather than fitting parametric curves with random effects, we included the baseline as a covariate and fit baseline adjusted mean response profile models34 also referred to as covariance pattern models,35 selecting appropriate covariance structures as well as missing data with maximum likelihood estimation.36 Site was included in initial analyses but was dropped from the final models because it did not alter the main effects. Because the baseline was statistically adjusted, treatment effects were evaluated with group main effects (i.e., differences in group mean response profiles). Two-tailed statistical tests were conducted and differences were considered statistically significant based on p≤.05. Effect sizes were computed using Cohen’s d and employing an ANCOVA approach to adjust for covariates and the correlation between baseline and 3-year outcomes. The following thresholds, defined by Cohen (1988), were used to determine whether an effect size was small (.20), moderate (.50) or large (.80).37 Positive effect sizes denote increases in HOPES relative to TAU, and negative effect sizes denote decreases in HOPES relative to TAU.38 Chi square analyses and number needed to treat (NNT)39 were used to evaluate the categorical outcomes of receipt of preventive health care. As determined in a prior study,40 NNT for one person to obtain preventive health screening, cancer screening or completion of advance directives was calculated as the inverse of the absolute risk reduction for not receiving either of these types of preventive health care.
Results
HOPES vs. TAU at baseline and 3-year follow-up
Participants assigned to HOPES did not differ significantly from those assigned to TAU on any demographic, diagnostic, or baseline measures, with the exception of a greater rate of asthma in HOPES compared to TAU group (n=18 vs. n=7; p=.017) (Table 1). We compared baseline demographics, functioning, and symptoms of participants who completed the 3-year assessment (n=129) to those lost at 3-year follow-up (n=54). Participants lost to follow-up were slightly older (mean age=62.7±9.3 vs. 59.1±7.1; t-test=2.58; df=181; p=.01), and had greater self-efficacy (mean R-SES score=72.5±17.9 vs. 65.6±19.0; t-test=-2.36; df=181; p=.02). Fewer than 2% of observations were missing at baseline for the CES-D, Charlson Comorbidity Index, and BPRS. At 3-year follow up, an average of 34% of observations were missing (range 31% to 39%), except for the Multnomah Scale, which had 50% missing observations. This rate of missing observations was expected as 30% (n=54) of participants were lost at 3-year follow up and the Multnomah requires locating and interviewing clinical providers who have detailed knowledge of participants’ functioning in the community.
Table 1.
Sample characteristics by group at baseline
| Characteristic | Total Sample (N=183) | TAU (N=93) | HOPES (N=90) | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N | % | N | % | N | % | |
| Age (M±SD) | 60.2±7.9 | 60.1±7.1 | 60.3±8.0 | |||
| Days in hospital (M±SD) | 20.7±39.6 | 21.1±45.1 | 20.2±31.1 | |||
| Gender | ||||||
| Female | 106 | 58 | 53 | 57 | 53 | 59 |
| Male | 77 | 42 | 40 | 43 | 37 | 41 |
| Ethnicity | ||||||
| White | 157 | 86 | 78 | 84 | 79 | 88 |
| Non-White | 26 | 14 | 15 | 16 | 11 | 12 |
| Latino | ||||||
| No | 171 | 93 | 88 | 95 | 83 | 92 |
| Yes | 12 | 7 | 5 | 5 | 7 | 8 |
| Marital Status | ||||||
| Never married | 118 | 65 | 59 | 63 | 59 | 66 |
| Married | 65 | 35 | 34 | 37 | 31 | 34 |
| Education | ||||||
| High school graduate | 134 | 73 | 64 | 69 | 70 | 78 |
| Less than high school | 49 | 27 | 29 | 31 | 20 | 22 |
| Residential | ||||||
| Living independently | 94 | 51 | 49 | 53 | 45 | 50 |
| Supervised/supported housing |
89 | 49 | 44 | 47 | 45 | 50 |
| Medical Diagnosis | ||||||
| Hypertension | 80 | 44 | 47 | 51 | 33 | 37 |
| Diabetes | 50 | 27 | 23 | 25 | 27 | 30 |
| COPD | 42 | 23 | 23 | 25 | 19 | 21 |
| Hypothyroidism | 32 | 18 | 18 | 19 | 14 | 16 |
| Asthma 1 | 25 | 14 | 7 | 8 | 18 | 20 |
| Cardiac Disease | 23 | 13 | 14 | 15 | 9 | 10 |
| Psychiatric Diagnosis | ||||||
| Schizoaffective | 52 | 28 | 28 | 30 | 24 | 27 |
| Schizophrenia | 51 | 28 | 26 | 28 | 25 | 28 |
| Depression | 44 | 24 | 20 | 22 | 24 | 27 |
| Bipolar | 36 | 20 | 19 | 20 | 17 | 19 |
Fisher’s 2-sided exact test=6.03; p=.017; no other comparisons were significant at p≤.05.
