Abstract
Background
Several epidemiological studies have examined the association between shortened telomere length and type 2 diabetes mellitus (T2DM), while the results remained conflicting. We conducted a meta-analysis to derive a more precise estimation of the relationship between them.
Methods
We systematically reviewed the databases of PubMed, EMBASE, and Web of Science for all studies on the association between telomere length and T2DM. We conducted this study assessed by STATA 11.0. Data were summarized using random-effects or fixed-effects meta-analysis. The heterogeneity and publication bias among studies were examined by using χ2-based Q statistic test and Egger’s test, respectively.
Results
Nine cohorts consisting of 5759 cases and 6518 controls were selected into the meta-analysis. The results indicated that shortened telomere length was significantly associated with T2DM risk (OR: 1.291; 95% CI: 1.112, 1.498; P<0.001) with heterogeneity (I2 = 71.6%). When three cohorts responsible for the heterogeneity were excluded, the pooled OR for the remaining cohorts indicated a significant association between shortened telomere length and T2DM (OR: 1.117; 95% CI: 1.002, 1.246; P = 0.045) without heterogeneity.
Conclusion
We found a statistically significant association between shortened telomere length and T2DM.
Introduction
Telomeres are the DNA-protein structures capped at the ends of chromosomes in eukaryotic cells, which are very important for chromosome stability and integrity [1]. They shorten during each cycle of DNA replication and therefore have been implicated as a biomarker for cell aging [2]. It has been proved that shortened telomere length is associated with various age-related diseases, such as myocardial infarction [3], stroke [4], peripheral arterial disease (PAD) [5] and Alzheimer’s disease [6]. T2DM is one of the most common chronic diseases in the world. It has been proved to be an age-related disease, and therefore might be associated with telomere length as well. Jeanclos et al firstly reported the association between shortened telomere length and T2DM in 1998 [7], [8]. After the initial association discovery, several subsequent replication studies have been conducted in different population cohorts including Europeans, Asians and Africans [9], [10], [11], [12], [13], [14], [15]. However, the results remained conflicting. Most of the studies found that T2DM patients had shorter telomere length, while others reported a negative association between telomere length and T2DM [9], [12]. Considering that most of these studies have been conducted on relatively small numbers of subjects and no meta-analysis has specifically examined the relationship between shortened telomere length and T2DM, we conducted the meta-analysis expecting to give a more precise estimate of the relationship between telomere length and T2DM.
Methods
Search Strategy of Articles
We systematically searched MEDLINE, EMBASE, and Web of Science for all articles on the association between “diabetes” and “telomere” from their starting dates to Oct 31, 2012. We limited our search by English. In addition, hand searching was performed to identify potentially relevant studies.
Selection Criteria and Exclusion Criteria
The literatures which meet the following criteria were selected into this meta-analysis: (1) case–control study or cohort study; (2) study aims at the association between telomere length and T2DM; and (3) study provides odds ratios (ORs) or sufficient information to estimate odds ratios (ORs) and their 95% confidence intervals (CIs).
The exclusion criteria were: (1) studies without sufficient information we need; (2) case subjects with multiple diseases; (3) case-only studies, review articles, commentaries, editorials, or case reports; (4) the studies have a sample size less than two hundred and haven’t given the number of cases and controls grouped by the median of the relative telomere length.
Quality Assessment
The quality of studies was independently assessed by two authors (JZ and KM) using the following criteria: (1) Representativeness of cases, (2) Representativeness of controls, (3) Ascertainment of T2DM, (4) Ascertainment of controls, (5) Measuring telomere length, (6) Distribution of telomere length, (7) Association assessment (Table S1). Total scores ranged from 0 (worst) to 13 (best). Higher score represented a better quality.
