Abstract
A Solitary Fibrous Tumor (Sft) Is A Rare Neoplasm Originated From The Pleura, But They Can Occur In A Variety Of Extrathoracic Regions. Although Many Cases Of Primary Sft Have Been Reported, There Are Extremely Rare Repots To Date Of A Malignant Sft In The Spine Or Skull. A 54-year-woman Visited Our Hospital Due To Low Back Pain And Both Leg Radiating Pain. Several Imaging Studies Including Magnetic Resonance Imaging And Computed Tomography Revealed Expansive Enhanced Lesions In The Occipital Bone, T8, S1-2, And Ilium, With Neural Tissue Compression. We Performed Surgical Resection Of The Tumor In Each Site, And Postoperative Radiosurgery And Chemotherapy Were Performed. However, After Six Months, Tumors Were Recurred And Metastasized In Multiple Regions Including Whole Spine And Lung. The Authors Report Here The First Case Of Patient With Malignant Sft Of Tandem Lesions In The Various Bony Structures, Including Skull, Thoracic Spine, And Sacral Spine, With A Rapid Recurrence And Metastasis. Although Malignant Sft Is Extremely Rare, It Should Be Considered In The Differential Diagnosis And Carful Follow-up Is Needed.
Keywords: Solitary fibrous tumors, Metastasis, Skull, Spine
INTRODUCTION
Solitary fibrous tumor (SFT) is a rare tumor which was first described as originating from the pleura and occurring most commonly in the thoracic cavity3,9,12). However, it is now recognized that these rare tumors can occur throughout the body7,14). There have been only 8 case reports of extradural spinal SFT, also only 8 case reports of head and neck SFT involving bony structures4,5). Although the majority of SFT are benign, sometimes these tumors show aggressive clinical course including recurrence or multiple invasions, report on a malignant SFT of the spine or skull is extremely rare5).
We report on a first case of malignant SFT of tandem lesions in the skull and spine with a rapid recurrence and metastasis.
CASE REPORT
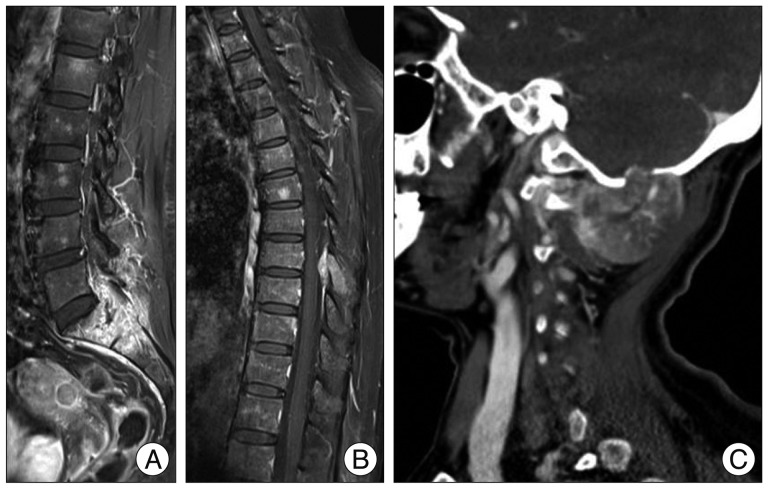
A 54-year-old female patient was referred to our spine center with a two-month history of lower back pain and radiating pain in both legs, which was persistent even at night. She had constipation with a symmetric leg muscle power grade of IV/V. Lumbo-sacral magnetic resonance imaging (MRI) showed a heterogeneously enhanced 6.7 cm sized lesion involving the sacrum, sacroiliac joint, and both illium with extraosseous mass formation containing a necrotic portion (Fig. 1A).
Fig. 1.
Image findings on admission. A : Sagittal lumbar enhanced MRI shows strongly enhanced lesion in S1, S2, and epidural space with encroachment of the bilateral nerve root of L5, S1, and S2. B : Sagittal thoracic enhanced MRI shows an extraosseous mass formation in the posterior element of T8. C : Enhanced neck CT shows heterogeneous enhancing extraosseous mass with occipital bone destruction and prominent collateral feeding vessels.