Functioning, symptom, and health status outcomes
Tables 2 and 3 show results of the intent-to-treat analyses of outcomes for community functioning, psychiatric symptoms, and health status at 1-, 2- and 3-year follow-up. HOPES compared to TAU was associated with greater improvement in the weighted combination of the three approaches to measuring community living skills using the MANOVA approach (F(2,151)=5.10, p=.007). Next, we conducted independent tests on each of the three dependent variables showing significant differences favoring HOPES over TAU for participant self-report, observed functioning in the community, and performance on standardized simulated tasks. HOPES contributed to greater overall self-efficacy compared to TAU. In testing for interactions by diagnostic group, we found that improved self-efficacy was greatest among participants with mood disorders. No other interactions between group and diagnosis were observed for any other outcome variables. For psychiatric symptoms, we compared both groups on the primary outcome of overall psychiatric symptom severity (i.e., BPRS total) and found lower overall symptom severity for HOPES. We then separately evaluated primary symptom outcomes between groups, finding lower negative symptom severity and a trend for lower depression among HOPES participants.
Table 2.
Community functioning outcomes at 3-year follow-up in HOPES compared to TAU
| Measures | Data Source | Group | Baseline | 1 Year | 2 Year | 3 Year | Group main effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| M | SD | M | SD | M | SD | M | SD | ES | F | df | p | |||
| Community living skills | ||||||||||||||
| Self-Reported | Participant Interview |
HOPES | .66 | .10 | .68 | .09 | .68 | .11 | .68 | .11 | .25 | 4.64 | 1, 161 | .033 |
| Independent Living Skills Survey (ILSS Total) |
TAU | .65 | .11 | .66 | .11 | .65 | .12 | .62 | .14 | |||||
|
Observed Functioning in
the Community |
Case Manager/ Clinician |
HOPES | 3.66 | .51 | 3.76 | .45 | 3.78 | .51 | 3.83 | .44 | .26 | 5.23 | 1, 150 | .024 |
| Multnomah Community Ability Scale Total |
TAU | 3.69 | .51 | 3.58 | .50 | 3.61 | .53 | 3.72 | .48 | |||||
| Simulated Performance | Performance- based Observation |
HOPES | 72.85 | 15.93 | 76.04 | 13.47 | 77.32 | 14.35 | 77.77 | 15.15 | .27 | 6.13 | 1, 163 | .014 |
| UCSD Performance Skills Assessment (UPSA Total) |
TAU | 68.54 | 19.04 | 68.97 | 19.88 | 69.44 | 21.06 | 70.30 | 19.48 | |||||
| Social Behaviors and Self-Efficacy | ||||||||||||||
| Social Behaviors | Participant Interview/ Observation |
HOPES | 51.42 | 8.94 | 49.30 | 8.54 | 46.74 | 8.41 | 48.56 | 10.06 | −.08 | 1.73 | 1, 162 | .190 |
| Social Behavior Survey (SBS Total) |
TAU | 51.17 | 7.97 | 50.75 | 9.23 | 49.14 | 9.58 | 50.29 | 10.39 | |||||
| Self-Efficacy | Participant Interview |
HOPES | 66.24 | 19.16 | 71.35 | 17.70 | 71.46 | 16.37 | 72.20 | 15.89 | .33 | 6.85 | 1, 156 | .010 |
| Revised Self-efficacy Scale (R-SES Total) |
TAU | 68.99 | 18.61 | 68.76 | 17.74 | 71.33 | 18.49 | 69.45 | 21.08 | |||||
Table 3.