Data Extraction
Two reviewers (JZ and MK) extracted data from the selected articles independently. They compared the results and reached consistencies on all items. Characters extracted from the studies were as follows: journal, author, year of publication, cohort/study name, study type, geographic location of study, ethnicity of the study population, total number of cases and controls, average age at baseline, participants’ gender (male, female, combined), methods for telomere length measurement, the length of telomere and their standard error, unadjusted odds ratios (ORs) for individuals in the 50th percentile of shorter telomeres compared with those in the longer and the 95% CI of the ORs. Ethnic groups (categorized as Caucasian, Asian, or others) were extracted separately for studies consisting different racial descent subjects.
Statistical Analysis
We performed the meta-analyses using the STATA 11.0 software (StataCorp, College Station, Texas, USA). We collected the unadjusted ORs for individuals in the 50th percentile of shorter telomeres compared with those in the longer and their 95% CI of the studies selected in the analysis. For the studies which did not provide ORs and 95% CI, we used the methods described by Wentzensen to compute the OR and 95% CI [16]. We assumed that the distribution of telomere length was normal, and divided the case and control subjects into two groups (the longer group and the shorter group) by using the mean telomere length of controls as a cut-point, and then we computed the probabilities among cases to fall into the shorter subgroup and longer subgroup, and after that we multiplied these probabilities by the total number of cases. We computed the ORs for the shorter compared with the longer group and their 95% CI using the χ2 test. With this method we converted the reported means and standard errors in cases and controls into ORs. However, studies with small sample size may be inappropriate to compute the OR in this way. So studies which didn’t provide the numbers of cases and controls grouped by the median of the relative telomere length and have a sample size less than two hundred were systematically excluded. Heterogeneity was evaluated by χ2-based Q statistic and I2 statistic. The percentages of I2 around 25%, 50%, and 75% indicate low, medium, and high heterogeneity, respectively [17]. The fixed-effects model was used to calculate the pooled OR when there was no significant heterogeneity (P more than 0.10 and I2 less than 50%) among the included studies; otherwise, we used the random-effects model [18]. We conducted subgroup analyses according to ethnicity (Asian, Caucasian, and African), article quality (more than 8 and less than 8), sample size (more than 1000 and less than 1000), mean age (older than 60 and younger than 60), and gender (male, female and mixed). We performed