We performed positron emission tomography/computed tomography (PET/CT) and bone scan to assess for metastatic lesion. PET/CT and bone scan revealed hot uptake in the sacro-pelvic area, T8, and occipital skull area. Thoracic MRI showed an epidural enhancing 1.7 cm sized lesion in the posterior element of T8 (Fig. 1B). In addition, findings on brain imaging studies showed an enhancing mass measuring 3.8 cm with occipital bone destruction and mild brain compression (Fig. 1C). Percutaneous biopsy of S1 was performed for diagnosis. Pathology of the biopsy indicated a type undetermined malignant neoplasm, suspected as a malignant spindle cell tumor.
On angiography, large feeding vessels were observed in sacral lesion, and tumor embolization using Gelfoam was performed prior to perform an open surgery. Partial removal of the tumor in sacroiliac area was done; then, gross total removal of the tumor at T8 was subsequently performed in a single stage. The tumor was extremely hypervascular and was not well demarcated. For the remnant tumor of the sacroiliac area, postoperative stereotactic radiosurgery using Novalis Tx (BrainLAB, Inc., Ammerthalstrabe, Germany) was administered at a dose of 4 fractionated 32 Gy, which encompassed 100% of the tumor volume. After open surgery and radiosurgery, her lower back pain and radiating pain showed improvement. Three weeks after first open surgery, gross total removal of the tumor in the occipital skull was performed after tumor embolization.
Tumors of the sacro-pelvic area, T8, and occipital area showed identical histologic features. The tumors consisted of fusiform or spindle cells and showed a hemangiopericytoma-like perivascular pattern or a so-called patternless pattern, with intervening irregular hyalinzed collagen bundles, typical for a solitary fibrous tumor. The tumors showed multifocal necrosis, high cellularity, and marked cellular atypia. Mitotic figures were frequently observed [up to 10/10 high-power fields (HPFs)]. By immunohistochemistry, the tumor cells were positive for CD34, CD99, Bcl-2 and EMA (focal-like +). As a result, the tumors were diagnosed as malignant solitary fibrous tumor (Fig. 2).
Fig. 2.

Photomicrographs of the malignant solitary fibrous tumor. A : Hemangiopericytoma-like perivascular pattern (H&E, ×100). B : Patternless pattern with intervening irregular hyalinzed collagen bundles (H&E, ×100). C : Tumor shows high cellularity and marked cellular atypia (H&E, ×400). D : Tumor cells are positive for CD34 (immunostain, ×400).
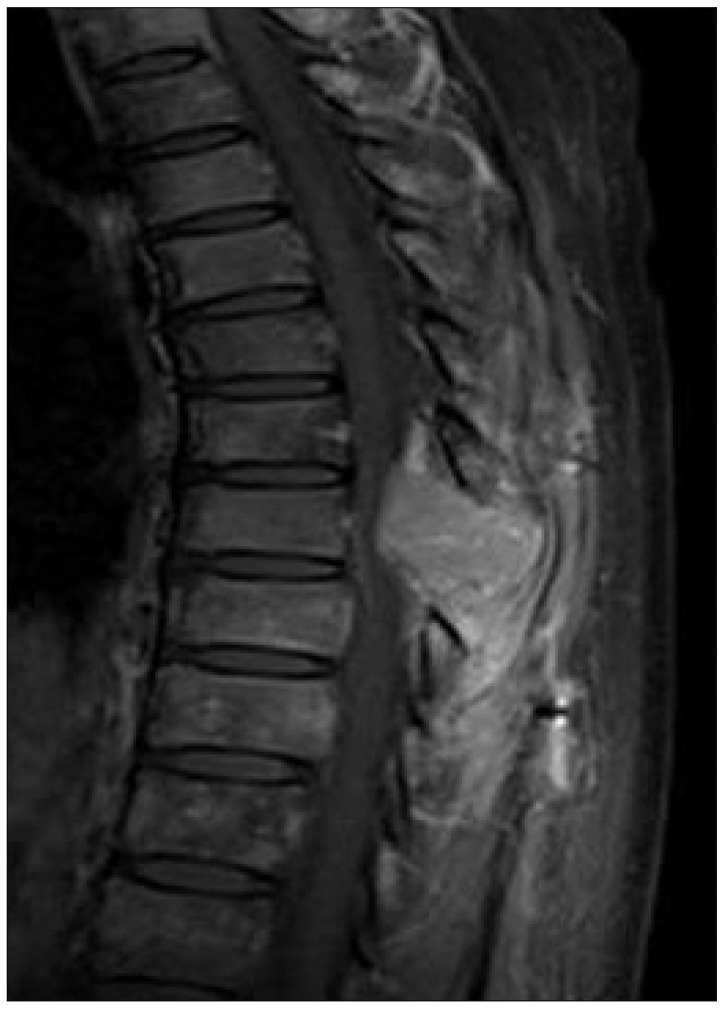
Although there was a lack of clinical evidence about adjuvant chemotherapy or radiotherapy, adjuvant chemotherapy with adriamycin and cisplatin was administered. However, just 2 days after starting adjuvant chemotherapy, one month after the initial surgery, she complained of aggravated upper back pain and paraparesis. Findings on follow-up thoracic MRI showed a recurred expansive posterior epidural mass with cord compression at T8 (Fig. 3). For the recurred tumor at T8, stereotactic radiosurgery was administered at a dose of 4 fractionated 32 Gy. After radiosurgery, her upper back pain and paraparesis showed improvement.