Psychiatric symptoms and health status outcomes at 3-year follow-up in HOPES compared to TAU
| Measures | Data Source | Group | Baseline | 1 Year | 2 Year | 3 Year | Group main effect | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| M | SD | M | SD | M | SD | M | SD | ES | F | df | P | |||
| Psychiatric Symptoms | ||||||||||||||
| Psychiatric Symptoms | Participant Interview/ Observation |
HOPES | 55.54 | 13.86 | 52.82 | 12.34 | 50.43 | 13.75 | 49.92 | 13.75 | −.17 | 3.97 | 1, 157 | .048 |
| Brief Psychiatric Rating Scale (BPRS Total) |
TAU | 54.23 | 12.75 | 54.34 | 13.66 | 51.09 | 13.84 | 50.53 | 14.98 | |||||
| Negative Symptoms | Participant Interview/ Observation |
HOPES | 2.42 | .54 | 2.29 | .49 | 2.26 | .55 | 2.21 | .64 | −.27 | 3.70 | 1, 155 | .053 |
| Scale for the Assessment of Negative Symptoms (SANS Total) |
TAU | 2.50 | .54 | 2.51 | .57 | 2.52 | .65 | 2.50 | .65 | |||||
| Depression | Participant Interview |
HOPES | 23.85 | 12.99 | 20.01 | 11.86 | 12.38 | 10.68 | 19.52 | 11.64 | −.22 | 3.26 | 1, 158 | .073 |
| Center for Epidemiologic Studies Depression Scale (CES-D Total) |
TAU | 20.83 | 12.82 | 20.63 | 11.42 | 19.59 | 13.18 | 19.43 | 14.27 | |||||
| Mental functioning | Participant Interview |
HOPES | 38.99 | 11.38 | 41.77 | 11.42 | 40.65 | 10.61 | 41.99 | 11.86 | .05 | .01 | 1, 159 | .936 |
| SF-36 Mental Component Score (MCS Total) |
TAU | 41.25 | 13.81 | 41.27 | 13.11 | 42.93 | 13.27 | 41.99 | 14.88 | |||||
| Health Status | ||||||||||||||
| Physical functioning | Participant Interview |
HOPES | 46.90 | 11.56 | 45.54 | 11.97 | 47.94 | 12.22 | 46.09 | 13.25 | .07 | .12 | 1, 159 | .732 |
| SF-36 Physical Component Score (PCS Total) |
TAU | 47.04 | 11.79 | 46.83 | 11.02 | 47.26 | 11.76 | 48.50 | 11.73 | |||||
| Medical Severity | Participant Interview Medical Records |
HOPES | 3.03 | 2.49 | 2.77 | 2.48 | 2.72 | 2.52 | 2.73 | 2.43 | −.22 | .26 | 1, 156 | .614 |
| Charlson Severity Index | TAU | 2.30 | 2.13 | 2.54 | 2.36 | 2.41 | 2.34 | 2.22 | 2.04 | |||||
|
Total number of
diseases |
Medical Records |
HOPES | 5.48 | 3.99 | 8.55 | 5.44 | 8.78 | 4.68 | 10.43 | 8.42 | −.14 | .10 | 1, 94 | .749 |
| TAU | 5.84 | 3.77 | 8.07 | 6.00 | 10.14 | 7.68 | 13.17 | 9.81 | ||||||
At 3-year follow-up, no significant differences were found between groups for behavior or mental/physical functioning outcomes as measured by the SF-36, or with respect to medical severity or total number of medical conditions.