meta-regression to find out the source of heterogeneity by fitting a co-variant (ethnicity, study quality, sample size, age and gender). Meanwhile, we also used the HETRED module of the STATA software to trace the responsible study for heterogeneity. Sensitivity analysis was performed to investigate the influence of each study on the overall result. In addition, publication bias was evaluated by funnel plot and Egger’s tests. All statistical testing was 2-sided and a P<0.05 was considered statistically significant.
Results
Literature Search
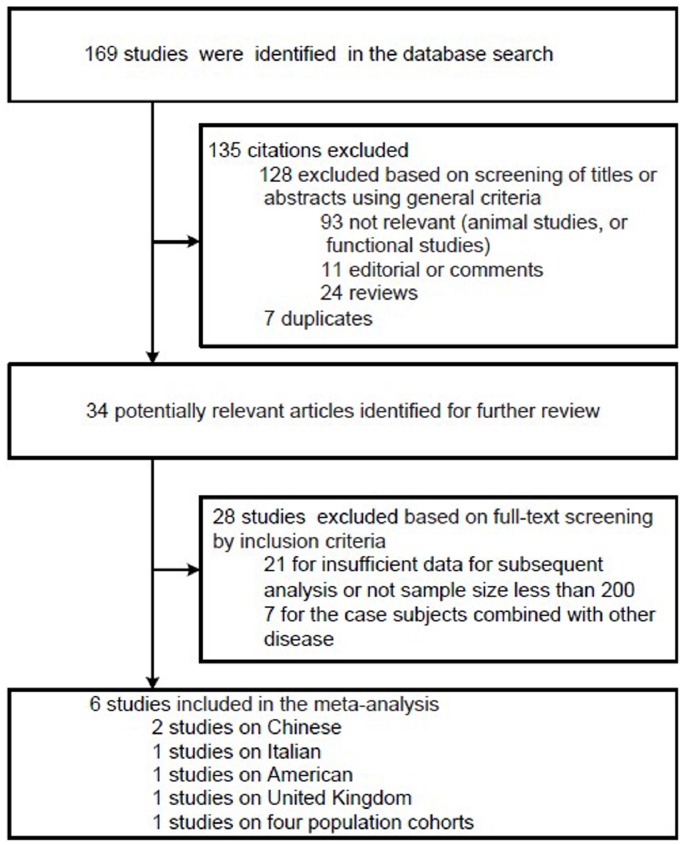
On the initial search, we identified 169 studies by the keywords mentioned in the search strategy, of which 135 was excluded for they were not aiming at the association between telomere length and T2DM. After the abstracts or full texts were reviewed, 28 articles were excluded for the case subjects combined with other disease or without sufficient data for subsequent analysis. Finally, 6 studies from 5 nationalities consisting 9 population cohorts were selected into the analysis [9], [10], [11], [12], [13], [14] (Figure 1).The characteristics of studies included in the analysis were listed in Table 1. Among these articles, 2 studies reported results on Chinese [10], [11], 1 on Italian [12], 1 on American [13] and 1 on United Kingdom [14].
Figure 1. Flow chart for the process of selecting eligible publications.
Table 1. Characteristics of studies examining the association between telomere length and T2DM included in the meta-analysis.
| Study | Year | Country | Design | Ethnicity | Case | Control | Quality assessment | |||||
| Total | Man | Age | Total | Man | Age | |||||||
| 1a | You et al 1 [9] | 2012 | USA | Prospective Study | Caucasian | 1012 | 0 | 63.96 | 1023 | 0 | 63.92 | 7 |
| 1b | You et al 2 [9] | 2012 | USA | Prospective Study | African | 400 | 0 | 60.97 | 819 | 0 | 60.95 | 7 |
| 1c | You et al 3 [9] | 2012 | USA | Prospective Study | Caucasian | 162 | 0 | 60.15 | 331 | 0 | 60.21 | 7 |
| 1d | You et al 4 [9] | 2012 | USA | Prospective Study | Asian | 101 | 0 | 63.37 | 207 | 0 | 63.58 | 7 |
| 2 | Shen et al [10] | 2012 | Chinese | Case-control study | Asian | 1936 | 30.2 | 64 | 2080 | 41.1 | 58 | 10 |
| 3 | Xiao et al [11] | 2011 | Chinese | Case-control study | Asian | 930 | 29.9 | 64.3 | 867 | 30.5 | 64.1 | 10 |
| 4 | Testa et al [12] | 2011 | Italy | Cross-sectional study | Caucasian | 217 | 59.7 | 65.6 | 400 | 55.6 | 65.1 | 9 |
| 5 | Zee et al [13] | 2009 | USA | Case-control study | Caucasian | 432 | 59.3 | 60 | 424 | 44.1 | 51 | 9 |
| 6 | Salpea et al [14] | 2009 | U.K | Case-control study | Caucasian | 569 | 59.4 | 70 | 367 | 100 | 53 | 9 |
Results of Meta-analysis
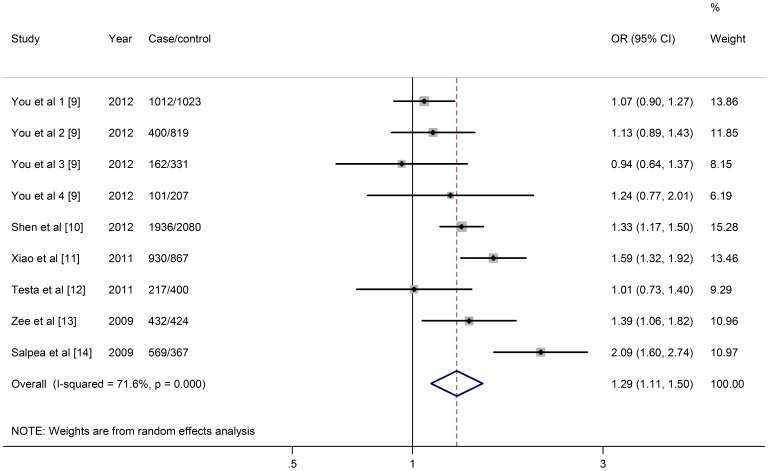
