Abstract
Previous studies have shown that cGMP-dependent protein kinase (PKG) act on several targets in the contractile pathway to reduce intracellular Ca2+ and/or augment RhoA-regulated myosin light chain phosphatase (MLCP) activity and cause muscle relaxation. Recent studies have identified a novel protein M-RIP that associates with MYPT1, the regulatory subunit of MLCP. Herein, we examine whether PKG enhance MLCP activity downstream of Ca2+ and RhoA via phosphorylation of M-RIP in gastric smooth muscle cells. Treatment of permeabilized muscle cells with 10 μM Ca2+ caused an increase in MLC20 phosphorylation and muscle contraction, but had no effect on Rho kinase activity. Activators of PKG (GSNO or cGMP) decreased MLC20 phosphorylation and contraction in response to 10 μM Ca2+, implying existence of inhibitory mechanism independent of Ca2+ and RhoA. The effect of PKG on Ca2+-induced MLC20 phosphorylation was attenuated by M-RIP siRNA. Both GSNO and 8-pCPT-cGMP induced phosphorylation of M-RIP; phosphorylation was accompanied by an increase in the association of M-RIP with MYPT1 and MLCP activity. Taken together, these results provide evidence that PKG induces phosphorylation of M-RIP and enhances its association with MYPT1 to augment MLCP activity and MLC20 dephosphorylation and inhibits muscle contraction, downstream of Ca2+- or RhoA-dependent pathways.
1. Introduction
Contraction of smooth muscle is dependent on phosphorylation of 20 kDa myosin light chain phosphorylation (MLC20) at Ser19, which stimulates the ATPase activity of the smooth muscle myosin [1-3]. The levels of MLC20 are regulated by opposing activities of MLC kinase (MLCK) and MLC phosphatase (MLCP). Contractile agonists stimulate MLCK, a Ca2+/calmodulin-dependent enzyme, mainly by increasing cytosolic Ca2+ and inhibit MLCP. Inhibition of MLCP is mediated via phosphorylation of CPI-17, and endogenous inhibitor of MLCP, by protein kinase C, and the regulatory subunit of MLCP by Rho kinase [1, 2, 4-6]. MYPT1 acts as a regulator of the catalytic subunit by targeting MLCP to myosin filaments and enhancing substrate specificity towards myosin. The N-terminal of MYPT1 is composed of eight repeat sequences that correspond to the sequences of an ankyrin repeat that are important for regulation and targeting of MLCP. The holoenzyme of MLCP has higher activity than its catalytic subunit suggesting that the binding of the regulatory subunit increases MLCP activity. Phosphorylation of MYPT1 by RhoA/Rho kinase pathway was shown to dissociate MYPT1 from myosin and, hence may decrease the dephosphorylating activity of MLCP toward myosin [5, 7, 8].
Recent studies have identified a new protein termed myosin phosphatase-rho interacting protein (M-RIP) that interact directly and simultaneously with RhoA and MYPT1 and thus, forms a ternary complex [9-14]. M-RIP lacks a catalytic domain, but contains several protein-protein interaction domains including two pleckstrin homology domains, two proline-rich sequences, and a C-terminal coiled-coil domain [9-11]. MYPT1 and RhoA bind on distinct sites of M-RIP and hence do not interfere with each other. RhoA interacting region resides in the first coiled region, whereas MYPT1 interacting region resides in the second/third coiled-coil region [15]. Suppression of M-RIP blocks inhibition of MLCP activity in response to contractile agonist suggesting that M-RIP plays an important role in the regulation of MLCP activity and MLC20 phosphorylation [6]. M-RIP facilitates the myosin/MLCP interaction and stimulates the activity of the holoenzyme toward myosin without affecting the catalytic subunit alone [16]. The N-terminus of M-RIP binds to actin and thus targets MLCP to the acto-myosin contractile filaments to dephosphorylate myosin [11, 12, 14]. Interaction of MLCP, via leucine zipper domain at C-terminus of MYPT1, with cGMP-dependent protein kinase (PKG) leads to the association of the M-RIP with PKGIα [15, 17-23]. It is therefore conceivable that M-RIP may also be a target of PKGIα. Consistent with this possibility M-RIP is a phosphoprotein and co-precipitates with PKGIα [13, 24]. However, phosphorylation of M-RIP and the regulatory role of phosphorylation are not known. In the present study, we set out to examine the effect of PKG on M-RIP-regulated MLCP activity. We show that activation of PKG: (i) increases phosphorylation of M-RIP ; ii) increases the association of M-RIP with MYPT1; (iii) increases MLCP activity, and (iv) decreases Ca2+-induced MLC20 phosphorylation and muscle contraction downstream of Ca2+ and RhoA. We conclude that pM-RIP is a target of PKG to mediate MLC20 dephosphorylation and muscle relaxation.
2. Materials and methods
2.1. Materials
[32P]orthophosphate was obtained from PerkinElmer Life Sciences (Boston, MA). Y27632 was obtained from Calbiochem (La Jolla, CA). Polyclonal antibodies to M-RIP, MYPT1, and p-MLC20 were from Santa Cruz Biotechnology (Santa Cruz, CA). pSIREN-DNR-DsRed vector was obtained from Clontech Laboratories, Inc. CA. Lipofectamine™ 2000 transfection reagent, SuperScript™ II Reverse Transcriptase kit, DH5-α competent cells, were obtained from Invitrogen (Carlsbad, CA). Western blotting materials were obtained from Bio-Rad Laboratories (Hercules, CA). Collagenase and soybean trypsin inhibitor were obtained from Worthington Biochemical (Freehold, NJ). All other reagents were obtained from Sigma.
