Abstract
The recessive nuclear gene iojap of Zea mays conditions a permanent, heritable deficiency in the ability of the plastid to differentiate. iojap-affected plastids contain a normal genome as evidenced by comparison of the restriction endonuclease digestion patterns of affected and normal plastids. iojap-affected plastids contain neither detectable ribosomes nor high molecular weight RNA; the affected plastids do not incorporate exogenous amino acids into protein. The lesion in plastid ribosome content occurs early in organ ontogeny because iojap-mediated albino stripes can occupy entire clones within affected leaves.
Keywords: chloroplast DNA, chloroplast ribosome, differentiation, meristem, leaf development
Full text
PDF

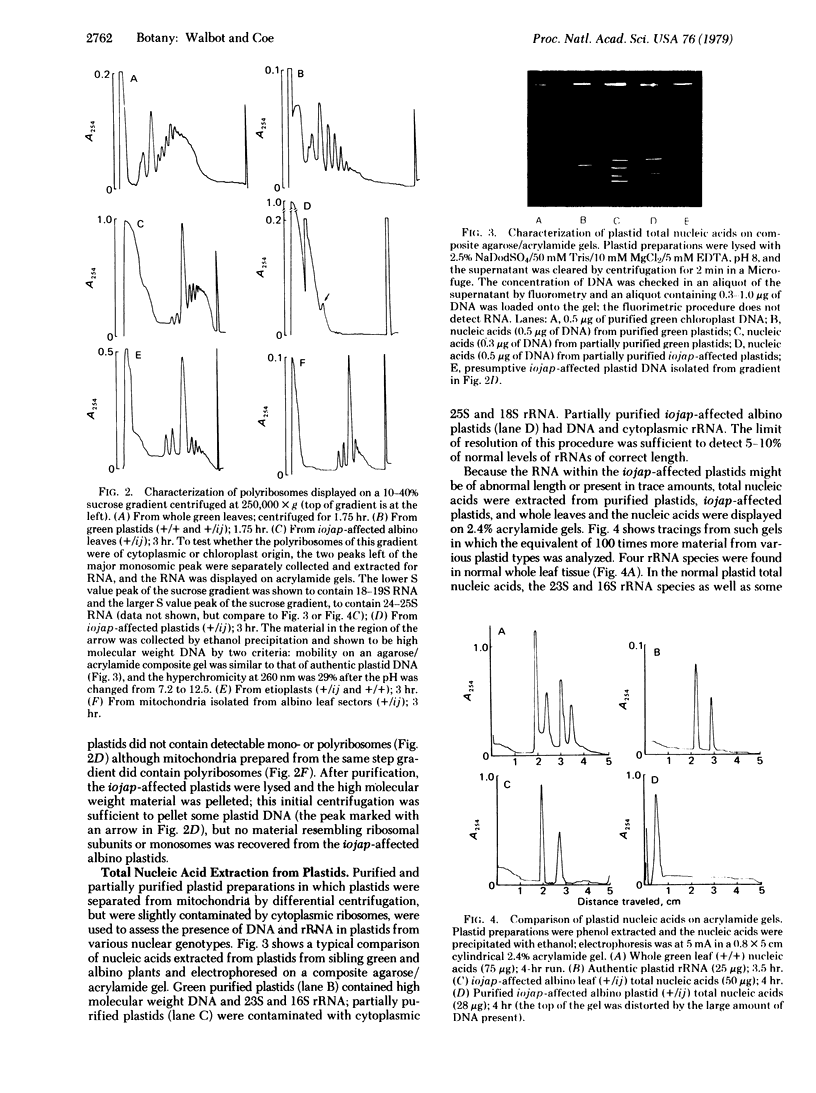


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beachy R. N., Thompson J. F., Madison J. T. Isolation of polyribosomes and messenger RNA active in in vitro synthesis of soybean seed proteins. Plant Physiol. 1978 Feb;61(2):139–144. doi: 10.1104/pp.61.2.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden J. C., Jr Quantitation of submicrogram quantities of protein by an improved protein-dye binding assay. Biochim Biophys Acta. 1978 Apr 26;533(2):525–529. doi: 10.1016/0005-2795(78)90398-7. [DOI] [PubMed] [Google Scholar]
- Bedbrook J. R., Bogorad L. Endonuclease recognition sites mapped on Zea mays chloroplast DNA. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4309–4313. doi: 10.1073/pnas.73.12.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedbrook J. R., Kolodner R., Bogorad L. Zea mays chloroplast ribosomal RNA genes are part of a 22,000 base pair inverted repeat. Cell. 1977 Aug;11(4):739–749. doi: 10.1016/0092-8674(77)90288-4. [DOI] [PubMed] [Google Scholar]
- Börner T., Herrmann F., Hagemann R. Plastid ribosome deficient mutants of Pelargonium zonale. FEBS Lett. 1973 Dec 1;37(2):117–119. doi: 10.1016/0014-5793(73)80438-7. [DOI] [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Highfield P. E., Ellis R. J. Protein synthesis in chloroplasts. VII. Initiation of protein synthesis in isolated intact pea chloroplasts. Biochim Biophys Acta. 1976 Sep 20;447(1):20–27. doi: 10.1016/0005-2787(76)90091-5. [DOI] [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pring D. R., Levings C. S. Heterogeneity of Maize Cytoplasmic Genomes among Male-Sterile Cytoplasms. Genetics. 1978 May;89(1):121–136. doi: 10.1093/genetics/89.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid E. E., Lyttle C. R., Canvin D. T., Dennis D. T. Pyruvate dehydrogenase complex activity in proplastids and mitochondria of developing castor bean endosperm. Biochem Biophys Res Commun. 1975 Jan 6;62(1):42–47. doi: 10.1016/s0006-291x(75)80402-5. [DOI] [PubMed] [Google Scholar]
- Upholt W. B. Estimation of DNA sequence divergence from comparison of restriction endonuclease digests. Nucleic Acids Res. 1977;4(5):1257–1265. doi: 10.1093/nar/4.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]