The raw data we extracted from the 9 population cohorts consists of 5759 cases and 6518 controls. When we pooled all eligible publications into the meta-analysis, significant association was found between shortened telomere length and T2DM (OR: 1.291; 95% CI: 1.112, 1.498; P<0.001) with heterogeneity (I 2 = 71.6%, χ2 = 28.13, P<0.001) (Figure 2). Subgroup analysis was further performed among these studies by ethnicity, study quality, sample size, age and gender (Table 2). The pooled ORs were increased in several subgroups, including: high quality studies, studies with younger control subjects and the mixed gender studies. High quality studies have an increased risk (OR: 1.452; 95% CI: 1.204, 1.753) compared with low quality studies (OR: 1.081; 95% CI: 0.952, 1.228). The control subjects of 3 reports (3647 control subjects) had an average age over 60, whereas other 7 cohorts (2871 control subjects) had an average age below 60. The pooled OR for younger subjects (OR: 1.543; 95% CI: 1.179, 2.018) was higher than the pooled OR for older (OR: 1.166; 95% CI: 0.975, 1.395). Meanwhile, the pooled OR for mixed gender studies (OR: 1.452; 95% CI: 1.204, 1.753) was higher than the pooled OR for studies focused on women (OR: 1.081; 95% CI: 0.952, 1.228).
Figure 2. Meta-analysis of the association between telomere length and T2DM.
Odds ratios (ORs) and 95% confidence intervals (CIs) for overall T2DM risk associated with relative telomere length (shorter vs. longer).
Table 2. Subgroup analyses of the meta-analysis.
| Variables | NO of cohorts | Sample | Shorter vs. longerOR (95% CI) | Q Statistic | P for Heterogeneity | I-squared Value | P Value Between Groups | |
| Case | Control | |||||||
| All | 9 | 5759 | 6518 | 1.291(1.112,1.498) | 28.13 | P<0.001 | 71.6% | NA |
| HETRED * | 6 | 2317 | 3183 | 1.117(1.002,1.246) | 4.07 | 0.539 | 0% | NA |
| Ethnicity | ||||||||
| Asian | 3 | 2967 | 3154 | 1.409(1.230,1.615) | 2.78 | 0.249 | 28.2% | |
| Caucasian | 5 | 2392 | 2545 | 1.252(0.945,1.658) | 21.45 | P<0.001 | 81.4% | |
| Others | 1 | 400 | 819 | 1.126(0.885,1.431) | 0.00 | NA | NA | P = 0.14 |
| Quality assessment | ||||||||
| Over 8 | 5 | 4084 | 4138 | 1.452(1.204,1.753) | 14.66 | 0.005 | 72.7% | |
| Under 8 | 4 | 1675 | 2380 | 1.081(0.952,1.228) | 0.98 | 0.805 | 0.0% | P<0.001 |
| Sample size | ||||||||
| More than 1000 | 4 | 4278 | 4789 | 1.270(1.075,1.500) | 10.74 | 0.013 | 72.1% | |
| Less than 1000 | 5 | 1481 | 1729 | 1.299(0.958,1.762) | 16.76 | 0.002 | 76.1% | P = 0.43 |
| Age | ||||||||
| Over 60 | 6 | 2822 | 3647 | 1.166(0.975,1.395) | 13.43 | 0.02 | 62.8% | |
| Under 60 | 3 | 2937 | 2871 | 1.543(1.179,2.018) | 9.10 | 0.011 | 78.0% | P = 0.02 |
| Gender | ||||||||
| Female | 4 | 1675 | 2380 | 1.081(0.952,1.228) | 0.98 | 0.805 | 0.0% | |
| Mixed | 5 | 4084 | 4138 | 1.452(1.204,1.753) | 14.66 | 0.005 | 72.7% | P<0.001 |
NA, not applicable;
After excluding the key contributor studies to heterogeneity (Studies of Xiao et al, Shen et al and Salpea et al).