2.2. Gastric smooth muscle cell culture
Smooth muscle cells were isolated from the circular muscle layer of rabbit stomach by sequential enzymatic digestion in 25 mM HEPES medium as described previously [25] by collagenase digestion. Dispersed muscle cells were harvested by filtration through 500-μm Nitex and then centrifuged twice at 350 g for 10 min. For permeabilization, dispersed smooth muscle cells were treated for 5 min with saponin (35 μg/ml) and resuspended in low-Ca2+ (100 nM) medium as previously described [26]. In some experiments, the cells were placed in culture in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum until they attained confluence [25].
2.3. Transfection of M-RIP siRNA
The RNAi-Ready pSIREN-DNR-DsRed-Express Vector encoding M-RIP small-interfering RNA was inserted between BamH1 and EcoR1 restriction sites and transfected into cultured gastric smooth muscle cells with lipofectamine™2000 reagent (Invitrogen) according to the manufacturer's recommendation. To check the specificity of the siRNA, empty vector without the siRNA sequence was used as control. Successful knockdown of M-RIP protein was verified by western blot and immunofluorescence microscopy [25].
2.4. Phosphorylaiton of M-RIP
Phosphorylation of M-RIP was determined from the amount of 32P incorporated by immunoprecipitation with specific antibody to M-RIP. Briefly, freshly dispersed cells were incubated with [32P]orthophosphate for 4 h and samples (3 × 106 cells/ml) were then incubated with S-nitrosoglutathione (GSNO, 10 μM) or [8-(4-chlorophenylthio) guanosine 3′,5′-cyclic monophosphate (8-pCPT-cGMP, 10 μM) for 10 min in the presence or absence of PKG inhibitor guanosine 3’,5’-cyclic monophosphorothioate, Rp isomer (Rp-cGMPS, 10 μM). Cell lysates were separated by centrifugation at 13,000 g for 10 min at 4°C, precleared with 40 μl of protein A-Sepharose, and incubated with M-RIP antibody for 2 h at 4°C and with 40 μl of protein A-Sepharose for another 1 h. The immunoprecipitates were extracted with Laemmli sample buffer and separated by electrophoresis on SDS-PAGE. After transfer to polyvinylidene difluoride (PVDF) membranes, [32P]M-RIP was visualized by autoradiography, and the amount of radioactivity in the band was measured using liquid scintillation. The results were expressed as counts per minute (cpm/mg protein) [25, 27].
2.5. Phosphorylation of MLC20
Permeabilized muscle cells were treated for 10 min with GSNO (10 μM) or cGMP (10 μM) followed by addition of Ca2+ (10 μM) for 30 s. Phosphorylation of MLC20 was determined by immunoblot analysis using a phospho-Ser19-specific antibody as described previously [25].
2.6. Immunoblot analysis of M-RIP association with MYPT1
Smooth muscle cells (3 × 106 cell/ml) were treated with GSNO (10 μM) or 8-pCPT-cGMP (10 μM) and the cell lysates were used to obtain MYPT1 immunoprecipitates. The immunoprecipitates were separated by SDS-PAGE, transferred to PVDF membranes, and probed with antibody to M-RIP. After incubation with secondary antibody, the proteins were visualized. The intensity of the protein band on ECL film was determined using densitometry [27].
2.7. Assay for Rho Kinase activity
Rho kinase activity was measured by an immunokinase assay as previously described [25]. Twenty microliters of Rho kinase immunoprecipitates were added to the reaction mixture containing 100 mM Tris-HCl (pH 7.4), 1 M KCl, 50 mM MgCl2, 1 mM DTT, 1 mM ATP, and 10 μCi of [γ-32P] ATP (3,000 Ci/mol) along with 5 μg of myelin basic protein, followed by incubation for 15 min at 37 °C. Phosphorylation of myelin basic protein was measured by liquid scintillation. The results are expressed as counts per minute per milligram of protein.
2.8. Measurement of MLCP activity
MLCP activity in myosin-enriched fractions prepared from permeabilized muscle cells was assayed using [32P]-labeled MLC20 as substrates [28]. [32P]-labeled MLC20 was prepared as described previously [29] by incubating MLC20 (0.8 mg/ml) with purified MLCK (50 μg/ml), 0.1 mg/ml calmodulin, and 50 μmol/l [γ-32P]ATP.
2.9. Measurement of contraction in dispersed smooth muscle cells
Permeabilized muscle cells were treated for 10 min with GSNO (10 μM) or cGMP (10 μM) followed by addition of Ca2+ (10 μM) for 30 s. The reaction was terminated with 1% acrolein. The mean cell length of 50 muscle cells treated with Ca2+ alone or in the presence of GSNO or cGMP was measured using scanning micrometry and was compared with the length of untreated muscle cells (mean control cell length 96±4 μm). Contraction was measured as decrease in muscle cell length in response to Ca2+ and relaxation was measured as inhibition of contraction [25, 26].