Fig. 3.
Follow-up enhanced thoracic MRI at postoperative one month. Sagittal enhanced MRI reveals a recurred expansive posterior epidural mass with cord compression at T8.
Gradually, she complained of progressive whole body pain. Despite chemotherapy, follow-up whole spine MRI and chest radiography revealed metastasis in multiple vertebrae and both lungs. Although palliative cervical and thoracic spine radiotherapy (dose of 5 fractionated 15 Gy) was performed for relieving intractable pain, her symptoms did not show improvement and the patient finally died due to poor general condition six months after first open surgery.
DISCUSSION
Classically, SFT, also known as localized fibrous tumor, has been known as a rare spindle-cell neoplasm originating from the pleura. Since the first case report in 1931, studies of SFT from the pleura were prolific in the 1980s12,15). Although SFT occurs mainly in the pleura, it can be seen in various organs, and has recently been considered to be a mesenchymal neoplasm originating from ubiquitous dendritic interstitial cells2). According to previous studies, approximately 30% of SFT arise in extrathoracic locations11). Reported extrathoracic locations include the mediastinum, pericardium, peritoneum, retroperitoneal space, pelvis, adrenal gland, kidney, liver, periosteum, salivary gland, thyroid gland, lacrimal gland, breast, nasopharynx, orbit, urogenital system, skin, meninges, and spinal cord10).
Due to overlapping histologic features, differentiation of SFT from other soft tissue tumors may be difficult. Performance of immunohistochemical staining is necessary in order to rule out other differential diagnoses19). Typical immunohistochemical features of SFT are a positive result for CD34, Bcl-2, and CD99, and a negative result for α-SMA, desmin, pan-cytokeratin, and S-100 protein11). However, these markers are frequently overexpressed in a range of other soft tissue tumors including hemangiopericytoma4). As a result, a differential diagnosis of hemangiopericytoma with SFT is difficult, even though their clinical courses are obviously different16). Therefore, 2002 World Health Organization criteria for soft-tissue tumors treats those two neoplastic entities as a single section, and many lesions that were called hemangiopericytomas prior to 1990 could now be called SFT5,10). However, in general, hemangiopericytomas are more cellular and have higher Ki-67 rated of 5-10%6). In the current case, light microscopic and immunohistochemical features were typical findings for malignant SFT rather than hemangiopericytoma.
Most thoracic SFTs are asymptomatic at presentation and are diagnosed as incidental finding on radiographs and CT images of the chest9). Extrathoracic SFTs, however, are usually symptomatic; depending on location, the manifestations are a painless mass or local pressure effects. In addition, an association of approximately 5% of SFTs with hypoglycemia due to secretion of insulin-like growth factors has been reported20). In the current case, initially, the patient complained lower back pain with radiating pain in both legs due to compression of nerve roots of S1 and S2.