Preventive health care screening and advanced directives
Table 4 compares HOPES to TAU with respect to preventive health care across three categories of indicators: 1) routine preventive care (blood pressure, eye examination, visual acuity test, hearing test, serum cholesterol, flu shot); 2) cancer screening (colon cancer, mammogram, PAP smear); and 3) advance directives. A higher percentage of HOPES participants received eye exams, visual acuity, and hearing tests compared to TAU, with the greatest between group difference found for receipt of mammograms and PAP smears (NNT=5.5 and 3.5, respectively). Finally, a nearly two-fold difference was found between HOPES and TAU for completing advance directives (NNT=3.6). Excluding the relationship between female gender and mammograms or PAP smears, there were no other gender differences with respect to receipt of the different preventive health care screens or completion of advanced directives. We also explored possible interactions between years of education, cognitive status and receipt of preventive health care screens or advanced directives, and no significant relationship emerged.
Table 4.
Preventive health care, screening and advance directives at 3-year follow-up in HOPES compared to TAU1
| Preventive Healthcare and Advance Directives |
HOPES (N=90) | TAU (N=93) | Number Needed to Treat |
Effect Size |
Chi Square2 |
P value | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| N | % | N | % | |||||
| Routine Preventive Care | ||||||||
| Blood Pressure | 87 | 100 | 89 | 99 | 90.9 | .00 | 1.00 | .508 |
| Eye exam | 84 | 97 | 79 | 88 | 11.2 | .36 | 4.96 | .048 |
| Visual acuity test | 74 | 85 | 65 | 72 | 7.8 | .32 | 4.39 | .045 |
| Hearing test | 52 | 60 | 40 | 44 | 6.5 | .32 | 4.18 | .051 |
| Cholesterol | 84 | 97 | 84 | 93 | 30.3 | .13 | .97 | .497 |
| Flu shot | 75 | 86 | 68 | 76 | 9.4 | .26 | 3.28 | .087 |
| Cancer Screening | ||||||||
| Colon cancer screen | 71 | 82 | 76 | 84 | −35.73 | −.07 | .25 | .690 |
| Mammogram (women) | 45 | 85 | 34 | 67 | 5.5 | .43 | 4.81 | .039 |
| PAP smear (women) | 41 | 77 | 25 | 49 | 3.5 | .59 | 9.16 | .004 |
| Care Planning | ||||||||
| Advance Directives | 51 | 61 | 28 | 33 | 3.6 | .59 | 13.20 | <.001 |
Percent receiving preventive health care screening at least once over 3-year study period and presence of documented advance directives.
Fisher’s 2-sided Exact Test.
As a slightly greater proportion of TAU participants received colon cancer screening compared to HOPES, the NNT is a negative value, more appropriately interpreted as number needed to harm (NNH).
Acute health service use
Greater decreases were observed from baseline to 3-year follow-up for HOPES compared to TAU with respect to the proportion of participants who had at least one psychiatric hospitalization, medical hospitalization, or emergency room visit, though these differences were not statistically significant. The proportion of participants experiencing at least one acute psychiatric hospitalization decreased 7% for HOPES compared to 3% for TAU (HOPES: 22% (n=17) baseline and 15% (n=11) at 3-year follow-up; TAU: 26% (n=22) baseline and 23% (n=16) at 3-year follow-up). The proportion of participants experiencing at least one acute medical hospitalization decreased by 3% for HOPES compared to an increase of 3% for TAU (HOPES: 30% (n=24) baseline and 27% (n=20) at 3-year follow-up; TAU: 27% (n=23) baseline and 30% (n=21) at 3-year follow-up). Finally, there was a 14% decrease in the proportion of participants experiencing at least one emergency room visit for HOPES compared to a 5% decrease for TAU (HOPES: 55% (n=43) at baseline and 41% (n=31) at 3-year follow-up; TAU: 49% (n=42) at baseline and 44% (n=31) at follow-up).
Discussion
Participation in HOPES was associated with improved community living skills at 3-year follow-up from three perspectives: participant self-report, case manager observation of functioning in the community, and performance on simulated tasks of independent living skills. HOPES contributed to greater self-efficacy, as well as decreased overall severity of psychiatric and negative symptoms. These results demonstrate the persistence of improved outcomes at one-year post-intervention. Integrated preventive health care was also associated with greater receipt of preventive health screening and greater completion of advanced directives.