2.10. Statistical analysis
The results were expressed as means ± S.E. of n experiments and analyzed for statistical significance using Student's t-test for paired and unpaired values. Each experiment was done on cells obtained from different animals. A probability of p< 0.05 was considered significant.
3. Results
3.1. Selective Phosphorylation of M-RIP by PKG
Treatment of freshly dispersed smooth muscle cells with GSNO (10 μM) caused phosphorylation of M-RIP (3256±502 cpm/mg protein compared to control phosphorylation of 425±86 cpm/mg protein). M-RIP phosphorylation by GSNO was abolished by the selective PKG inhibitor Rp-cGMPS (10 μM, 82±5% inhibition) (Fig. 1A) suggesting that phosphorylation is mediated by PKG. Rp-cGMPS (10 μM) alone had no effect on basal M-RIP phosphorylation (483±67 cpm/mg protein). Treatment of cells with a nonhydrolyzable membrane-permeable analog of cGMP, 8-pCPT-cGMP (10 μM) that selectively activates PKG also induced phosphorylation of M-RIP (2865±425 cpm/mg protein) and the extent of phosphorylation is similar to that induced by GSNO (Fig. 1A). The effect of 8-pCPT-cGMP was inhibited by Rp-cGMPS (568±102 cpm/mg protein).
Figure 1. Phosphorylation of M-RIP and increased association of M-RIP with MYPT1 by activators of PKG.
(A) Gastric smooth muscle cells labeled with 32P were incubated with GSNO (10 μM) in the presence or absence of PKG inhibitor Rp-cGMPS (RpG, 10 μM), or 8-cCPT-cGMP (8pCPT, 10 μM) for 10 min. Immunoprecipitates derived from 500 μg of protein using M-RIP antibody were separated on SDS-PAGE. Bands corresponding to [32P]M-RIP were identified using autoradiography. Radioactivity in the bands is expressed as cpm. Immunoblot analysis showed equal amounts of loaded protein. Rp-cGMPS (10 μM) alone had no effect on basal M-RIP phosphorylation (483±67 cpm/mg protein). Values are means ± SE of 4 experiments. **P < 0.01, significant increase of M-RIP phosphorylation. (B) Dispersed smooth muscle cells were incubated with GSNO (10 μM) in the presence or absence of PKG inhibitor Rp-cGMPS (RpG (10 μM), or 8-cCPT-cGMP (8pCPT, 10 μM) for 10 min. Immunoprecipitates derived from 500 μg of protein using MYPT1 antibody were separated on SDS-PAGE and immunoblotted using M-RIP antibody. Rp-cGMPS (10 μM) alone had no effect on basal association of M-RIP with MYPT1(data not shown). Values are means ± SE of 4 experiments. **P < 0.01, significant increase in M-RIP and MYPT1 association.
3.2. Effect of PKG-mediated phosphorylation on association of M-RIP with MYPT1
We examined whether phosphorylation by PKG augmented association of M-RIP with MYPT1 by immunoprecipitation of MYPT1 followed by western blot analysis with M-RIP antibody. In the basal state M-RIP was associated with MYPT1. Rp-cGMPS (10 μM) alone had no effect on basal association of M-RIP with MYPT1 (data not shown). Treatment of cells with GSNO (10 μM) or 8-pCPT-cGMP (10 μM) increased the amount of MYPT1 associated with M-RIP. Increase in M-RIP association with MYPT1 by GSNO was abolished by the PKG inhibitor Rp-cGMPS (81±4% inhibition), suggesting that phosphorylation of M-RIP by PKG augmented the binding of M-RIP to MYPT1 (Fig. 1B). Similar increase in association was obtained by immunoprecipitation of M-RIP followed by western blot analysis with MYPT1 antibody (data not shown).
3.3. Inhibition of Ca2+ induced MLC20 phosphorylation and muscle contraction by PKG
We next examined whether increased association of M-RIP with MYPT1 results in increased MLCP activity. In these experiments, the ability of PKG activators to stimulate MLCP activity was examined in permeabilized muscle cells in the presence of Rho kinase inhibitor (Y27632, 10 μM) to exclude the effect of PKG on RhoA and RhoA-dependent MLC phosphatase activity. Pretreatment of cells with GSNO (10 μM) or cGMP (10 μM) caused significant increase in MLC phosphatase activity. Stimulation of MLCP activity by GSNO was abolished by the PKG inhibitor Rp-cGMPS (Fig. 2A). Rp-cGMPS (10 μM) alone had no effect on basal MLCP activity (data not shown).
Figure 2. Stimulation of MLCP activity by activators of PKG.
(A) Permeabilized muscle cells were treated with GSNO (10 μM) in the presence or absence of PKG inhibitor Rp-cGMPS (RpG, 10 μM), or 8-cCPT-cGMP (8pCPT, 10 μM) for 10 min. MLCP activity was measured using [32P]-labeled MLC as substrate. Rp-cGMPS (10 μM) alone had no effect on MLCP activity (data not shown). Results are expressed fold increase over basal levels. Values are means ± SE of 4 experiments. **P < 0.01, significant increase in MLCP activity. (B) Permeabilized muscle cells were treated with GSNO (10 μM) or cGMP (10 μM) for 10 min and then stimulated with Ca2+ (10 μM) for 30 s. MLC20 phosphorylation was determined by western blot analysis using phosphospecific (Ser19) antibody. Decrease in MLC20 phosphorylation in the presence of GSNO or cGMP reflects dephosphorylation of Ca2+ induced MLC20 phosphorylaition via PKG-dependent mechanisms. Values are means ± SE of 3 experiments. **P < 0.01, significant inhibition of Ca2+-induced MLC20 phosphorylation.
