Abstract
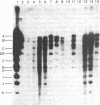
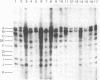
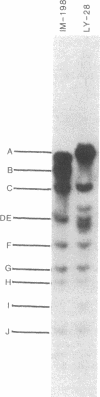
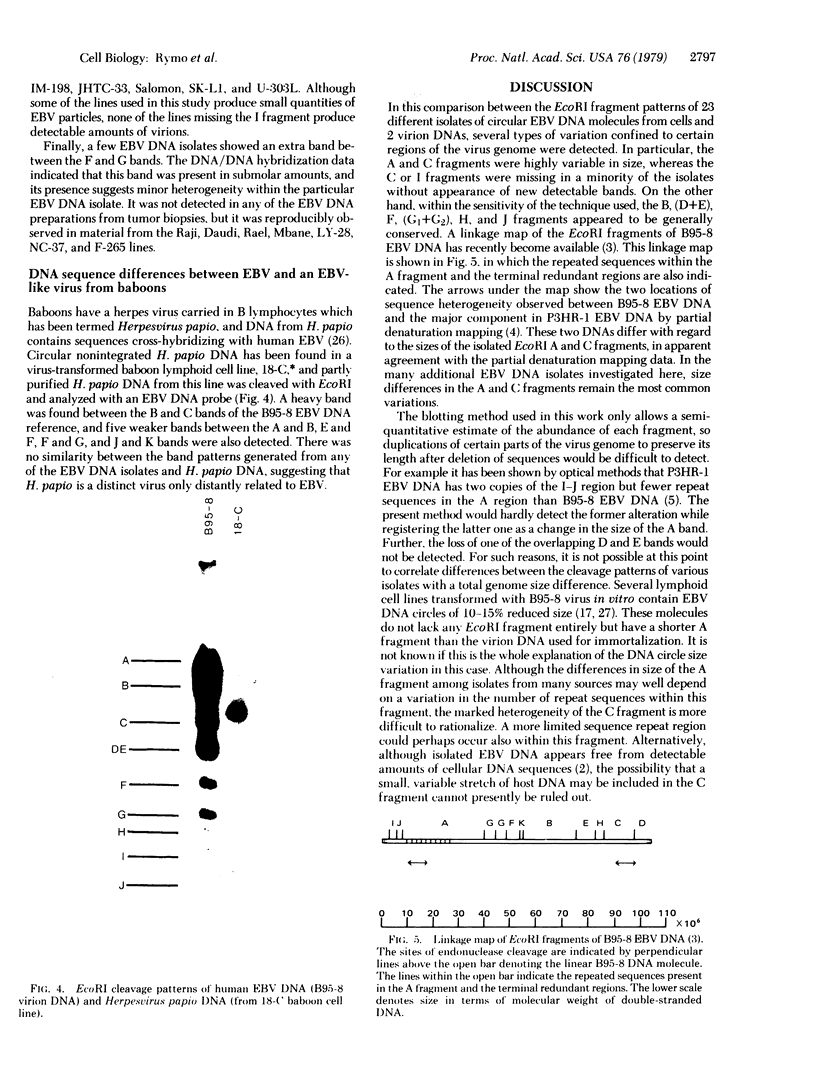
The intracellular Epstein-Barr virus (EBV) DNA present in virus-transformed cells was partly purified from 23 cell lines or biopsies of Burkitt lymphoma, nasopharyngeal carcinoma, infectious mononucleosis, or healthy carrier origin. Such DNA was cleaved in fragments (A-K) of molecular weights between 1 x 10(6) and 30 x 10(6) with restriction enzyme EcoRI, and these fragments were analyzed by standard methods involving agarose gel electrophoresis, transfer to nitrocellulose filters, and hybridization with radioactive EBV DNA or complementary RNA. Sequence variability among different EBV DNA isolates was largely confined to the A, C, and I fragments. These results are discussed in relation to the linkage map of the EcoRI fragments of EBV DNA. The EcoRI cleavage pattern of intracellular viral DNA of an EBV-like virus from baboon cells, Herpesvirus papio, was entirely different from that of human EBV isolates.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams A., Bjursell G., Gussander E., Koliais S., Falk L., Lindahl T. Size of the intracellular circular Epstein-Barr virus DNA molecules in infectious mononucleosis-derived human lymphoid cell lines. J Virol. 1979 Feb;29(2):815–817. doi: 10.1128/jvi.29.2.815-817.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams A., Bjursell G., Kaschka-Dierich C., Lindahl T. Circular Epstein-Barr virus genomes of reduced size in a human lymphoid cell line of infectious mononucleosis origin. J Virol. 1977 May;22(2):373–380. doi: 10.1128/jvi.22.2.373-380.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- De-Thé G., Ho H. C., Kwan H. C., Desgranges C., Favre M. C. Nasopharyngeal carcinoma (NPC). I. Types of cultures derived from tumour biopsies and non-tumorous tissues of Chinese patients with special reference to lymphoblastoid transformation. Int J Cancer. 1970 Sep 15;6(2):189–206. doi: 10.1002/ijc.2910060206. [DOI] [PubMed] [Google Scholar]
- Delius H., Bornkamm G. W. Heterogeneity of Epstein-Barr virus. III. Comparison of a transforming and a nontransforming virus by partial denaturation mapping of their DNAs. J Virol. 1978 Jul;27(1):81–89. doi: 10.1128/jvi.27.1.81-89.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk L., Deinhardt F., Nonoyama M., Wolfe L. G., Bergholz C. Properties of a baboon lymphotropic herpesvirus related to Epstein-Barr virus. Int J Cancer. 1976 Dec 15;18(6):798–807. doi: 10.1002/ijc.2910180611. [DOI] [PubMed] [Google Scholar]
- Given D., Kieff E. DNA of Epstein-Barr virus. IV. Linkage map of restriction enzyme fragments of the B95-8 and W91 strains of Epstein-Barr Virus. J Virol. 1978 Nov;28(2):524–542. doi: 10.1128/jvi.28.2.524-542.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser R., Nonoyama M. Host cell regulation of induction of Epstein-Barr virus. J Virol. 1974 Jul;14(1):174–176. doi: 10.1128/jvi.14.1.174-176.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Adams A., Lindahl T., Bornkamm G. W., Bjursell G., Klein G., Giovanella B. C., Singh S. Intracellular forms of Epstein-Barr virus DNA in human tumour cells in vivo. Nature. 1976 Mar 25;260(5549):302–306. doi: 10.1038/260302a0. [DOI] [PubMed] [Google Scholar]