These results contribute to a limited empirical research literature consisting of the Functional Adaptation Skills Training (FAST) and Cognitive Behavioral Social Skills Training (CBSST) programs. These interventions focus on middle-aged and older adults with schizophrenia and do not include an integrated component of preventive health care management. FAST is a 6-month intervention to improve skills for independent living, communication, and psychiatric illness management.10 In a randomized trial (n=240), FAST was associated with greater improvement compared to usual care in negative symptoms and in performance of community living skills.11 CBSST is a 3-month intervention combining cognitive-behavioral therapy (e.g., cognitive restructuring) with social skills training.11 A randomized trial (n=76) found that CBSST contributed to greater improvement in 6-month outcomes for insight and on the leisure and transportation subscales of the ILSS,23 but in contrast to HOPES, no differences were found between groups in the total scores for living skills.8 Our 3-year findings from the current study suggest that skills training can result in sustained improvements in psychosocial functioning and symptom severity for a heterogeneous group of older adults with SMI.
In addition to achieving improved functioning and decreased psychiatric symptoms, HOPES participants compared to TAU experienced greater preventive health care screening. It is noteworthy that the greatest improvement was found for mammograms, PAP smears, and advance care planning. However, we did not observe greater improvement in subjective health status (as measured by the SF-36), or lower ratings of medical severity. Of interest, the number of identified medical diseases approximately doubled (rather than decreased) for both HOPES and TAU over the three year period of study, most likely reflecting the impact of increased attention to comorbid medical disorders resulting from repeated participant interviews, clinician ratings, and requests for primary care medical records. Finally, decreases in the proportion of participants who were hospitalized or who had an emergency room visit were greater for HOPES compared to TAU, though the sample size may not have been adequate to demonstrate statistically significant differences.
Several limitations warrant consideration when interpreting the results. First, our study sample was predominantly white (86%; n=157), suggesting the need to evaluate HOPES in diverse populations, and to explore the need for cultural adaptations.41 Second, as HOPES consists of both skills training and preventive health care, we are unable to attribute the study outcomes to either component. Third, the nurse component emphasized preventive health care and health care coordination. We found improved quality of preventive health care (a proximal outcome), but did not demonstrate significant improvement in health status (a distal outcome) as measured by the SF-36. More targeted disease management (as opposed to general care coordination) and a longer follow-up period may be needed to demonstrate improved health outcomes.
In addition, as HOPES consists of seven discrete modules delivered over one year, followed by a second year of monthly booster sessions, our study was not designed to assess the comparative or incremental contributions of the individual modules to improved outcomes. A more targeted and individually tailored approach may be more effective and efficient. We are currently engaged in pilot studies exploring the feasibility and potential effectiveness of matching participant need and preference for selected components of HOPES. Lastly, while we found statistically significant improvements on several measures of functioning and symptoms over 3 years, the clinical significance of these improvements is uncertain. For most of the measures, standards on clinical significance have not been established. However, the UPSA has an established cutoff for clinically significant improvement in functioning: a score of ≥75 is predictive of residential independence.42 At three-year follow-up two-thirds of HOPES participants achieved ≥75 on the UPSA (67%; n=37) compared to slightly more than half of those receiving TAU (54%; n=30) (NNT=7.3).
These findings advance the evidence base on effective interventions for older adults with SMI in several ways. Our study demonstrates the feasibility and effectiveness of integrating group-based skills training and preventive health care targeting older adults with SMI. An important strength was that HOPES demonstrated improved independent living skills and community functioning from multiple perspectives including self-report, case manager observation, and role-play assessments of skills. We also found that integrated preventive health care coordination by an embedded nurse can significantly improve adherence to preventive screening, especially for procedures such as mammograms and PAP smears, in addition to substantially improving advance care planning. Finally, HOPES participants maintained gains in improved functioning and symptoms at 3-year follow-up. To our knowledge, this improvement in functioning provides the longest and largest demonstration of the effectiveness of psychosocial skills training for persons with SMI, regardless of age group.