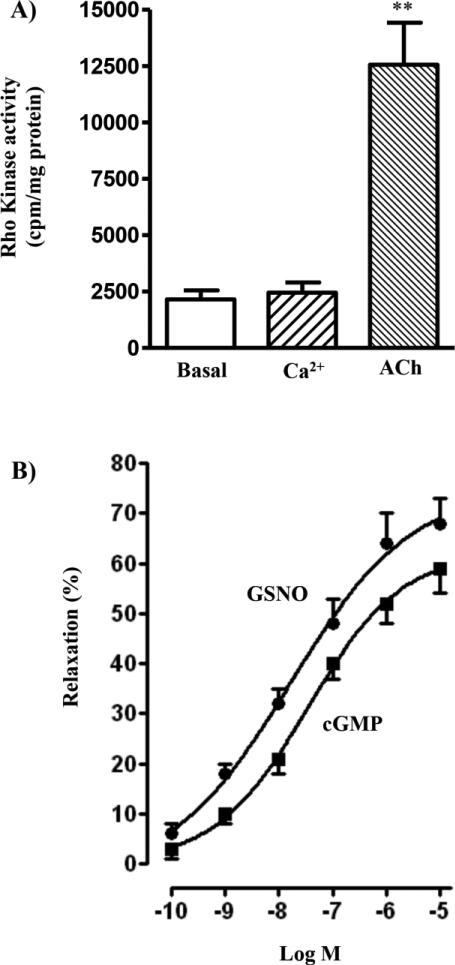
We also examined the increase in MLCP activity as inhibition of Ca2+-induced MLC20 phosphorylation. Increase in MLC20 phosphorylation by Ca2+ in permeabilized muscle cells reflects activation of Ca2+/calmodulin-dependent activation of MLCK activity and inhibition of MLCP activity via RhoA/Rho kinase-dependent pathway [1-4]. Treatment of permeabilized smooth muscle cells with Ca2+ (10 μM) in the presence of Rho kinase inhibitor Y27632 (10 μM) caused phosphorylation of MLC20 at Ser19. Pretreatment of cells for 10 min with GSNO (10 μM) or cGMP (10 μM) inhibited Ca2+-induced MLC20 phosphorylation reflecting stimulation of MLCP activity (Fig. 2B). Control studies showed that treatment of permeabilized cells with Ca2+ had no effect on Rho kinase activity (Fig. 3A). In contrast, treatment of cells with the contractile agonist, acetylcholine caused stimulation of Rho kinase activity (Fig. 3A).
Figure 3. Inhibition of Ca2+-induced muscle contraction by activators of PKG independent of RhoA.
(A) Permenabilized muscle cells were treated with Ca2+ (10 μM) or aceycholine (ACh, 1 μM) for 10 min and Rho kinase activity was measured by immunokinase assay as described in the methods. Results are expressed as cpm/mg protein. Values are means ± SE of 4 experiments. **P < 0.01, significant increase in Rho kinase activity. (B) Permeabilized muscle cells were treated with various concentrations of GSNO or cGMP in the presence of Rho kinase inhibitor Y27632 (10 μM) for 10min, and then stimulated with 10 μM Ca2+ for 30 s to induce muscle contraction. In control experiments the cells were treated for 30 s with Ca2+ in the presence of Y27632 without prior addition of GSNO or cGMP. Relaxation was expressed as % inhibition of Ca2+-induced contraction (31.3±2.8 μm decrease from control cell length of 96.2±4.3 μm). Values are means ± SE of 4–5 experiments.
Treatment of permeabilized smooth muscle cells with Ca2+ (10 μM) in the presence of Rho kinase inhibitor Y27632 (10 μM) caused contraction (32±3% decrease in cell length). Pretreatment of cells for 10 min with GSNO (10 μM) or cGMP (10 μM) inhibited Ca2+-induced contraction (i.e., induced muscle relaxation) in a concentration-dependent manner (Fig. 3B). These results suggest that inhibition of Ca2+-induced MLC20 phosphorylation and contraction by PKG was probably due to increased association of phosphorylated M-RIP with MYPT1 and subsequent stimulation of MLCP activity.
