Abstract
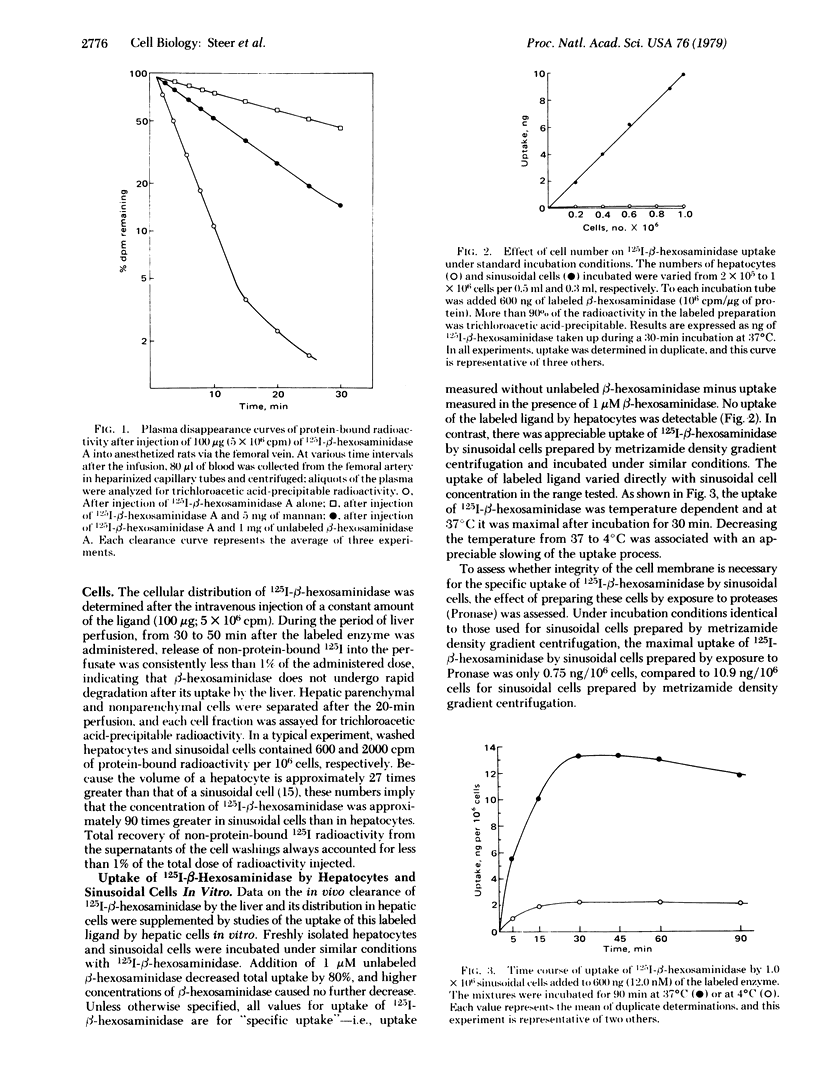
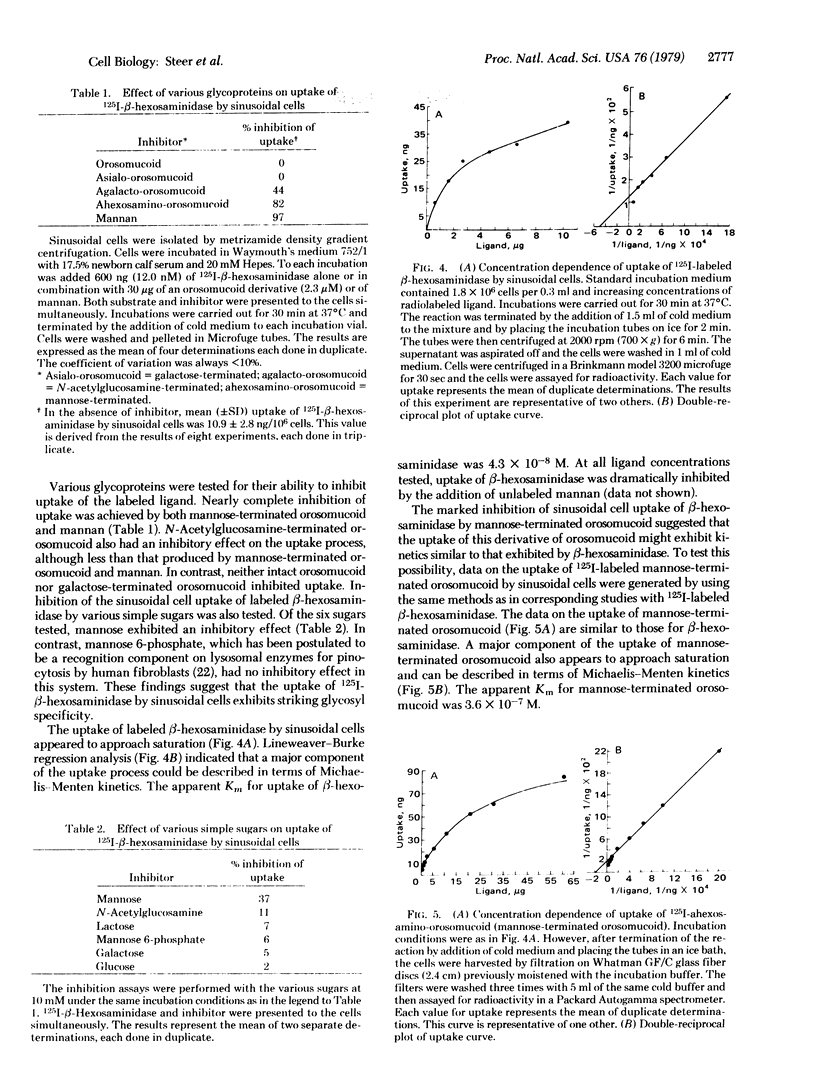
Intravenously administered 125I-labeled human β-hexosaminidase A (β-N-acetylglucosaminidase; 2-acetamido-2-deoxy-β-D-glucoside acetamidodeoxyglucohydrolase, EC 3.2.1.30) was rapidly cleared from the circulation of rats and accumulated in the liver. When hepatic cells were subsequently isolated, the label was recovered from both sinusoidal cells and, to a lesser extent, hepatocytes. Clearance was inhibited by the simultaneous infusion of mannan but not by a galactose-terminated glycoprotein. Studies in vitro, in which 125I-β-hexosaminidase was incubated with isolated hepatic cells, detected no uptake of the labeled ligand by hepatocytes. In contrast, uptake by sinusoidal cells was shown to be temperature dependent and approached saturability. Prior treatment of sinusoidal cells with Pronase resulted in markedly decreased uptake of 125I-β-hexosaminidase by these cells. Mannan and partially deglycosylated glycoproteins bearing terminal nonreducing N-acetylglucosamine or mannose residues were shown to be potent inhibitors of the cellular uptake of 125I-β-hexosaminidase; native orosomucoid and desialylated (galactoseterminated) orosomucoid were not inhibitory. Of six simple sugars tested, including N-acetylglucosamine, only mannose was an effective inhibitor of the cellular uptake of 125I-β-hexosaminidase. The kinetics of uptake of β-hexosaminidase and mannose-terminated orosomucoid by sinusoidal cells were shown to be similar. These findings suggest that the hepatic uptake of the lysosomal glycosidase β-hexosaminidase A is mediated by a receptor on sinusoidal cells which recognizes and binds mannose-terminated glycoproteins.
Keywords: receptor-mediated uptake, glycoproteins, β-N-acetylglucosaminidase
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achord D. T., Brot F. E., Bell C. E., Sly W. S. Human beta-glucuronidase: in vivo clearance and in vitro uptake by a glycoprotein recognition system on reticuloendothelial cells. Cell. 1978 Sep;15(1):269–278. doi: 10.1016/0092-8674(78)90102-2. [DOI] [PubMed] [Google Scholar]
- Achord D. T., Brot F. E., Sly W. S. Inhibition of the rat clearance system for agalacto-orosomucoid by yeast mannans and by mannose. Biochem Biophys Res Commun. 1977 Jul 11;77(1):409–415. doi: 10.1016/s0006-291x(77)80213-1. [DOI] [PubMed] [Google Scholar]
- Ashwell G., Morell A. G. The role of surface carbohydrates in the hepatic recognition and transport of circulating glycoproteins. Adv Enzymol Relat Areas Mol Biol. 1974;41(0):99–128. doi: 10.1002/9780470122860.ch3. [DOI] [PubMed] [Google Scholar]