In the meta-regression analysis for heterogeneity, no participant characteristic (ethnicity, study quality, sample size, age and gender) was significantly correlated with the magnitude of genetic effect (all P>0.05). Next, we also used the HETRED module of the STATA software to find out the responsible study for heterogeneity. The studies of Xiao et al, Shen et al and Salpea et al were determined as the key contributors to the heterogeneity. After excluding these three studies, the overall heterogeneity decreased from 71.6% to 0% according to the I2 assessment (Table 2). The pooled OR for the remaining six cohorts indicates a significant association between shortened telomere length and T2DM (OR: 1.117; 95% CI: 1.002, 1.246; P = 0.045).
Sensitivity Analysis and Publication Bias
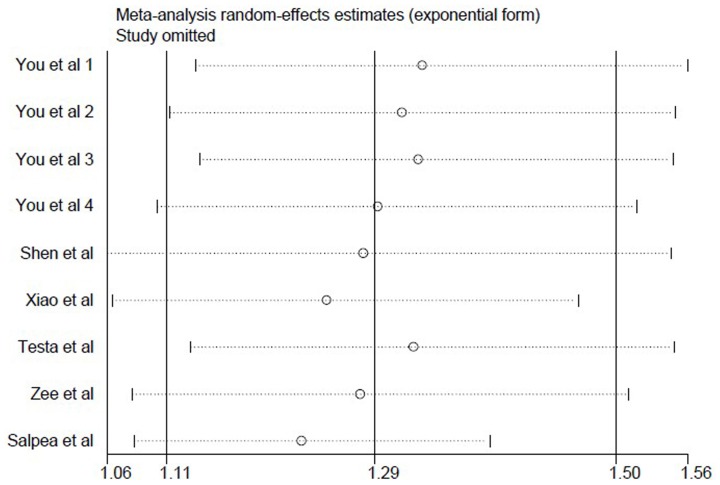
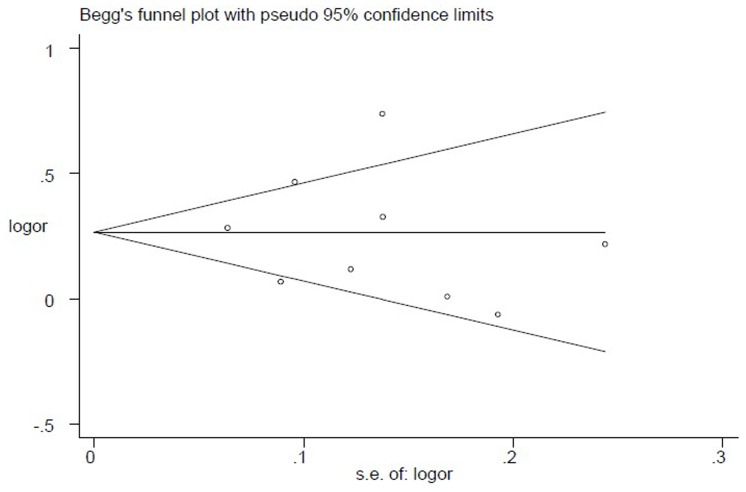
Sensitivity analysis (exclusion of 1 study at a time) indicated that no single study changed the pooled ORs qualitatively (Figure 3), which suggested that the results of the meta-analysis were stable and reliable. Egger’s test suggested no publication bias in the current meta-analysis (P = 0.764) and the shape of the funnel plots seemed symmetrical (Figure 4). Thus publication bias might not have a significant influence on the result of this meta-analysis.