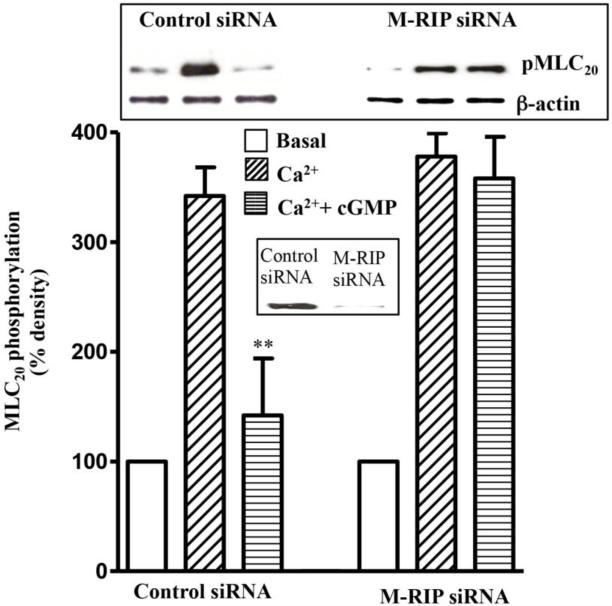
3.4. M-RIP dependent inhibition of MLC20 phosphorylation
The specific involvement of M-RIP in PKG-mediated inhibition of MLC20 phosphorylation was analysed by transfection of cells with M-RIP siRNA. Western blot analysis revealed downregulation of M-RIP5 expression in cells transfected with M-RIP siRNA (Fig. 4). Ca2+ induced MLC20 phosphorylation was not significantly affected (8±5% inhibition) by transfection of cells with M-RIP siRNA, however, GSNO-induced inhibition of MLC20 phosphorylation was significantly attenuated (80±8% inhibition) by transfection of M-RIP siRNA (Fig. 4)
Figure 4. M-RIP-dependent inhibition of MLC20 phosphorylation by PKG.
Cultured muscle cells were transfected with control siRNA or M-RIP siRNA for 48 h. Permeabilized muscle cells treated with cGMP (10 μM) for 10 min and then stimulated with Ca2+ (10 μM) for 30 s. MLC20 phosphorylation was determined by western blot analysis using phosphospecific (Ser19) antibody. cGMP significantly inhibited MLC20 phosphorylation in control cells (80±8% inhibition), but not in cells transfected with M-Rip siRNA (8±5% inhibition). Inset: Downregulation of M-RIP expression in cells transfected with M-RIP siRNA compared to control siRNA. Values are means ± SE of 3 experiments. **P < 0.01, significant inhibition of Ca2+-induced MLC20 phosphorylation.
4. Discussion
MLCP plays an important role in the regulation of MLC20 phosphorylation and thus, smooth muscle contraction [5, 8, 30]. Contractile agonists induce MLC20 phosphorylation and contraction with simultaneous inhibition of MLCP by phosphorylation of MYPT1 and CPI-17 [1, 2, 4, 5]. In contrast, relaxant agonists stimulate MLCP activity and induce MLC20 dephopshorylation and relaxation via production of cAMP and cGMP and activation of PKA and PKG activity, respectively [2, 21, 31]. Inhibition of RhoA/Rho kinase-dependent contraction by PKA and/or PKG reflects their action on various targets culminating in stimulation of MLCP activity. Three such targets have been identified: RhoA, telokin and MYPT1 [1, 32-34].
RhoA, via activation of Rho kinase and phosphorylation of the MYPT1, plays an important role in the smooth muscle contraction. Phosphorylation of MYPT1 at Thr696/Thr853 by Rho kinase inhibits the activity of MLCP and promotes MLC20 phosphorylation and contraction [1, 34]. Phosphorylaiton of RhoA (at Ser188) by PKGIα, the main isoform of PKG in smooth muscle, and by PKA leads to translocation of RhoA and inhibition of Rho kinase activity [25, 32]. Recent studies have demonstrated that binding of activated PKGIα, but not inactive PKGIα to RhoA via leucine-zipper domain is required for the inhibition of RhoA activity [35]. Telokin is an endogenous regulator of MLCP activity [33, 35]. Phosphorylation of telokin by PKA and PKG facilitates its interaction with phospho-MYPT1 (Thr696/Thr853) and/or phospho MLC20 to accelerate the dephosphorylation of the latter [1, 36]. MYPT1 is the targeting subunit of MLC phosphatase. Phosphorylation of MYPT1 at Ser695 by PKG precludes inhibitory phosphorylation by Rho kinase and aids in disinhibition of MLCP activity [34]. A newly identified member of MYPT family, MYPT3, which lacks the C-terminal leucin-zipper domain is phosphorylated by cAMP-dependent protein kinase leading to activation of the catalytic activity protein phosphatase 1 (PP1c) [37]. The functional significance of RhoA phosphorylation is well established, whereas the significance of MYPT1 and telokin phosphorylation remains to be determined.