- Berg T., Boman D. Distribution of lysosomal enzymes between parenchymal and Kupffer cells of rat liver. Biochim Biophys Acta. 1973 Oct 10;321(2):585–596. doi: 10.1016/0005-2744(73)90201-5. [DOI] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T. L., Henderson L. A., Thorpe S. R., Baynes J. W. The effect of alpha-mannose-terminal oligosaccharides on the survival of glycoproteins in the circulation. Rapid uptake and catabolism of bovine pancreatic ribonuclease B by nonparenchymal cells of rat liver. Arch Biochem Biophys. 1978 Jun;188(2):418–428. doi: 10.1016/s0003-9861(78)80026-5. [DOI] [PubMed] [Google Scholar]
- Emeis J. J., Planqué B. Heterogeneity of cells isolated from rat liver by pronase digestion: ultrastructure, cytochemistry and cell culture. J Reticuloendothel Soc. 1976 Jul;20(1):11–29. [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems R., Ross B. D., Berry M. N., Krebs H. A. Gluconeogenesis in the perfused rat liver. Biochem J. 1966 Nov;101(2):284–292. doi: 10.1042/bj1010284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones E. A., Vergalla J., Steer C. J., Bradley-Moore P. R., Vierling J. M. Metabolism of intact and desialylated alpha 1-antitrypsin. Clin Sci Mol Med. 1978 Aug;55(2):139–148. doi: 10.1042/cs0550139. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Fischer D., Achord D., Sly W. Phosphohexosyl recognition is a general characteristic of pinocytosis of lysosomal glycosidases by human fibroblasts. J Clin Invest. 1977 Nov;60(5):1088–1093. doi: 10.1172/JCI108860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki T., Ashwell G. Isolation and characterization of an avian hepatic binding protein specific for N-acetylglucosamine-terminated glycoproteins. J Biol Chem. 1977 Sep 25;252(18):6536–6543. [PubMed] [Google Scholar]
- Kawasaki T., Etoh R., Yamashina I. Isolation and characterization of a mannan-binding protein from rabbit liver. Biochem Biophys Res Commun. 1978 Apr 14;81(3):1018–1024. doi: 10.1016/0006-291x(78)91452-3. [DOI] [PubMed] [Google Scholar]
- Kusiak J. W., Toney J. H., Quirk J. M., Brady R. O. Specific binding of 125I-labeled beta-hexosaminidase A to rat brain synaptosomes. Proc Natl Acad Sci U S A. 1979 Feb;76(2):982–985. doi: 10.1073/pnas.76.2.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBadie J. H., Chapman K. P., Aronson N. N., Jr Glycoprotein catabolism in rat liver: Lysosomal digestion of iodinated asialo-fetuin. Biochem J. 1975 Nov;152(2):271–279. doi: 10.1042/bj1520271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunney J., Ashwell G. A hepatic receptor of avian origin capable of binding specifically modified glycoproteins. Proc Natl Acad Sci U S A. 1976 Feb;73(2):341–343. doi: 10.1073/pnas.73.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munthe-Kaas A. C. Phagocytosis in rat Kupffer cells in vitro. Exp Cell Res. 1976 May;99(2):319–327. doi: 10.1016/0014-4827(76)90589-9. [DOI] [PubMed] [Google Scholar]