Figure 3. Sensitivity analyses of selected studies.
Each line showed the recalculated pooled relative risk of remaining studies by omitting one study listed in the left volume a time.
Figure 4. Begg’s funnel plot of selected studies.
Asymmetry of the plot indicates publication bias. The horizontal line represents the meta-analysis summary estimate, the diagonal lines pseudo-95% CI limits about the effect estimate. logor, natural logarithm of the OR; s.e. of logor, standard error of logor.
Discussion
In this meta-analysis, we combined all together 9 cohorts consisting of 5759 T2DM cases and 6518 control subjects to evaluate the association between shortened telomere length and T2DM risk. The combined results showed that shortened telomere length is significantly associated with T2DM risk.
Previous studies have demonstrated that oxidative stress was a principal mechanism in the development and progression of T2DM [19], [20]. Elevated cellular glucose promotes the cellular oxidative stress [21]. Increased oxidative stress mediated cellular injury destroys the beta cells in the pancreas and decreases insulin sensitivity [22]. Furthermore, cellular injury and the decreased insulin sensitivity also promote the presence of cellular oxidative stress [23], [24]. In brief, oxidative stress was increased in T2DM patients. Clinical and experimental studies have indicated that oxidative stress elicits the deletion of telomeres [25], [26]. The G triplet of telomere is very sensitive to oxidative stress, and high-intensity stresses can cause telomere shorten by inducing telomeric double-strand breaks at high frequency [27], [28], [29]. So the telomere shortening in T2DM patients might be caused by increased oxidative stress and further studies are needed to elucidate the exact mechanisms underlying the association between telomere length and T2DM.
Although this meta-analysis showed significant association between shortened telomere length and T2DM, some results from subgroup analysis remind us of drawing the conclusion with caution. Firstly, previous studies have reported the variety of telomere between males and females [30]. Although the information is insufficient to compare gender difference in this analysis, the mixed gender studies had a higher OR than the studies focused on women subjects (1.452 vs. 1.081). Several hypotheses has explained the gender difference in telomere length and T2DM subjects, such as the higher stress exposure for males and the protective effect of estrogen in females. Secondly, age is another essential factor involved in telomere shortening [2]. All the studies have an average age between 60 and 70 in case subjects, so stratification analyses were conducted by the average age of control subjects (older than 60 or younger than 60). Both subgroups showed an association between telomere and T2DM, while the pooled OR for the studies with a younger control cohorts higher than the studies with an older control cohorts (1.543 vs. 1.166). It further identified that age has an influence on strength of association between telomere length and T2DM. Thirdly, several studies have reported the heterogeneity of telomere length within different population cohorts [9], [31], [32]. The results indicated that shortened telomere length was associated with T2DM in Asians, while not in Caucasians. The current study includes only one African cohort, and therefore the association in Africans should be evaluated in further studies with larger sample size.
Several limitations of the current meta-analysis should be noted. First, the publication bias might exist. In our meta-analysis, only studies indexed by the selected databases were included. Negative studies were less likely to be published in journals, resulting in potential overestimation of effect size. In addition, the exclusion of studies conducted in non-English speaking countries would have also introduced a considerable degree of bias. Second, telomere shortening is a cause or a consequence of diabetes remained to be clarified. Third, although all of the selected studies used the quantitative PCR based method to measure telomere length, the absolute telomere length was reported in different ways, including T/S ratio and KB, and therefore might provide us with less reliable evidence to make our conclusion. Fourth, seven of nine studies selected in the meta-analysis reported the adjusted OR. After adjusted for confounding variables (including BMI), their directions toward significant associations remain unchanged. While the detailed information on traditional risk factors was not available for analysis, the effects of those factors could not be adequately addressed in the present meta-analysis.