Although, M-RIP was initially isolated as a RhoA interacting protein, subsequent studies showed that under physiological conditions, M-RIP is not likely to interact directly with RhoA and regulate RhoA or Rho kinase activity [6, 14, 15]. The functional significance of M-RIP and RhoA association is unclear. The location of the scaffolding phosphoprotein M-RIP within the contractile filaments containing myosin and its interaction with the C-terminus leucine zipper domain of MYPT1, where PKGIα is shown to associate, makes it an ideal target of PKGIα, to modulate MLCP activity and MLC20 dephosphorylation [17, 20, 31, 38]. We hypothesized that phosphorylation of M-RIP by PKG increases MLCP activity by increasing the association of MYPT1 and M-RIP. The present data support this hypothesis by showing that activators of PKG induced phosphorylation of M-RIP and subsequent increase in the association of M-RIP with MYPT1 leading to increase in MLCP activity, and decrease in Ca2+-induced MLC20 phosphorylation and muscle contraction. The main findings of the present study are that activators of PKG (i) induced phosphorylation of M-RIP; (ii) increased the association of M-RIP with MYPT1; (iii) increased MLCP activity and induced muscle relaxation (inhibition of contraction) downstream of Ca2+ and RhoA. The inhibitory effect of PKG on Ca2+-induced MLC20 phosphorylation was attenuated by suppression of M-RIP. Relaxation in response to PKG activation involves phosphorylation of several targets including M-RIP. In the present study, the effect of PKG activators examined in permeabilized cells contracted with fixed Ca2+ concentrations (10 μM) and in the presence of Rho kinase inhibitor Y27632. Under these conditions contraction was mediated by MLCK and independent of Rho kinase/MYPT1 pathway. This experimental procedure excludes the possible effects of PKG on targets involved in the regulation of Ca2+ and RhoA/Rho kinase. Permeabilization also excludes the possibility of telokin as one of the targets as treatment of cells with permeabilizing agents was shown to result in the loss of telokin from the cytosol into the extracellular medium [33]. However, the temporal relationship between M-RIP phosphorylation and MLCP activity was not examined. Thus, it is speculated that under these experimental conditions relaxation could be due to phosphorylation of M-RIP by PKG and activation of MLCP activity.
MLCP is a holoenzyme consisting of three subunits; a 20 kDa subunit of unknown function, an approximately 38-kDa catalytic subunit and a 130 kDa myosin targeting subunit (MYPT1). There are 4 distinct isoforms of MYPT1: 2 MYPT1 isoforms with inclusion or exclusion of 41 amino acid central insert, and 2 isoforms with inclusion (LZ+MYPT1) and exclusion (LZ-MYPT1) of C-terminal leucine zipper domain [19, 20]. Previous studies showed requirement of LZ domain to interact with PKGIα and PKGIα-mediated phosphorylation of MYPT1 at Ser695 [20, 22, 38]. Although the involvement of LZ domain of MYPT1 in the binding to PKGIα and M-RIP is established, direct interaction of PKGIα with M-RIP is not known. However, anchoring of both M-RIP and PKG-Iα via MYPT1 provides a scaffold for PKG-Iα to target M-RIP facilitating phosphorylation of M-RIP.
In summary, our results demonstrate that in gastrointestinal smooth muscle, M-RIP phosphorylation is mediated by PKG-Iα. Phosphorylation of M-RIP enhances its association with MYPT1 and MLC20 dephosphorylation resulting in muscle relaxation. Given the complexity and the importance of MLCP, we provide evidence that M-RIP, which cololalizes with myosin and MYPT1 complex, is a target of PKG-Iα to regulate MLCP activity and muscle contraction. The present studies do not identify the singular contribution of M-RIP to mediate muscle relaxation in response to relaxant transmitters. It is possible that M-RIP along with other targets of PKG either cooperate dynamically to maintain muscle tone or serve as backup system under conditions where other systems are compromised. The importance of M-RIP to mediate muscle relaxation may depend on the expression levels. Recent studies have shown that the expression of M-RIP was higher in phasic (antrum) than tonic (fundus) muscle [39]. Further studies are needed to examine the physiological significance of M-RIP as regulator of MLCP activity in different regions of the gastrointestinal tract.
Acknowledgements
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant to KSM (DK28300).
References
- 1.Murthy KS. Signaling for contraction and relaxation in smooth muscle of the gut. Annu Rev Physiol. 2006;68:345–374. doi: 10.1146/annurev.physiol.68.040504.094707. [DOI] [PubMed] [Google Scholar]
- 2.Somlyo AP, Somlyo AV. Ca2+ sensitivity of smooth muscle and nonmuscle myosin II: modulated by G proteins, kinases, and myosin phosphatase. Physiol Rev. 2003;83(4):1325–58. doi: 10.1152/physrev.00023.2003. [DOI] [PubMed] [Google Scholar]
