Abstract
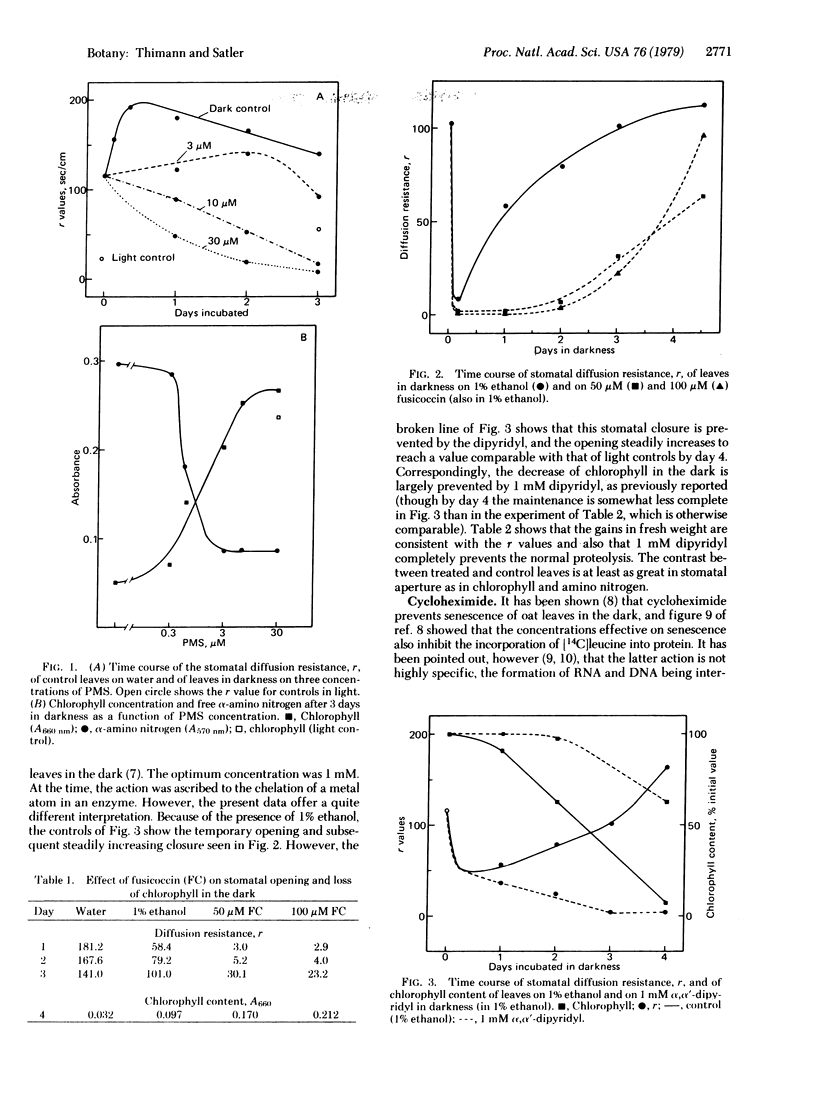
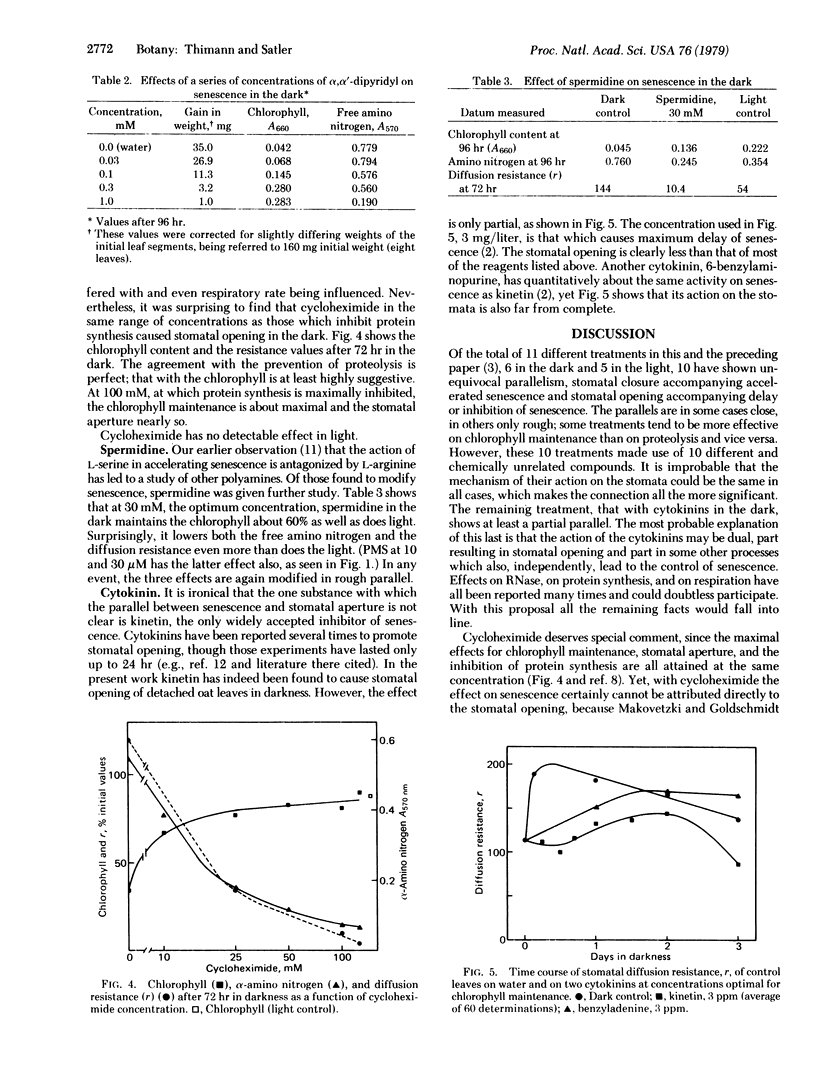
The senescence (proteolysis and loss of chlorophyll) of isolated leaves of oat seedlings in the dark is inhibited or delayed by compounds of six different types: phenazine methosulfate, fusicoccin, α,α′-dipyridyl, cycloheximide, spermidine, and two cytokinins. In every case but the last, these compounds in optimum concentration caused the stomata to open and remain partly or completely open throughout the 72- or 96-hr experimental period. The cytokinins caused only a partial opening, which is ascribed to their exerting two different effects. Taken together with the previous report that five different treatments that accelerated or promoted senescence in the light caused stomatal closure or occlusion, these data establish a general parallel between stomatal aperture and senescence, with strong indication that the stomatal aperture is the causal factor. A possible explanation of the relationship is proposed.
Keywords: abscisic acid, cycloheximide, cytokinins, diffusion resistance, dipyridyl, fusicoccin, phenazine methosulfate, spermidine
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Kaur-Sawhney R., Galston A. W. Stabilization of Oat Leaf Protoplasts through Polyamine-mediated Inhibition of Senescence. Plant Physiol. 1977 Oct;60(4):570–574. doi: 10.1104/pp.60.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemasu E. T., Thurtell G. W., Tanner C. B. Design calibration and field use of a stomatal diffusion porometer. Plant Physiol. 1969 Jun;44(6):881–885. doi: 10.1104/pp.44.