In summary, this meta-analysis provides evidence that shortened telomere length is associated with T2DM risk. Further prospective studies with larger sample size are needed to clarify whether telomere shortening is a cause or a consequence of diabetes.
Supporting Information
Quality assessment of association studies between telomere length and T2DM.
(DOC)
PRISMA checklist.
(DOC)
Funding Statement
This work was supported by grants from National “973” projects (Nos.2012CB518004), “863” project (No.2012AA02A510), Ministry of Education of China for Outstanding Young Teachers in University (20110142120012), and Key Project of Health Ministration. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Blackburn EH (1991) Structure and function of telomeres. Nature 350: 569–573. [DOI] [PubMed] [Google Scholar]
- 2. Vasa-Nicotera M, Brouilette S, Mangino M, Thompson JR, Braund P, et al. (2005) Mapping of a major locus that determines telomere length in humans. Am J Hum Genet 76: 147–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Maubaret CG, Salpea KD, Jain A, Cooper JA, Hamsten A, et al. (2010) Telomeres are shorter in myocardial infarction patients compared to healthy subjects: correlation with environmental risk factors. J Mol Med (Berl) 88: 785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ding H, Chen C, Shaffer JR, Liu L, Xu Y, et al. (2012) Telomere length and risk of stroke in Chinese. Stroke 43: 658–663. [DOI] [PubMed] [Google Scholar]
- 5. Zhang W, Chen Y, Yang X, Fan J, Mi X, et al. (2012) Functional haplotypes of the hTERT gene, leukocyte telomere length shortening, and the risk of peripheral arterial disease. PLoS One 7: e47029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hochstrasser T, Marksteiner J, Humpel C (2012) Telomere length is age-dependent and reduced in monocytes of Alzheimer patients. Exp Gerontol 47: 160–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jeanclos E, Krolewski A, Skurnick J, Kimura M, Aviv H, et al. (1998) Shortened telomere length in white blood cells of patients with IDDM. Diabetes 47: 482–486. [DOI] [PubMed] [Google Scholar]
- 8. Hamel FG (2010) Telomeres and type 2 diabetes. Transl Res 155: 164–165. [DOI] [PubMed] [Google Scholar]
- 9.You NC, Chen BH, Song Y, Lu X, Chen Y, et al.. (2012) A Prospective Study of Leukocyte Telomere Length and Risk of Type 2 Diabetes in Postmenopausal Women. Diabetes. [DOI] [PMC free article] [PubMed]
- 10. Shen Q, Zhao X, Yu L, Zhang Z, Zhou D, et al. (2012) Association of leukocyte telomere length with type 2 diabetes in mainland Chinese populations. J Clin Endocrinol Metab 97: 1371–1374. [DOI] [PubMed] [Google Scholar]
- 11. Xiao F, Zheng X, Cui M, Shi G, Chen X, et al. (2011) Telomere dysfunction-related serological markers are associated with type 2 diabetes. Diabetes Care 34: 2273–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Testa R, Olivieri F, Sirolla C, Spazzafumo L, Rippo MR, et al. (2011) Leukocyte telomere length is associated with complications of type 2 diabetes mellitus. Diabet Med 28: 1388–1394. [DOI] [PubMed] [Google Scholar]
- 13. Zee RY, Castonguay AJ, Barton NS, Germer S, Martin M (2010) Mean leukocyte telomere length shortening and type 2 diabetes mellitus: a case-control study. Transl Res 155: 166–169. [DOI] [PubMed] [Google Scholar]