There are several potential implications of these findings. First, as underscored by the 2012 Institute of Medicine Report on the mental health workforce for older adults,5 the burgeoning population of older adults with SMI will require more providers trained to provide evidence-based practices for this high-risk group.43 HOPES adds to the small number of psychosocial interventions validated for use among older adults with SMI8, 11 and confirms that the effectiveness of psychosocial rehabilitation is not limited to younger adults. These interventions may also provide a strategy for responding to the US Supreme Court Olmstead Decision mandating that states provide services supporting the preference of adults with disabilities to reside in non-institutional settings.2 Finally, there remains a dramatic gap between science and services provided in the community. To respond to the rapidly growing population of older adults with SMI, a future research agenda should include identifying successful strategies for implementing and sustaining effective integrated rehabilitation and health care services in the community.
Acknowledgements
The study was supported by NIMH grant R01 MH62324. We wish to thank the following individuals for their assistance in conducting this project: Kay Allen, Therese Andrews, Rachel Berman, Sarah Bishop-Horton, Alice Cassidy, Sara Castillo, Martha Curtis, Vanessa D’Anna, Meghan Driscoll, Carol Farmer, Susan Fitzpatrick, Anne Fletcher, Carol Furlong, Severina Haddad, Carol Johnson, Sarah Kelly, Lisa Kennedy, Meghan Santos, Cynthia Meddich, Katie Merrill, Krystal Murray, Brenda Nickerson, Thomas Patterson, Reni Poulakos, Christina Riggs, Brenda Wilbert, Joanne Wojcik, and Valerie Zelonis.
Grant Support: Supported by the National Institute of Mental Health (R01 MH62324).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure: No disclosures to report.
References
- 1.Bartels SJ, Miles KM, Dums AR, et al. Factors associated with community mental health service use by older adults with severe mental illness. Journal of Mental Health and Aging. 2003;9:123–135. [Google Scholar]
- 2.Bartels SJ. Commentary: the forgotten older adult with serious mental illness: the final challenge in achieving the promise of Olmstead? Journal of Aging and Social Policy. 2011;23:244–257. doi: 10.1080/08959420.2011.579497. [DOI] [PubMed] [Google Scholar]
- 3.United States Census Bureau . National population projections released 2008: table 12: projections of the population by age and sex for the United States: 2010 to 2050. United States Census Bureau; Washington, DC: 2008. [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration . MedTEAM: how to use the evidence-based practices KITs. Center for Mental Health Services, Substance Abuse and Mental Health Services Administration, U.S. Department of Health and Human Services; Rockville, MD: 2010. [Google Scholar]
- 5.Institute of Medicine . The mental health and substance use workforce for older adults: in whose hands? The National Academies Press; Washington, DC: 2012. [PubMed] [Google Scholar]
- 6.Granholm E, Holden J, Link PC, et al. Randomized controlled trial of cognitive behavioral social skills training for older consumers with schizophrenia: defeatist performance attitudes and functional outcome. Am J Geriatr Psychiatry. 2013;21:251–262. doi: 10.1016/j.jagp.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granholm E, McQuaid JR, McClure FS, et al. Randomized controlled trial of cognitive behavioral social skills training for older people with schizophrenia: 12-month follow-up. Journal of Clinical psychiatry. 2007;68:730–737. doi: 10.4088/jcp.v68n0510. [DOI] [PubMed] [Google Scholar]
- 8.Granholm E, McQuaid JR, McClure FS, et al. A randomized, controlled trial of cognitive behavioral social skills training for middle-aged and older outpatients with chronic schizophrenia. American Journal of Psychiatry. 2005;162:520–529. doi: 10.1176/appi.ajp.162.3.520. [DOI] [PubMed] [Google Scholar]