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. Role of Protein Synthesis in the Senescence of Leaves: II. The Influence of Amino Acids on Senescence. Plant Physiol. 1972 Oct;50(4):432–437. doi: 10.1104/pp.50.4.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C., Thimann K. V. The role of protein synthesis in the senescence of leaves: I. The formation of protease. Plant Physiol. 1972 Jan;49(1):64–71. doi: 10.1104/pp.49.1.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon D. Cycloheximide is not a specific inhibitor of protein synthesis in vivo. Plant Physiol. 1975 May;55(5):815–821. doi: 10.1104/pp.55.5.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley R. M., Thimann K. V. The Metabolism of Oat Leaves during Senescence: I. Respiration, Carbohydrate Metabolism, and the Action of Cytokinins. Plant Physiol. 1974 Sep;54(3):294–303. doi: 10.1104/pp.54.3.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetley R. M., Thimann K. V. The Metabolism of Oat Leaves during Senescence: IV. The Effects of alphaalpha'-Dipyridyl and other Metal Chelators on Senescence. Plant Physiol. 1975 Jul;56(1):140–142. doi: 10.1104/pp.56.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Satler S. O. Relation between leaf senescence and stomatal closure: Senescence in light. Proc Natl Acad Sci U S A. 1979 May;76(5):2295–2298. doi: 10.1073/pnas.76.5.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thimann K. V., Tetley R. M., Krivak B. M. Metabolism of Oat Leaves during Senescence: V. Senescence in Light. Plant Physiol. 1977 Mar;59(3):448–454. doi: 10.1104/pp.59.3.448. [DOI] [PMC free article] [PubMed] [Google Scholar]