- 14. Salpea KD, Talmud PJ, Cooper JA, Maubaret CG, Stephens JW, et al. (2010) Association of telomere length with type 2 diabetes, oxidative stress and UCP2 gene variation. Atherosclerosis 209: 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Olivieri F, Lorenzi M, Antonicelli R, Testa R, Sirolla C, et al. (2009) Leukocyte telomere shortening in elderly Type2DM patients with previous myocardial infarction. Atherosclerosis 206: 588–593. [DOI] [PubMed] [Google Scholar]
- 16. Wentzensen IM, Mirabello L, Pfeiffer RM, Savage SA (2011) The association of telomere length and cancer: a meta-analysis. Cancer Epidemiol Biomarkers Prev 20: 1238–1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 19. Yang H, Jin X, Kei Lam CW, Yan SK (2011) Oxidative stress and diabetes mellitus. Clin Chem Lab Med 49: 1773–1782. [DOI] [PubMed] [Google Scholar]
- 20. Eckers A, Altschmied J, Haendeler J (2012) [Oxidative stress in endothelial cells and in diabetes type 2]. Z Gerontol Geriatr 45: 90–94. [DOI] [PubMed] [Google Scholar]
- 21. Wolff SP, Jiang ZY, Hunt JV (1991) Protein glycation and oxidative stress in diabetes mellitus and ageing. Free Radic Biol Med 10: 339–352. [DOI] [PubMed] [Google Scholar]
- 22. Sampson MJ, Winterbone MS, Hughes JC, Dozio N, Hughes DA (2006) Monocyte telomere shortening and oxidative DNA damage in type 2 diabetes. Diabetes Care 29: 283–289. [DOI] [PubMed] [Google Scholar]
- 23. Maiese K, Morhan SD, Chong ZZ (2007) Oxidative stress biology and cell injury during type 1 and type 2 diabetes mellitus. Curr Neurovasc Res 4: 63–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Henriksen EJ, Diamond-Stanic MK, Marchionne EM (2011) Oxidative stress and the etiology of insulin resistance and type 2 diabetes. Free Radic Biol Med 51: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. von Zglinicki T (2000) Role of oxidative stress in telomere length regulation and replicative senescence. Ann N Y Acad Sci 908: 99–110. [DOI] [PubMed] [Google Scholar]
- 26. von Zglinicki T (2002) Oxidative stress shortens telomeres. Trends Biochem Sci 27: 339–344. [DOI] [PubMed] [Google Scholar]
- 27. Bar-Or D, Thomas GW, Rael LT, Lau EP, Winkler JV (2001) Asp-Ala-His-Lys (DAHK) inhibits copper-induced oxidative DNA double strand breaks and telomere shortening. Biochem Biophys Res Commun 282: 356–360. [DOI] [PubMed] [Google Scholar]
- 28. Kanvah S, Schuster GB (2005) The sacrificial role of easily oxidizable sites in the protection of DNA from damage. Nucleic Acids Res 33: 5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Oikawa S, Kawanishi S (1999) Site-specific DNA damage at GGG sequence by oxidative stress may accelerate telomere shortening. FEBS Lett 453: 365–368. [DOI] [PubMed] [Google Scholar]
- 30. Harte AL, da Silva NF, Miller MA, Cappuccio FP, Kelly A, et al. (2012) Telomere length attrition, a marker of biological senescence, is inversely correlated with triglycerides and cholesterol in South Asian males with type 2 diabetes mellitus. Exp Diabetes Res 2012: 895185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Eisenberg DT, Salpea KD, Kuzawa CW, Hayes MG, Humphries SE (2011) Substantial variation in qPCR measured mean blood telomere lengths in young men from eleven European countries. Am J Hum Biol 23: 228–231. [DOI] [PubMed] [Google Scholar]
- 32. Diez Roux AV, Ranjit N, Jenny NS, Shea S, Cushman M, et al. (2009) Race/ethnicity and telomere length in the Multi-Ethnic Study of Atherosclerosis. Aging Cell 8: 251–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Quality assessment of association studies between telomere length and T2DM.
(DOC)
PRISMA checklist.
(DOC)