In imaging studies, including MRI and CT, imaging features of SFT are relatively nonspecific. However, in general, SFTs have discrete margins, and the majority of SFTs have shown a lobulated contour. SFTs were typically well defined, tending to displace adjacent structures, and local invasion is not common. However, in our case, the tumor margin was not well defined, and local invasion was obvious. These findings strongly implied that tumor should be malignant1,5,20). As in the current case, a useful distinguishing imaging feature of SFTs is the presence of large collateral feeding vessels. However, the presence of these vessels does not appear to be related to the histologic subtype of the tumor20). On the other hand, the tumor occurred in multiple tandem regions, including the skull, thoracic spine, lumbo-sacral spine, and plevic bone. The current report is first case about the malignant SFT of multiple tandem lesions.
At present, due to the rarity of the disease, standard therapies for malignant SFT have not been well established. Surgical en bloc removal has been recommended as the treatment of choice for SFT9). Recurrence occurred commonly in cases involving incomplete excision, possibly caused by the level of difficulty in achievement complete resection. In these cases, adjuvant chemotherapy or radiotherapy may play a role in prevention of recurrence4). However, no previous studies have demonstrated the effect of chemotherapy or radiotherapy10).
The majority of SFTs are benign, and the malignant form accounts for 9-22%6,11). Based on previous case reports, malignant SFT showed rapid local recurrence and distant metastasis6). Some authors have suggested that the size of a SFT is one of the best indicators of malignancy1,13,17). In addition, findings such as nuclear atypia, increased cellularity, necrosis, and greater than 4 mitoses/10 HPFs, are suggestive of the malignant potential of SFTs18). Otherwise, some studies have reported that the prognosis of an SFT is most likely dependent upon complete resection rather than histologic findings8). Hence, careful follow-up may be needed, even if the tumor is small and benign in appearance at the time of presentation5,8,11).
The current patient showed malignant characteristics with respect to tumor size, multiple lesions, local invasion, impossibility of complete resection, and histopathologic findings. Fortunately, at initial presentation, the current patient showed a significant response to open surgery and radiosurgery. However, despite multidisciplinary treatment including adjuvant chemotherapy and radiotherapy, the tumor showed rapid local recurrence and aggressive metastasis to the whole spine and lung.
CONCLUSION
The authors report here the first case of patient with malignant SFT of tandem lesions in the skull and spine with a rapid recurrence and metastasis. Although malignant SFT is extremely rare, it should be considered in the differential diagnosis of tumors in the spine and skull, and carful follow-up is needed.
References
- 1.Briselli M, Mark EJ, Dickersin GR. Solitary fibrous tumors of the pleura : eight new cases and review of 360 cases in the literature. Cancer. 1981;47:2678–2689. doi: 10.1002/1097-0142(19810601)47:11<2678::aid-cncr2820471126>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 2.Chan JK. Solitary fibrous tumour--everywhere, and a diagnosis in vogue. Histopathology. 1997;31:568–576. doi: 10.1046/j.1365-2559.1997.2400897.x. [DOI] [PubMed] [Google Scholar]
- 3.Chang ED, Lee EH, Won YS, Kim JM, Suh KS, Kim BK. Malignant solitary fibrous tumor of the pleura causing recurrent hypoglycemia; immunohistochemical stain of insulin-like growth factor i receptor in three cases. J Korean Med Sci. 2001;16:220–224. doi: 10.3346/jkms.2001.16.2.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cox DP, Daniels T, Jordan RC. Solitary fibrous tumor of the head and neck. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2010;110:79–84. doi: 10.1016/j.tripleo.2010.01.023. [DOI] [PubMed] [Google Scholar]