- 3.Kamm KE, Stull JT. Signaling to myosin regulatory light chain in sarcomeres. J Biol Chem. 2011;286(12):9941–7. doi: 10.1074/jbc.R110.198697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Godoy MA, Rattan S. Role of rho kinase in the functional and dysfunctional tonic smooth muscles. Trends Pharmacol Sci. 2011;32:384–393. doi: 10.1016/j.tips.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ito M, Nakano T, Erdodi F, Hartshorne DJ. Myosin phosphatase: structure, regulation and function. Mol Cell Biochem. 2004;259:197–209. doi: 10.1023/b:mcbi.0000021373.14288.00. [DOI] [PubMed] [Google Scholar]
- 6.Riddick N, Ohtani K, Surks HK. Targeting by myosin phosphatase-RhoA interacting protein mediates RhoA/ROCK regulation of myosin phosphatase. J Cell Biochem. 2008;103:1158–1170. doi: 10.1002/jcb.21488. [DOI] [PubMed] [Google Scholar]
- 7.Grassie ME, Moffat LD, Walsh MP, MacDonald JA. The myosin phosphatase targeting protein (MYPT) family: a regulated mechanism for achieving substrate specificity of the catalytic subunit of protein phosphatase type 1delta. Arch Biochem Biophys. 2011;510:147–159. doi: 10.1016/j.abb.2011.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Matsumura F, Hartshorne DJ. Myosin phosphatase target subunit: Many roles in cell function. Biochem Biophys Res Commun. 2008;369:149–156. doi: 10.1016/j.bbrc.2007.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gebbink MF, Kranenburg O, Poland M, van Horck FP, Houssa B, Moolenaar WH. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mulder J, Poland M, Gebbink MF, Calafat J, Moolenaar WH, Kranenburg O. p116Rip is a novel filamentous actin-binding protein. J Biol Chem. 2003;278:27216–27223. doi: 10.1074/jbc.M302399200. [DOI] [PubMed] [Google Scholar]
- 11.Mulder J, Ariaens A, van den Boomen D, Moolenaar WH. p116Rip targets myosin phosphatase to the actin cytoskeleton and is essential for RhoA/ROCK-regulated neuritogenesis. Mol Biol Cell. 2004;15:5516–5527. doi: 10.1091/mbc.E04-04-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulder J, Ariaens A, van Horck FP, Moolenaar WH. Inhibition of RhoA-mediated SRF activation by p116Rip. FEBS Lett. 2005;579:6121–6127. doi: 10.1016/j.febslet.2005.09.083. [DOI] [PubMed] [Google Scholar]
- 13.Surks HK, Richards CT, Mendelsohn ME. Myosin phosphatase-Rho interactingprotein. A new member of the myosin phosphatase complex that directly binds RhoA. J Biol Chem. 2003;278:51484–51493. doi: 10.1074/jbc.M305622200. [DOI] [PubMed] [Google Scholar]
- 14.Surks HK, Riddick N, Ohtani K. M-RIP targets myosin phosphatase to stress fibers to regulate myosin light chain phosphorylation in vascular smooth muscle cells. J Biol Chem. 2005;280:42543–42551. doi: 10.1074/jbc.M506863200. [DOI] [PubMed] [Google Scholar]
- 15.Surks HK, Mendelsohn ME. Dimerization of cGMP-dependent protein kinase 1alpha and the myosin-binding subunit of myosin phosphatase: role of leucine zipper domains. Cell Signal. 2003;15:937–944. doi: 10.1016/s0898-6568(03)00057-3. [DOI] [PubMed] [Google Scholar]
- 16.Koga Y, Ikebe M. p116Rip decreases myosin II phosphorylation by activating myosin light chain phosphatase and by inactivating RhoA. J Biol Chem. 2005;280:4983–4991. doi: 10.1074/jbc.M410909200. [DOI] [PubMed] [Google Scholar]
- 17.Sharma AK, Zhou GP, Kupferman J, Surks HK, Christensen EN, Chou JJ, Mendelsohn ME, Rigby AC. Probing the interaction between the coiled coil leucine zipper of cGMP-dependent protein kinase Iα and the C terminus of the myosin binding subunit of the myosin light chain phosphatase. J Biol Chem. 2008;283:32860–32869. doi: 10.1074/jbc.M804916200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou GP. The structural determinations of the leucine zipper coiled-coil domains of the cGMP-dependent protein kinase Ialpha and its interaction with the myosin binding subunit of the myosin light chains phosphase. Protein Pept Lett. 2011;18:966–978. doi: 10.2174/0929866511107010966. [DOI] [PubMed] [Google Scholar]
- 19.Huang QQ, Fisher SA, Brozovich FV. Unzipping the role of myosin light chain phosphatase in smooth muscle cell relaxation. J Biol Chem. 2004;279:597–603. doi: 10.1074/jbc.M308496200. [DOI] [PubMed] [Google Scholar]
- 20.Yuen S, Ogut O, Brozovich FV. MYPT1 protein isoforms are differentially phosphorylated by protein kinase G. J Biol Chem. 2011;286:37274–37279. doi: 10.1074/jbc.M111.282905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakamura M, Ichikawa K, Ito M, Yamamori B, Okinaka T, Isaka N, Yoshida Y, Fujita S, Nakano T. Effects of the phosphorylation of myosin phosphatase by cyclic GMP-dependent protein kinase. Cell Signal. 1999;11:671–676. doi: 10.1016/s0898-6568(99)00036-4. [DOI] [PubMed] [Google Scholar]
- 22.Surks HK, Mochizuki N, Kasai Y, Georgescu SP, Tang KM, Ito M, Lincoln TM, Mendelsohn ME. Regulation of myosin phosphatase by a specific interaction with cGMP- dependent protein kinase Ialpha. Science. 1999;286:1583–1587. doi: 10.1126/science.286.5444.1583. [DOI] [PubMed] [Google Scholar]