- 9.Berry K, Purandare N, Drake R, et al. A mixed-methods evaluation of a pilot psychosocial intervention group for older people with schizophrenia. Behavioural and Cognitive Psychotherapy. 2013:1–12. doi: 10.1017/S1352465812001075. [DOI] [PubMed] [Google Scholar]
- 10.Patterson TL, McKibbin C, Taylor M, et al. Functional Adaptation Skills Training (FAST): a pilot psychosocial intervention study in middle-aged and older patients with chronic psychotic disorders. Am J Geriatr Psychiatry. 2003;11:17–23. [PubMed] [Google Scholar]
- 11.Patterson TL, Mausbach BT, McKibbin C, et al. Functional Adaptation Skills Training (FAST): a randomized trial of a psychosocial intervention for middle-aged and older patients with chronic psychotic disorders. Schizophrenia Research. 2006;86:291–299. doi: 10.1016/j.schres.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 12.Bartels SJ, Clark RE, Peacock WJ, et al. Medicare and Medicaid costs for schizophrenia patients by age cohort compared with costs for depression, dementia, and medically ill patients. Am J Geriatr Psychiatry. 2003;11:648–657. doi: 10.1176/appi.ajgp.11.6.648. [DOI] [PubMed] [Google Scholar]
- 13.Bartels SJ, Miles KM, Dums AR, et al. Are nursing homes appropriate for older adults with severe mental illness? conflicting consumer and clinician views and implications for the Olmstead decision. Journal of the American Geriatrics Society. 2003;51:1571–1579. doi: 10.1046/j.1532-5415.2003.51508.x. [DOI] [PubMed] [Google Scholar]
- 14.Hendrie HC, Lindgren D, Hay DP, et al. Comorbidity profile and healthcare utilization in elderly patients with serious mental illnesses. Am J Geriatr Psychiatry. 2013 doi: 10.1016/j.jagp.2013.01.056. [Epub ahead of print]: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Druss BG, Rosenheck RA, Desai MM, et al. Quality of preventive medical care for patients with mental disorders. Medical Care. 2002;40:129–136. doi: 10.1097/00005650-200202000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Bartels SJ, Forester B, Mueser KT, et al. Enhanced skills training and health care management for older persons with severe mental illness. Community Mental Health Journal. 2004;40:75–90. doi: 10.1023/b:comh.0000015219.29172.64. [DOI] [PubMed] [Google Scholar]
- 17.Pratt SI, Bartels SJ, Mueser KT, et al. Helping Older People Experience Success: an integrated model of psychosocial rehabilitation and health care management for older adults with serious mental illness. American Journal of Psychiatric Rehabilitation. 2008;11:41–60. [Google Scholar]
- 18.Mueser KT, Pratt SI, Bartels SJ, et al. Randomized trial of social rehabilitation and integrated health care for older people with severe mental illness. J Consult Clin Psychol. 2010;78:561–573. doi: 10.1037/a0019629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pratt SI, Mueser KT, Bartels SJ, et al. The impact of skills training on cognitive functioning in older people with serious mental illness. Am J Geriatr Psychiatry. 2013;21:242–250. doi: 10.1097/JGP.0b013e31826682dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DMS-IV Axis-I Disorders - Patient Edition (SCID-I/P , Version 2.0) Biometrics Research Department; New York: 1996. [Google Scholar]
- 21.Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 22.Bellack AS. Skills training for people with severe mental illness. Psychiatr Rehabil J. 2004;27:375–391. doi: 10.2975/27.2004.375.391. [DOI] [PubMed] [Google Scholar]
- 23.Wallace CJ, Liberman RP, Tauber R, et al. The Independent Living Skills Survey: a comprehensive measure of the community functioning of severely and persistently mentally ill individuals. Schizophrenia Bulletin. 2000;26:631–658. doi: 10.1093/oxfordjournals.schbul.a033483. [DOI] [PubMed] [Google Scholar]