- Kaschka-Dierich C., Falk L., Bjursell G., Adams A., Lindahl T. Human lymphoblastoid cell lines derived from individuals without lymphoproliferative disease contain the same latent forms of Epstein-Barr virus DNA as those found in tumor cells. Int J Cancer. 1977 Aug 15;20(2):173–180. doi: 10.1002/ijc.2910200203. [DOI] [PubMed] [Google Scholar]
- Ketner G., Kelly T. J., Jr Integrated simian virus 40 sequences in transformed cell DNA: analysis using restriction endonucleases. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1102–1106. doi: 10.1073/pnas.73.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Dombos L. Relationship between the sensitivity of EBV-carrying lymphoblastoid lines to superinfection and the inducibility of the resident viral genome. Int J Cancer. 1973 Mar 15;11(2):327–337. doi: 10.1002/ijc.2910110210. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koliais S., Bjursell G., Adams A., Lindahl T., Klein G. State of Epstein-Barr virus DNA in an American Burkitt's lymphoma line. J Natl Cancer Inst. 1978 May;60(5):991–994. doi: 10.1093/jnci/60.5.991. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Bjursell G., Bornkamm G. W., Kaschka-Dierich C., Jehn U. Covalently closed circular duplex DNA of Epstein-Barr virus in a human lymphoid cell line. J Mol Biol. 1976 Apr 15;102(3):511–530. doi: 10.1016/0022-2836(76)90331-4. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathans D., Danna K. J. Studies of SV40 DNA. 3. Differences in DNA from various strains of SV40. J Mol Biol. 1972 Mar 14;64(2):515–518. doi: 10.1016/0022-2836(72)90515-3. [DOI] [PubMed] [Google Scholar]
- Nonoyama M., Pagano J. S. Homology between Epstein-Barr virus DNA and viral DNA from Burkitt's lymphoma and nasopharyngeal carcinoma determined by DNA-DNA reassociation kinetics. Nature. 1973 Mar 2;242(5392):44–47. doi: 10.1038/242044a0. [DOI] [PubMed] [Google Scholar]
- Raab-Traub N., Pritchett R., Kieff E. DNA of Epstein-Barr virus. III. Identification of restriction enzyme fragments that contain DNA sequences which differ among strains of Epstein-Barr virus. J Virol. 1978 Aug;27(2):388–398. doi: 10.1128/jvi.27.2.388-398.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rymo L., Forsblom S. Cleavage of Epstein-Barr virus DNA by restriction endonucleases EcoRI, HindIII and BamI. Nucleic Acids Res. 1978 Apr;5(4):1387–1402. doi: 10.1093/nar/5.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherberg N. H., Refetoff S. The preparation of carrier-free iodine isotope-substituted cytosine nucleotides. Biochim Biophys Acta. 1974 Apr 10;340(4):446–451. doi: 10.1016/0005-2787(74)90065-3. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sugden B. Comparison of Epstein-Barr viral DNAs in Burkitt lymphoma biopsy cells and in cells clonally transformed in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4651–4655. doi: 10.1073/pnas.74.10.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden B., Summers W. C., Klein G. Nucleic acid renaturation and restriction endonuclease cleavage analyses show that the DNAs of a transforming and a nontransforming strain of Epstein-Barr virus share approximately 90% of their nucleotide sequences. J Virol. 1976 May;18(2):765–775. doi: 10.1128/jvi.18.2.765-775.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]