- 23.Given AM, Ogut O, Brozovich FV. MYPT1 mutants demonstrate the importance of aa 888-928 for the interaction with PKGIalpha. Am J Physiol Cell Physiol. 2007;292:C432–439. doi: 10.1152/ajpcell.00175.2006. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Palaia T, Ragolia L. Impaired insulin-stimulated myosin phosphatase Rho-interacting protein signaling in diabetic Goto-Kakizaki vascular smooth muscle cells. Am J Physiol Cell Physiol. 2012;302:C1371–1381. doi: 10.1152/ajpcell.00254.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murthy KS, Zhou H, Grider JR, Makhlouf GM. Inhibition of sustained smooth muscle contraction by PKA and PKG preferentially mediated by phosphorylation of RhoA. Am J Physiol Gastrointest Liver Physiol. 2003;284:G1006–1016. doi: 10.1152/ajpgi.00465.2002. [DOI] [PubMed] [Google Scholar]
- 26.Murthy KS, Makhlouf GM. Opioid mu, delta, and kappa receptor-induced activation of phospholipase C-beta 3 and inhibition of adenylyl cyclase is mediated by Gi2 and G(o) in smooth muscle. Mol Pharmacol. 1996;50:870–877. [PubMed] [Google Scholar]
- 27.Murthy KS. Contractile agonists attenuate cGMP levels by stimulating phosphorylation of cGMP-specific PDE5; an effect mediated by RhoA/PKC-dependent inhibition of protein phosphatase 1. Br J Pharmacol. 2008;153:1214–1224. doi: 10.1038/sj.bjp.0707686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begum N, Duddy N, Sandu O, Reinzie J, Ragolia L. Regulation of myosin-bound protein phosphatase by insulin in vascular smooth muscle cells: evaluation of the role of Rho kinase and phosphatidylinositol-3-kinase-dependent signaling pathways. Mol Endocrinol. 2000;14:1365–76. doi: 10.1210/mend.14.9.0522. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu H, Ito M, Miyahara M, Ichikawa K, Okubo S, Konishi T, Naka M, Tanaka T, Hirano K, Hartshorne DJ, Nakano T. Characterization of the myosin-binding subunit of smooth muscle myosin phosphatise. J Biol Chem. 1994;269:30407–30411. [PubMed] [Google Scholar]
- 30.Wu Y, Muranyi A, Erdodi F, Hartshorne DJ. Localization of myosin phosphatase target subunit and its mutants. J Muscle Res Cell Motil. 2005;26:123–134. doi: 10.1007/s10974-005-2579-5. [DOI] [PubMed] [Google Scholar]
- 31.Khatri JJ, Joyce KM, Brozovich FV, Fisher SA. Role of myosin phosphatase isoforms in cGMP-mediated smooth muscle relaxation. J Biol Chem. 2001;276:37250–37257. doi: 10.1074/jbc.M105275200. [DOI] [PubMed] [Google Scholar]
- 32.Sauzeau V, Le Jeune H, Cario-Toumaniantz C, Smolenski A, Lohmann SM, Bertoglio J, Chardin P, Pacaud P, Loirand G. Cyclic GMP-dependent protein kinase signaling pathway inhibits RhoA-induced Ca2+ sensitization of contraction in vascular smooth muscle. J Biol Chem. 2000;275:21722–9. doi: 10.1074/jbc.M000753200. [DOI] [PubMed] [Google Scholar]
- 33.Wu X, Haystead TA, Nakamoto RK, Somlyo AV, Somlyo AP. Acceleration of myosin light chain dephosphorylation and relaxation of smooth muscle by telokin. Synergism with cyclic nucleotide-activated kinase. J Biol Chem. 1998;273:11362–11369. doi: 10.1074/jbc.273.18.11362. [DOI] [PubMed] [Google Scholar]
- 34.Wooldridge AA, MacDonald JA, Erdodi F, Ma C, Borman MA, Hartshorne DJ, Haystead TA. Smooth muscle phosphatase is regulated in vivo by exclusion of phosphorylation of threonine 696 of MYPT1 by phosphorylation of Serine 695 in response to cyclic nucleotides. J Biol Chem. 2004;279:34496–34504. doi: 10.1074/jbc.M405957200. [DOI] [PubMed] [Google Scholar]
- 35.Kato M, Blanton R, Wang GR, Judson TJ, Abe Y, Myoishi M, Karas RH, Mendelsohn ME. Direct Binding and Regulation of RhoA by Cyclic GMP-dependent Protein Kinase Ialpha. J Biol Chem. 2012 doi: 10.1074/jbc.M112.421040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khromov AS, Wang H, Choudhury N, McDuffie M, Herring BP, Nakamoto R, Owens GK, Somlyo AP, Somlyo AV. Smooth muscle of telokin-deficient mice exhibits increased sensitivity to Ca2+ and decreased cGMP-induced relaxation. Proc Natl Acad Sci USA. 2006;103(7):2440–5. doi: 10.1073/pnas.0508566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yong J, Tan I, Lim L, Leung T. Phosphorylation of myosin phosphatase targeting subunit 3 (MYPT3) and regulation of protein phosphatase 1 by protein kinase A. J Biol Chem. 2006;281:31202–31211. doi: 10.1074/jbc.M607287200. [DOI] [PubMed] [Google Scholar]
- 38.Lee E, Hayes DB, Langsetmo K, Sundberg EJ, Tao TC. Interactions between the leucine-zipper motif of cGMP-dependent protein kinase and the C-terminal region of the targeting subunit of myosin light chain phosphatase. J Mol Biol. 2007;373:1198–1212. doi: 10.1016/j.jmb.2007.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhetwal BP, An CL, Fisher SA, Perrino BA. Regulation of basal LC20 phosphorylation by MYPT1 and CPI-17 in murine gastric antrum, gastric fundus, and proximal colon smooth muscles. Neurogastroenterol Motil. 2011;23:e425–436. doi: 10.1111/j.1365-2982.2011.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]