- Munthe-Kaas A. C., Seglen P. O. The use of Metrizamide as a gradient medium for isopycnic separation of rat liver cells. FEBS Lett. 1974 Aug 1;43(3):252–256. doi: 10.1016/0014-5793(74)80654-x. [DOI] [PubMed] [Google Scholar]
- Regoeczi E., Debanne M. T., Hatton M. C., Koj A. Elimination of asialofetuin and asialoorosomucoid by the intact rat. Quantitative aspects of the hepatic clearance mechanism. Biochim Biophys Acta. 1978 Jul 3;541(3):372–384. doi: 10.1016/0304-4165(78)90196-4. [DOI] [PubMed] [Google Scholar]
- Schlesinger P. H., Doebber T. W., Mandell B. F., White R., DeSchryver C., Rodman J. S., Miller M. J., Stahl P. Plasma clearance of glycoproteins with terminal mannose and N-acetylglucosamine by liver non-parenchymal cells. Studies with beta-glucuronidase, N-acetyl-beta-D-glucosaminidase, ribonuclease B and agalacto-orosomucoid. Biochem J. 1978 Oct 15;176(1):103–109. doi: 10.1042/bj1760103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger P., Rodman J. S., Frey M., Lang S., Stahl P. Clearance of lysosomal hydrolases following intravenous infusion. The role of liver in the clearance of beta-glucuronidase and N-acetyl-beta-D-glucosaminidase. Arch Biochem Biophys. 1976 Dec;177(2):606–614. doi: 10.1016/0003-9861(76)90472-0. [DOI] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P., Schlesinger P. H., Rodman J. S., Doebber T. Recognition of lysosomal glycosidases in vivo inhibited by modified glycoproteins. Nature. 1976 Nov 4;264(5581):86–88. doi: 10.1038/264086a0. [DOI] [PubMed] [Google Scholar]
- Stahl P., Six H., Rodman J. S., Schlesinger P., Tulsiani D. R., Touster O. Evidence for specific recognition sites mediating clearance of lysosomal enzymes in vivo. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4045–4049. doi: 10.1073/pnas.73.11.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. The existence of a second route for the transfer of certain glycoproteins from the circulation into the liver. Biochem Biophys Res Commun. 1976 Feb 9;68(3):988–993. doi: 10.1016/0006-291x(76)91243-2. [DOI] [PubMed] [Google Scholar]
- Tallman J. F., Brady R. O., Quirk J. M., Villalba M., Gal A. E. Isolation and relationship of human hexosaminidases. J Biol Chem. 1974 Jun 10;249(11):3489–3499. [PubMed] [Google Scholar]
- Thomas P., Birbeck M. S., Cartwright P. A radioautographic study of the hepatic uptake of circulating carcinoembryonic antigen by the mouse. Biochem Soc Trans. 1977;5(1):312–313. doi: 10.1042/bst0050312. [DOI] [PubMed] [Google Scholar]