- 5.Demicco EG, Park MS, Araujo DM, Fox PS, Bassett RL, Pollock RE, et al. Solitary fibrous tumor : a clinicopathological study of 110 cases and proposed risk assessment model. Mod Pathol. 2012;25:1298–1306. doi: 10.1038/modpathol.2012.83. [DOI] [PubMed] [Google Scholar]
- 6.Fargen KM, Opalach KJ, Wakefield D, Jacob RP, Yachnis AT, Lister JR. The central nervous system solitary fibrous tumor : a review of clinical, imaging and pathologic findings among all reported cases from 1996 to 2010. Clin Neurol Neurosurg. 2011;113:703–710. doi: 10.1016/j.clineuro.2011.07.024. [DOI] [PubMed] [Google Scholar]
- 7.Fukunaga M, Naganuma H, Nikaido T, Harada T, Ushigome S. Extrapleural solitary fibrous tumor : a report of seven cases. Mod Pathol. 1997;10:443–450. [PubMed] [Google Scholar]
- 8.Gengler C, Guillou L. Solitary fibrous tumour and haemangiopericytoma : evolution of a concept. Histopathology. 2006;48:63–74. doi: 10.1111/j.1365-2559.2005.02290.x. [DOI] [PubMed] [Google Scholar]
- 9.Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ, et al. Clinicopathologic correlates of solitary fibrous tumors. Cancer. 2002;94:1057–1068. [PubMed] [Google Scholar]
- 10.Ha JK, Park BJ, Kim YH, Lim YJ. Orbital solitary fibrous tumor : a case report and diagnostic clues. J Korean Neurosurg Soc. 2009;46:77–80. doi: 10.3340/jkns.2009.46.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawamura S, Nakamura T, Oya T, Ishizawa S, Sakai Y, Tanaka T, et al. Advanced malignant solitary fibrous tumor in pelvis responding to radiation therapy. Pathol Int. 2007;57:213–218. doi: 10.1111/j.1440-1827.2007.02083.x. [DOI] [PubMed] [Google Scholar]
- 12.Klemperer P, Coleman BR. Primary neoplasms of the pleura. A report of five cases. Am J Ind Med. 1992;22:1–31. doi: 10.1002/ajim.4700220103. [DOI] [PubMed] [Google Scholar]
- 13.Mussak EN, Tu JJ, Voigt EP. Malignant solitary fibrous tumor of the hypopharynx with dysphagia. Otolaryngol Head Neck Surg. 2005;133:805–807. doi: 10.1016/j.otohns.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen GP, O'Connell JX, Dickersin GR, Rosenberg AE. Solitary fibrous tumor of soft tissue : a report of 15 cases, including 5 malignant examples with light microscopic, immunohistochemical, and ultrastructural data. Mod Pathol. 1997;10:1028–1037. [PubMed] [Google Scholar]
- 15.Okike N, Bernatz PE, Woolner LB. Localized mesothelioma of the pleura : benign and malignant variants. J Thorac Cardiovasc Surg. 1978;75:363–372. [PubMed] [Google Scholar]
- 16.Shnayder Y, Greenfield BJ, Oweity T, DeLacure MD. Malignant solitary fibrous tumor of the tongue. Am J Otolaryngol. 2003;24:246–249. doi: 10.1016/s0196-0709(03)00007-3. [DOI] [PubMed] [Google Scholar]
- 17.Sung SH, Chang JW, Kim J, Lee KS, Han J, Park SI. Solitary fibrous tumors of the pleura : surgical outcome and clinical course. Ann Thorac Surg. 2005;79:303–307. doi: 10.1016/j.athoracsur.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 18.Vallat-Decouvelaere AV, Dry SM, Fletcher CD. Atypical and malignant solitary fibrous tumors in extrathoracic locations : evidence of their comparability to intra-thoracic tumors. Am J Surg Pathol. 1998;22:1501–1511. doi: 10.1097/00000478-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Westra WH, Grenko RT, Epstein J. Solitary fibrous tumor of the lower urogenital tract : a report of five cases involving the seminal vesicles, urinary bladder, and prostate. Hum Pathol. 2000;31:63–68. doi: 10.1016/s0046-8177(00)80200-2. [DOI] [PubMed] [Google Scholar]
- 20.Wignall OJ, Moskovic EC, Thway K, Thomas JM. Solitary fibrous tumors of the soft tissues : review of the imaging and clinical features with histopathologic correlation. AJR Am J Roentgenol. 2010;195:W55–W62. doi: 10.2214/AJR.09.3379. [DOI] [PubMed] [Google Scholar]