- 24.Barker S, Barron N, McFarland BH, et al. A community ability scale for chronically mentally ill consumers: part I. reliability and validity. Community Mental Health Journal. 1994;30:363–379. doi: 10.1007/BF02207489. [DOI] [PubMed] [Google Scholar]
- 25.Patterson TL, Goldman S, McKibbin CL, et al. USCD Performance-based Skills Assessment: development of a new measure of everyday functioning for severely mentally ill adults. Schizophrenia Bulletin. 2001;27:235–245. doi: 10.1093/oxfordjournals.schbul.a006870. [DOI] [PubMed] [Google Scholar]
- 26.Wykes T, Sturt E. The measurement of social behaviour in psychiatric patients: an assessment of the reliability and validity of the SBS Schedule. British Journal of Psychiatry. 1986;148:1–11. doi: 10.1192/bjp.148.1.1. [DOI] [PubMed] [Google Scholar]
- 27.McDermott BE. Development of an instrument for assessing self-efficacy in schizophrenic spectrum disorders. Journal of Clinical Psychology. 1995;51:320–331. [PubMed] [Google Scholar]
- 28.Andersen J, Larsen JK, Korner A, et al. The brief psychiatric rating scale: schizophrenia, reliability and validity studies. Nordic Journal of Psychiatry. 1986;40:135–138. [Google Scholar]
- 29.Andreasen NC. Modified Scale for the Assessment of Negative Symptoms. U.S. Department of Health and Human Services; Bethesda, MD: 1984. [Google Scholar]
- 30.Lewinsohn PM, Seeley JR, Roberts RE, et al. Center for Epidemiologic Studies Depression Scale (CES-D) as a screening instrument for depression among community-residing older adults. Psychology and Aging. 1997;12:277–287. doi: 10.1037//0882-7974.12.2.277. [DOI] [PubMed] [Google Scholar]
- 31.Ware JE, Jr., Snow KK, Kosinski M, et al. SF-36 Health Survey Manual and Interpretation Guide. The Health Institute, New England Medical Center; Boston, MA: 1997. [Google Scholar]
- 32.Charlson M, Pompei P, Ales K, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 33.U.S. Preventive Services Task Force . Recommendations for adults. USPSTF Program Office; Rockville, Maryland: 2013. [Google Scholar]
- 34.Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. John Wiley & Sons; New York: 2004. [Google Scholar]
- 35.Hedeker D, Gibbons RD. Longitudinal data analysis. Wiley; New York: 2006. [Google Scholar]
- 36.Jennrich R, Schluchter M. Unbalanced repeated-measures models with structured covariance matrices. Biometrics. 1986;42:805–820. [PubMed] [Google Scholar]
- 37.Cohen J. Statistical power analysis for the behavioral sciences. second edition Lawrence Erlbaum Associates, Inc.; Hillsdale, New Jersey: 1988. [Google Scholar]
- 38.Kazis LE, Anderson JJ, Meenan RF. Effect sizes for interpreting changes in health status. Medical Care. 1989;27:S178–S189. doi: 10.1097/00005650-198903001-00015. [DOI] [PubMed] [Google Scholar]
- 39.Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. New England Journal of Medicine. 1988;318:1728–1733. doi: 10.1056/NEJM198806303182605. [DOI] [PubMed] [Google Scholar]
- 40.Crowther RE, Marshall M, Bond GR, et al. Helping people with severe mental illness to obtain work: systematic review. BMJ. 2001;322:204–208. doi: 10.1136/bmj.322.7280.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patterson TL, Bucardo J, McKibbin CL, et al. Development and pilot testing of a new psychosocial intervention for older Latinos with chronic psychosis. Schizophrenia Bulletin. 2005;31:922–930. doi: 10.1093/schbul/sbi036. [DOI] [PubMed] [Google Scholar]
- 42.Mausbach BT, Bowie CR, Harvey PD, et al. Usefulness of the UCSD Performance-Based Skills Assessment (UPSA) for predicting residential independence in patients with chronic schizophrenia. Journal of Psychiatric Research. 2008;42:320–327. doi: 10.1016/j.jpsychires.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartels SJ, Naslund JA. The underside of the silver tsunami—older adults and mental health care. New England Journal of Medicine. 2013;368:493–496. doi: 10.1056/NEJMp1211456. [DOI] [PubMed] [Google Scholar]